Characteristics, Treatment Patterns, and Outcomes of Patients with Multiple Myeloma, Including Those Who are Triple-Class Exposed: A Retrospective Cohort Study in England Using National Cancer Registry Data
Funding: This study was sponsored by Pfizer.
Additional supporting information can be found online in the Supporting Information section.
POST SUMMARY:
The aim of this study using real-world data was to generate up-to-date evidence on the management and outcomes of multiple myeloma (MM) in England, given the complex and rapidly evolving treatment landscape. This publication presents recent national data on demographic and clinical characteristics of patients with MM, and insights into their treatment patterns and outcomes.
ABSTRACT
Introduction
Multiple myeloma (MM) prognosis worsens once patients become triple-class exposed (TCE) to at least one treatment in each class: immunomodulators, proteasome inhibitors, and anti-CD38 monoclonal antibodies.
Methods
We conducted a retrospective study using the Cancer Analysis System database to assess characteristics, treatment patterns, and clinical outcomes for adults diagnosed with MM between 2014 and 2020. The main cohort included patients ≥18 years-old diagnosed with incident MM (including TCE patients) who had at least one record of systemic anticancer therapy treatment within 30 days prior to, on, or any time after their diagnosis.
Results
The main cohort comprised 14,990 patients, predominantly white and male, with a median age at diagnosis of 71 years. Of these, 848 (5.6%) became TCE. In the main cohort (n = 14,990), 57.2% of patients received only one line of therapy, and >50% of all first-line regimens included bortezomib. Median overall survival (OS) from diagnosis was 51.5 months. After becoming TCE (n = 848), median OS was 13.2 months and median time to next treatment or death was 5.7 months.
Conclusions
This study provides current evidence on real-world OS and management for patients with MM in England, including those who become TCE.
1 Introduction
Multiple myeloma (MM) is a plasma cell neoplasm associated with considerable morbidity and 5-year survival of approximately 50% [1-3]. Despite many available treatment combinations, the disease is considered incurable. Patients become increasingly refractory to treatment, with shorter duration of remission with each line of therapy (LoT) [1, 4, 5].
The treatment landscape for MM in England is complex and rapidly evolving, with many approvals of novel therapies and treatment combinations in recent years, including monoclonal antibodies (mAbs) and other new classes of drugs [1, 6-10]. Reimbursed therapies for treating MM in the UK currently include alkylating agents, immunomodulators (IMiDs), proteasome inhibitors (PIs), anti-CD38 mAbs, B-cell maturation antigen (BCMA) bispecific antibodies, histone deacetylase (HDAC) inhibitors, and exportin 1 (XPO1) inhibitors [1, 7, 11–13].
As newer treatment classes are licensed and increasingly used, patients may become exposed to at least one drug from each of the following classes: IMiD, PI, and anti-CD38 mAb. These patients are considered triple-class exposed (TCE) and tend to have poor prognosis and limited treatment options [14, 15]. The increasing availability of novel therapies and combinations throughout the treatment pathway has also resulted in patients becoming TCE in earlier LoTs [16]. Patients with TCE relapsed/refractory MM (RRMM) require additional management strategies due to their lower overall response rates and shorter progression-free survival (PFS), which contribute to a poorer prognosis [1, 15, 17]. Limited evidence exists on real-world treatments and outcomes for patients with RRMM in England, especially after becoming TCE [17].
We report results from an analysis of real-world electronic healthcare data from patients with MM in England over an 8-year period, using the Cancer Analysis System (CAS) database [18]. Patient characteristics, treatment patterns, and clinical outcomes are described.
2 Materials and Methods
2.1 Study Design
This was a retrospective, population-based cohort study using data from patients newly diagnosed with MM between January 1, 2014 and December 31, 2020 (identification period) and treated with systemic anticancer therapy (SACT) at National Health Service (NHS) hospitals in England. Patients were followed from the date of MM diagnosis until death, loss to follow-up, or May 31, 2022 (end of data availability), whichever occurred earliest.
2.2 Database
This work used data provided by patients and collected by the NHS as part of their care and support. The data are collated, maintained, and quality-assured by the National Disease Registration Service, which is part of NHS England. Access to these data was facilitated by the Simulacrum produced by Health Data Insight (HDI) Community Interest Company (Section 1, Supporting Information); analyses were conducted by HDI on anonymized data in consultation with the authors, and only aggregated data were shared with the authors. The CAS database contains anonymized patient-level data on nearly all cancer patients in England (Section 1, Supporting Information) and comprises the Cancer Outcomes and Services Dataset (for diagnoses, tumor characteristics, and death) and the SACT dataset (for treatments). CAS was also linked to the Hospital Episode Statistics (HES) database for stem cell transplant (SCT) data and to the Office for National Statistics database for death data. Diagnoses were recorded using International Classification of Diseases for Oncology 10th Revision (ICD-10) codes, and HES procedures recorded using Office of Population Censuses and Surveys (OPCS) Classification of Interventions and Procedures (OPCS-4) codes. Approval from an ethics committee was not needed since this study did not involve individually identifiable patient data.
Treatment information in CAS is recorded at the SACT regimen (i.e., mono-/combination therapy) level. This dataset captures nearly all cancer treatments in hospital inpatient and outpatient and community settings, including traditional chemotherapy drugs (infusion/injection/orals), biologics, immunotherapy, hormones, and drugs for patients treated in clinical trials. Treatments received as part of a clinical trial or that were not systemic treatments were excluded from the study. The remaining SACT treatments were combined into LoTs using a bespoke algorithm, based on published treatment guidance, expert clinical input (Section 2, Supporting Information) and previous research [19].
2.3 Study Populations
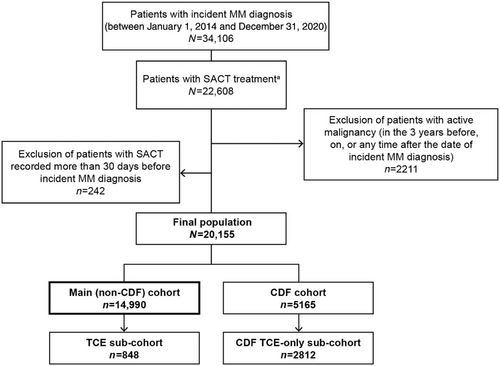
Patients were included in the study if they: (a) had an incident diagnosis of MM (ICD-10 code C90.0 for both symptomatic MM and asymptomatic Smouldering MM [SMM]) within the identification period; (b) were aged ≥18 years at diagnosis; and (c) had a record of SACT treatment ≤30 days before, on, or any time after incident MM diagnosis. Patients were excluded if they had: (a) a diagnosis of any other malignancy within 1095 days before, on, or any time after the diagnosis date (except for nonmelanoma skin cancer [ICD-10 code C44.x] or extramedullary disease, including plasma cell leukemia [ICD-10 code C90.1], extramedullary plasmacytoma [ICD-10 code C90.2], and solitary plasmacytoma [ICD-10 code C90.3]), or a diagnosis of MM in their entire record prior to incident diagnosis; (b) a record of any SACT treatment >30 days before incident MM diagnosis; or (c) no vital status reported at any point in their records.
During the study period, there was a restriction on analyzing treatment data for patients receiving therapies funded by the NHS England Cancer Drugs Fund (CDF) (detailed therapy list available in Section 3, Supporting Information and Table S3.1). The CDF program provides patients with early access to new treatments while collecting additional evidence of clinical effectiveness, typically through the SACT database [20]. Consequently, although the total number of patients could be reported, treatment patterns or outcomes for CDF-funded patients could not be analyzed. Thus, these patients were included separately in the demographic descriptions and excluded from the other analysis.
2.4 Study Outcomes and Analyses
Demographic and clinical characteristics were described for all cohorts and included age and sex at diagnosis, ethnicity, disease stage (International Staging System), Eastern Cooperative Oncology Group (ECOG) score, number of LoTs initiated during follow-up, and record of SCT. The distribution and sequencing of treatment regimens were assessed by LoT and described for both the main cohort and TCE sub-cohort.
Overall survival (OS), defined as time (months) from incident MM diagnosis to death due to any cause, was described from diagnosis for patients in the main cohort and from the start of the LoT in which a patient became TCE for patients in the TCE sub-cohort. OS from diagnosis was not reported in the TCE sub-cohort because of immortal time bias, since patients must have survived to later LoTs to become TCE. To further understand treatment outcomes in UK patients with RRMM, time to next treatment or death (TTNTD) was reported in the TCE sub-cohort; TTNTD was defined as the time from the LoT in which a patient became TCE to next treatment or death due to any cause, whichever occurred earliest. Censoring events for both OS and TTNTD were earliest occurrence of loss to follow-up or end of survival ascertainment period (May 31, 2022).
2.5 Statistics
Descriptive statistics were used to summarize patients’ characteristics. SACT regimens received after MM diagnosis were described at regimen- and class-level using Sankey diagrams. The seven treatment classes considered were IMiDs, PIs, anti-CD38 mAbs, alkylating agents, HDAC inhibitors, SCT, and “other” (i.e., treatments not belonging to any specified class). Corticosteroids and other supportive therapies were not analyzed owing to poor reporting in CAS.
Time-to-event analyses (OS and TTNTD) were performed using the Kaplan–Meier method. No imputation for missing data was performed. R version 4.1.2 or higher was used for all analyses.
3 Results
3.1 Demographic and Clinical Characteristics
In total, 20,155 eligible patients with MM (including SMM) were identified in CAS (Figure 1), of whom 5165 (25.6%) received a CDF drug (CDF cohort), and 14,990 (74.4%) did not (main cohort). Patients in the main cohort tended to be White and male, with a median age at diagnosis of 71 years (interquartile range [IQR]: 62–79; Table 1). Staging and ECOG score at diagnosis were not well recorded, with 62.8 and 49.8% of records missing, respectively.
| Characteristics |
Main cohort (n = 14,990) |
TCE sub-cohort (n = 848) |
|---|---|---|
| Age at diagnosis, median years (IQR Q1–Q3) | 71 (62–79) | 69 (60–75) |
| Sex at diagnosis, n (%) | ||
| Male | 8659 (57.8) | 499 (58.8) |
| Female | 6331 (42.2) | 349 (41.2) |
| Ethnicity, n (%) | ||
| White | 12,946 (86.4) | 735 (86.7) |
| Asian | 510 (3.4) | 21 (2.5) |
| Black | 697 (4.7) | 51 (6.0) |
| Other | 253 (1.7) | 17 (2.0) |
| Mixed | 80 (0.5) | –a |
| Missing | 504 (3.4) | –b |
| Diagnosed with EMD, n (%)c | 64 (0.4) | –a |
| Disease stage (ISS), n (%) | ||
| I | 1379 (9.2) | 65 (7.7) |
| II | 1592 (10.6) | 101 (11.9) |
| III | 1619 (10.8) | 123 (14.5) |
| Other staging system | 992 (6.6) | 38 (4.5) |
| Missing | 9408 (62.8) | 521 (61.4) |
| CCI score, n (%) | ||
| 0 | 8876 (59.2) | 562 (66.3) |
| 1 | 2820 (18.8) | 157 (18.5) |
| 2 | 1655 (11.0) | 62 (7.3) |
| 3 | 912 (6.1) | 43 (5.1) |
| 4+ | 727 (4.9) | 24 (2.8) |
| ECOG score, % | ||
| 0 | 2902 (19.4) | 187 (22.0) |
| 1 | 2873 (19.2) | 186 (21.9) |
| 2 | 1152 (7.7) | 57 (6.7) |
| 3 | 487 (3.3) | 21 (2.5) |
| 4 | 107 (0.7) | 6 (0.7) |
| Missing | 7469 (49.8) | 391 (46.1) |
| Number of LoTs initiated during follow-up, n (%) | ||
| 1 | 8566 (57.1) | 38 (4.5) |
| 2 | 3812 (25.4) | 24 (2.8) |
| 3 | 1532 (10.2) | 166 (19.6) |
| 4 | 617 (4.1) | 284 (33.5) |
| 5 | 285 (1.9) | 205 (24.2) |
| ≥6 | 178 (1.2) | 131 (15.5) |
| Record of SCT on or after MM diagnosis | ||
| Yes | 4140 (27.6) | 226 (26.7) |
| No | 10,850 (72.4) | 622 (73.4) |
- Abbreviations: CAS, Cancer Analysis System; CCI, Charlson Comorbidity Index; ECOG, Eastern Cooperative Oncology Group; EMD, extramedullary disease; IQR, interquartile range; ISS, International Staging System; LoT, line of therapy; MM, multiple myeloma; Q, quartile; SCT, stem cell transplant; TCE, triple-class exposed.
- a Numbers 1–5 are masked, as per CAS reporting rules.
- b Denotes secondary suppression to prevent back-calculation of masked (a) values.
- c This number incorporates EMD diagnoses before, during, and after MM diagnosis.
In total, 848 patients became TCE (5.7%) in the main cohort (TCE sub-cohort), and 2812 became TCE (54.4%) in the CDF cohort (CDF TCE sub-cohort). Demographic and clinical characteristics for the main cohort and the TCE sub-cohort are presented in Table 1. Demographic characteristics for the CDF cohort and the CDF TCE sub-cohort are presented in Section 4, Supporting Information and Table S4.1.
Median follow-up from diagnosis was 32.6 months (IQR: 18.1–53.4 months) in the main cohort and 52.1 months (IQR: 35.1–70.7 months) in the TCE sub-cohort. During follow-up, 57.2% patients in the main cohort received only one LoT and 27.6% of patients received an SCT. Median age at initiation of first LoT was 72 years (IQR: 63–79 years; Table 2) and median available follow-up time from first LoT was 27.0 months (IQR: 12.2–45.3 months), suggesting that first LoT was generally received within a year of diagnosis. The observed latency between diagnosis and first LoT may be linked to the inclusion of asymptomatic SMM patients, who only start treatment upon progression to symptomatic disease.
|
First LoT (n = 14,990) |
Second LoT (n = 6424) |
Third LoT (n = 2612) |
Fourth LoT (n = 1080) |
Fifth LoT (n = 463) |
Sixth LoT (n = 178) |
|
|---|---|---|---|---|---|---|
|
Age at diagnosis, median years (IQR Q1–Q3) |
71 (62–79) | 71 (62–78) | 71 (62–77) | 69 (60–75) | 68 (59–74) | 66 (56–71) |
|
Age at line initiation, median years (IQR Q1–Q3) |
72 (63–79) | 73 (64–80) | 73 (64–80) | 72 (63–79) | 72 (63–78) | 70 (60–76) |
| CCI score at diagnosis, n (%) | ||||||
| 0 | 8876 (59.2) | 3960 (61.6) | 1673 (64.1) | 729 (67.5) | 302 (65) | 115 (65) |
| 1 | 2820 (18.8) | 1221 (19.0) | 469 (18.0) | 188 (17.4) | 96 (21) | 37 (21) |
| 2 | 1655 (11.0) | 670 (10.4) | 248 (9.5) | 86 (8.0) | 31 (7) | 15 (8) |
| 3 | 912 (6.1) | 340 (5.3) | 131 (5.0) | 51 (4.7) | 24 (5) | –b |
| 4+ | 727 (4.9) | 233 (3.6) | 91 (3.5) | 26 (2.4) | 10 (2) | –a |
| ECOG score at line initiation, n (%) | ||||||
| 0 | 3208 (21.4) | 1276 (19.9) | 453 (17.3) | 166 (15.4) | 71 (15.3) | 23 (12.9) |
| 1 | 5283 (35.2) | 2424 (37.7) | 977 (37.4) | 425 (39.4) | 174 (37.6) | 78 (43.8) |
| 2 | 2177 (14.5) | 971 (15.1) | 486 (18.6) | 241 (22.3) | 104 (22.5) | 34 (19.1) |
| 3 | 659 (4.4) | 208 (3.2) | 100 (3.8) | – a | –b | 8 (4.5) |
| 4 | 127 (0.9) | 27 (0.4) | 11 (0.42) | –b | –a | 0 (0) |
| Missing | 3536 (23.6) | 1518 (23.6) | 585 (22.4) | 219 (20.3) | 97 (21.0) | 35 (19.7) |
- Abbreviations: CAS, Cancer Analysis System; CCI, Charlson Comorbidity Index; ECOG, Eastern Cooperative Oncology Group; IQR, interquartile range; LoT, line of therapy; Q, quartile.
- a Numbers 1–5 are masked, as per CAS reporting rules.
- b Denotes secondary suppression to prevent back-calculation of masked (a) values.
Patient demographics in the TCE sub-cohort were broadly similar to the main cohort, although patients were slightly younger at diagnosis (median age of 69 years [IQR: 60–75 years]). These patients had a median age of 72 years (IQR: 63–78 years) at the start of the LoT in which they became TCE, indicating that this took approximately 3 years from initial diagnosis, consistent with the finding that among patients who became TCE, this tended to occur at third (33.1%) or fourth LoTs (49.2%).
3.2 Distribution of SACT Regimens
In the main cohort (Table 3), bortezomib-based regimens accounted for over half of all first LoT regimens. Lenalidomide was mostly in second (30.0%) and third LoTs (34.6%), daratumumab in fourth (36.2%), and pomalidomide in fifth (41.3%), sixth, and subsequent LoTs (21.6%). Few regimens containing an SCT were observed beyond second LoT (see Supplementary Section 5, Table S5.1).
| Overall |
First LoT |
Second LoT |
Third LoT |
Fourth LoT |
Fifth LoT |
Sixth LoT + |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | |
| Total | 25,814 | 100.0% | 14,990 | 58.1% | 6424 | 24.9% | 2612 | 10.1% | 1080 | 4.2% | 463 | 1.8% | 245 | 1.0% |
| SACT regimen | ||||||||||||||
| Lenalidomide | 4088 | 15.8% | 1154 | 7.7% | 1930 | 30.0% | 904 | 34.6% | 85 | 7.9% | b | b | a | a |
| Bortezomib + cyclophosphamide | 3549 | 13.8% | 2993 | 20.0% | 490 | 7.6% | 54 | 2.1% | b | b | 0 | 0% | a | a |
| Bortezomib | 2544 | 9.9% | 1974 | 13.2% | 485 | 7.6% | 62 | 2.4% | 16 | 1.5% | a | a | a | a |
| Cyclophosphamide + thalidomide | 1965 | 7.6% | 1256 | 8.4% | 572 | 8.9% | 89 | 3.4% | 26 | 2.4% | 10 | 2.2% | 12 | 4.9% |
| Bortezomib + thalidomide | 1423 | 5.5% | 1275 | 8.5% | 133 | 2.1% | b | b | 0 | 0% | a | a | 0 | 0% |
| Bortezomib + melphalan | 1173 | 4.5% | 1095 | 7.3% | 68 | 1.1% | b | b | a | a | 0 | 0% | 0 | 0% |
| Pomalidomide | 879 | 3.4% | 14 | 0.1% | 107 | 1.7% | 285 | 10.9% | 229 | 21.2% | 191 | 41.3% | 53 | 21.6% |
| Daratumumab | 866 | 3.4% | 32 | 0.2% | 90 | 1.4% | 294 | 11.3% | 391 | 36.2% | 53 | 11.5% | 6 | 2.5% |
| Ixazomib + lenalidomide | 724 | 2.8% | 29 | 0.2% | 553 | 8.6% | 128 | 4.9% | b | b | a | a | 0 | 0% |
| Cyclophosphamide | 641 | 2.5% | 233 | 1.6% | 243 | 3.8% | 101 | 3.9% | 27 | 2.5% | 19 | 4.1% | 18 | 7.4% |
- Abbreviations: CAS, Cancer Analysis System; LoT, line of therapy; SACT, systemic anticancer treatment (dataset).
- a Numbers 1–5 are masked, as per CAS reporting rules.
- b Denotes secondary suppression to prevent back-calculation of masked (a) values.
A total of 3590 LoTs were recorded in the TCE sub-cohort; daratumumab was the most common, representing 20.1% of all LoTs, and occurring most frequently in fourth LoT (see Section 5, Supporting Information and Table S5.2). Regimens received in the TCE sub-cohort were similar to those received in the main cohort (Table 4).
| Overall |
First LoT |
Second LoT |
Third LoT |
Fourth LoT |
Fifth LoT |
Sixth LoT + |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | |
| Total | 3590 | 100.0% | 848 | 23.6% | 810 | 22.6% | 786 | 21.9% | 620 | 17.3% | 336 | 9.4% | 190 | 5.3% |
| SACT regimen | ||||||||||||||
| Daratumumab | 720 | 20.1% | a | a | 32 | 4.0% | 251 | 31.9% | 379 | 61.1% | 51 | 15.2% | a | b |
| Lenalidomide | 488 | 13.6% | 16 | 1.9% | 237 | 29.3% | 207 | 26.3% | 19 | 3.1% | a | a | a | a |
| Pomalidomide | 360 | 10.0% | a | a | b | b | 57 | 7.3% | 101 | 16.3% | 146 | 43.5% | 43 | 22.6% |
| Bortezomib + cyclophosphamide | 270 | 7.5% | 186 | 21.9% | 67 | 8.3% | 9 | 1.2% | a | a | 0 | 0% | a | a |
| Cyclophosphamide + thalidomide | 221 | 6.2% | 78 | 9.2% | 94 | 11.6% | 28 | 3.6% | b | b | a | a | 8 | 4.2% |
| Bortezomib | 214 | 6.0% | 128 | 15.1% | 64 | 7.9% | 14 | 1.8% | a | a | a | a | a | a |
| Bortezomib + panobinostat | 138 | 3.8% | 0 | 0% | a | a | b | b | 27 | 4.4% | 45 | 13.4% | 40 | 21.1% |
| Bortezomib + thalidomide | 130 | 3.6% | 107 | 12.6% | b | b | a | a | 0 | 0.0% | 0 | 0% | 0 | 0% |
| Cyclophosphamide + lenalidomide | 97 | 2.7% | a | a | 50 | 6.2% | 36 | 4.6% | a | a | a | a | 0 | 0% |
| Cyclophosphamide | 88 | 2.5% | 6 | 0.7% | 21 | 2.6% | 22 | 2.8% | 10 | 1.6% | 12 | 3.6% | 17 | 9.0% |
- Abbreviations: CAS, Cancer Analysis System; LoT, line of therapy; SACT, systemic anticancer treatment (dataset).
- a Numbers 1–5 are masked, as per CAS reporting rules.
- b Denotes secondary suppression to prevent back-calculation of masked (a) values.
3.3 Treatment Sequencing
Sankey diagrams for the main cohort and TCE sub-cohort are presented in Section 5, Supporting Information and Figures S5.1 and S5.2. In the main cohort, bortezomib plus thalidomide was the most common first LoT induction therapy regimen before SCT (n = 1614 [39.0% of all patients who received a SCT]). Among those who received first LoT, 4005 patients died (26.7%) and 4561 (30.4%) did not receive any further treatment before the end of follow-up. Among the patients who received a second LoT (n = 6424), the most frequent treatment at second LoT was lenalidomide (n = 1930 [30.0%]).
In the TCE sub-cohort, 261 patients died (30.8%) and 181 (21.3%) did not receive any further treatment during the study follow-up period after becoming TCE. Most patients (over 80.0%) received daratumumab as mono- or combination therapy in the LoT in which they became TCE. Among the patients who received a second LoT (n = 406), the most common subsequent regimens were pomalidomide (n = 238 [59%]) and bortezomib plus panobinostat (n = 44 [11%]). Sequences were described up to fourth LoT for the TCE sub-cohort (see Section S5.2, Supporting Information).
3.4 Overall Survival
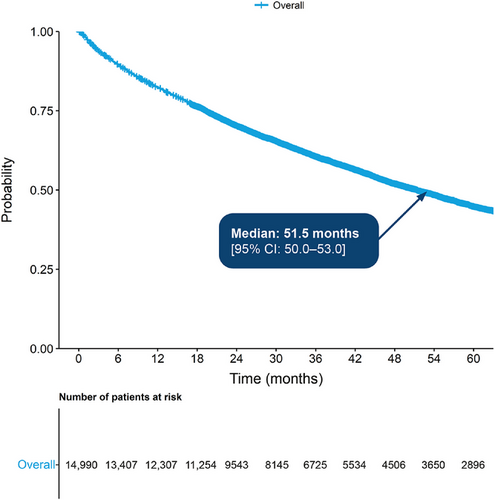
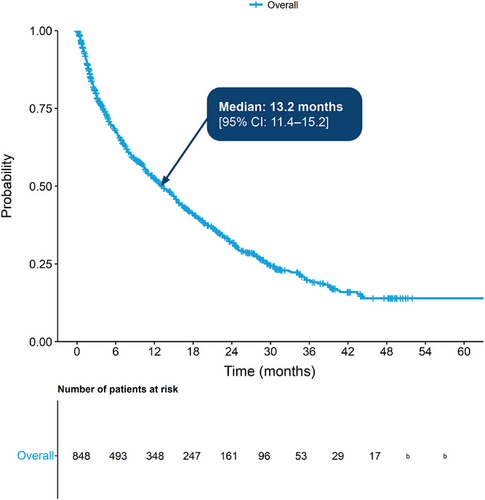
In the main cohort, median OS from diagnosis was 51.5 months (95% confidence interval [CI]: 50.0–53.0 months) (Figure 2). The probability of surviving beyond 12 months from diagnosis was 82.0%, while the probability of surviving beyond 60 months was 45.0% (95% CI: 0.4–0.5). In the TCE sub-cohort, median OS from the point of becoming TCE was 13.2 months (95% CI: 11.4–15.2 months), with 67.0% surviving beyond 6 months and 32.0% beyond 24 months (Figure 3).
3.5 Time to Next Treatment or Death
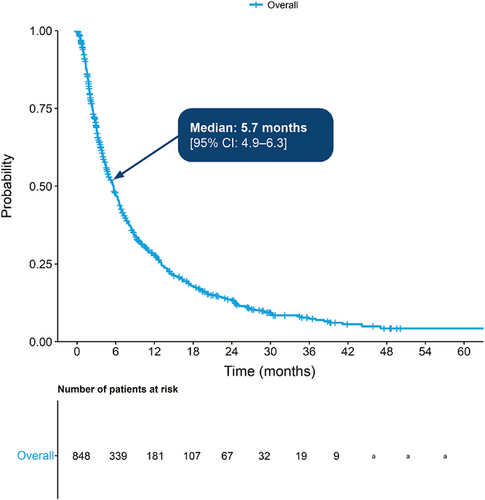
Median TTNTD from the start of the LoT in which a patient became TCE was 5.7 months (4.9–6.3 months), with 28.0% surviving beyond 12 months while on treatment (Figure 4).
4 Discussion
This study provides up-to-date, real-world evidence on the characteristics, treatment patterns, and outcomes of patients with MM in England. The main study cohort reflected the general MM population in England; on average, 4872 patients per year were identified from CAS (before applying study eligibility criteria), which aligns with Cancer Research UK (CRUK) estimates of 5041 new cases of MM per year in England [21]. Patients were predominantly White and male, with a median age at diagnosis of 71 years, which also aligns with CRUK estimates and previous research [22-24].
In this study, 30.4% of patients received only one LoT and remained alive until the end of follow-up, potentially indicating long term remission during the study period. Treatment regimens generally aligned with NHS treatment guidance, although some divergence can be expected due to the use of an algorithm to group treatments into LoTs. Lenalidomide was the most frequently used treatment in the main cohort across all LoTs, while bortezomib plus cyclophosphamide (first and third LoT) and lenalidomide (second LoT) were the most commonly used in patients who were TCE. There was low use of panobinostat–bortezomib–dexamethasone at third and fourth LoTs compared with fifth and sixth LoTs. Patients became TCE approximately 3 years after diagnosis, mostly at third or fourth LoT, indicated by high daratumumab use in these LoTs. This demonstrates that the majority of patients become TCE at third and fourth LoTs, as a result of daratumumab use at the fourth LoT.
Median OS in the main study cohort was 51.5 months with approximately 45% of patients surviving ≥5 years. This is slightly lower than published estimates of approximately 50% in the UK [3] and may be due to excluding patients from outcome analyses who received new drugs funded by the CDF, such as daratumumab (as 2nd LoT), ixazomib (as 3rd or 4th LoT), and isatuximab (as 4th LoT), which may be associated with improved outcomes. Accordingly, the CDF cohorts, which comprised one-quarter of the total patients, were generally younger and had fewer comorbidities at diagnosis than the main cohort.
Among TCE patients, only 32% survived beyond 24 months from the point of becoming TCE. The prognosis for patients once they become TCE remains poor, reflecting previous findings, as treatment options remain limited [4, 5, 14, 17]. In the TCE sub-cohort, median TTNTD was 5.68 months and median OS was 13.21 months from the point of becoming TCE. While TTNTD from the point of becoming TCE and published estimates of PFS are not directly comparable, our results are similar to the median PFS of 4.6 months and median OS of 12.4 months in the LocoMMotion study of TCE RRMM patients (n = 248) in Europe and the United States [14].
A retrospective study by Elsada et al. [17] reported survival in a UK cohort of 366 patients with TCE RRMM after three or more LoTs, using the same data source as the current study, but an older version (their study period was between January 1, 2013 and December 31, 2018). They reported median OS of 8.2 months and median TTNTD of 5.3 months in their TCE cohort [17]. Our study provides an additional 30 months of follow-up and while our cohort was less restricted (i.e., not limited to patients who received at least three prior LoTs), both studies found similarly poor outcomes, reiterating the ongoing need for effective treatments for TCE RRMM.
Differences between Elsada et al. [17] and our study may reflect changes in the treatment pathway since the Elsada et al. study was undertaken, with the introduction of novel regimens including greater use of anti-CD38 regimens [7, 16], such as daratumumab, bortezomib, thalidomide, and dexamethasone (DVTd) [7, 16, 25]. However, the TCE group in this study remains similar to the Elsada population as anti-CD38 regimens were not routinely available within the NHS in the first 4 years of our time period (2014–2020), and CDF restrictions on daratumumab plus bortezomib and dexamethasone (DVd) and isatuximab/pomalidomide regimen data were still in place. As daratumumab was recommended by NICE as monotherapy in 2018, it only featured in the final year of analysis in Elsada et al. [17, 25].
The MAMMOTH study comprised 275 US patients with MM who were refractory to anti-CD38 mAb therapy (daratumumab or isatuximab). Median OS was 8.6 (95% CI: 7.5–9.9) months, comparable with the current study, and median PFS and OS from the next regimen after becoming refractory were 3.4 and 9.3 months, respectively, highlighting unmet treatment needs for anti-CD38-refractory patients [15].
The treatment landscape for MM continues to evolve [1, 7, 9, 25]. DVd [26] was approved within the CDF during the study period, while DVTd and daratumumab plus lenalidomide and dexamethasone were approved later, close to study end [25, 27]. As a result, patients in the UK now can become TCE from first line onward in their treatment pathway [16]. Additional therapies, including BCMA bispecific antibodies and XPO1 inhibitors, were approved after the end of the study period [12, 13]. Analyses of the CAS database should therefore be repeated in the future to assess the impact of new treatment approvals on outcomes.
4.1 Strengths and Limitations
The CAS database used in this study captures data on nearly all patients diagnosed with and treated for MM in NHS hospitals in England. Some clinical information needed for richer characterization of patients with MM and their treatments is not comprehensively recorded in CAS, such as ECOG score, stage at diagnosis, supportive therapies, and steroid treatments. Furthermore, SCT eligibility could not be assessed with CAS or HES data, and no stratifications were undertaken by SCT status.
The LoT algorithm used here is an innovative methodology developed for this study; however, a degree of regimen misclassification cannot be ruled out based on the way the algorithm grouped treatments into LoTs. For example, daratumumab was not approved for use at third LoT, but some patients may have received a steroid-only regimen that did not show up in CAS; therefore, treatments used in the following LoT may have been incorrectly attributed to the third instead of the fourth LoT. A potential survival bias was observed in the TCE sub-cohort, as these patients were more likely than the main cohort to receive at least a fourth LoT during follow-up. This may be expected in a younger, fitter patient group who were likely to survive long enough to be exposed to multiple LoTs.
The exclusion of patients who received any drug on the CDF register (Section 3, Supporting Information and Table S3.1) at the time of analysis may have affected the results on treatment patterns and outcomes. As such, it was not possible to report these in approximately one-quarter of the main cohort and in three-quarters of identified TCE patients, limiting the generalizability of these findings to the real-world MM population in England.
Finally, while our results demonstrate continuing unmet need in patients with RRMM, future research on treatment patterns and outcomes would benefit from new data fields in the CAS database, specifically for refractoriness and disease progression to reduce reliance on proxy measures and increase the robustness of analyses.
5 Conclusion
The treatment pathway for MM in England remains complicated, with many treatment regimens used within and across LoTs. This study provides up-to-date evidence on patient characteristics, treatment patterns, and clinical outcomes for patients with MM in England from initial diagnosis onward, including those who eventually become TCE and for whom outcomes remain poor. However, analyses on treatments and outcomes were limited by CDF reporting restrictions in place during the study as well as missing ECOG score and disease staging for at least half of patients.
Author Contributions
Carmen Tsang, Kellyn Arnold, Charles Duffield, Stavros Oikonomou, Tarana Mehdikhanova, Samuel Wood, and Mamta Garg contributed to study design. Carmen Tsang, Kellyn Arnold, Stavros Oikonomou, and Tarana Mehdikhanova were responsible for data acquisition. Kellyn Arnold, Stavros Oikonomou, and Tarana Mehdikhanova contributed to data analysis. All authors contributed to interpretation of the results. All authors contributed to manuscript development and approved the final version of the manuscript for submission.
Acknowledgments
Support for medical writing, editorial assistance, and graphic design were provided by Rebecca Hopkins via IQVIA, and by Cristiana Miglio, Daria Renshaw, and Samila Sakhabuth of IQVIA, funded by Pfizer. Programming of study methodology and data analysis were provided by Alexandrina Lambova of IQVIA. Anne Broe and Saskia Hagenaars (senior epidemiologists) from IQVIA were involved in study design and interpretation. Eleanor Ralphs and Sarah Lay-Flurrie (senior statisticians) were involved in study methods and interpretation.
Ethics Statement
The study was conducted in accordance with legal and regulatory requirements, as well as with scientific purpose, value, and rigor and follow generally accepted research practices described by the International Society for Pharmacoepidemiology. There was no need to seek approval from an ethics commission for this study, since it did not involve the collection, use, or transmittal of individually identifiable patient data.
Conflicts of Interest
Carmen Tsang, Thomas Price, and Samuel Wood are employees of Pfizer and may hold stock or stock options. Charles Duffield was an employee of Pfizer at the time of the study and may hold stock or stock options. Kellyn Arnold, Stavros Oikonomou, and Tarana Mehdikhanova are employees of IQVIA, which received funding from Pfizer to conduct this study. Mamta Garg received consulting fees from Pfizer as the clinical advisor on this study but did not receive any payment from Pfizer in connection with the development of this manuscript. Mamta Garg has received honoraria to attend international or national meetings, advisory boards, educational lectures from various pharmaceutical companies, namely, Amgen, BMS, Celgene, J&J, Menarini, Novartis, Pfizer, and Stemline.
Consent
As this study involved anonymized structured data, which according to applicable legal requirements do not contain data subject to privacy laws, obtaining informed consent from patients was not required.
Clinical Trial Registration
The authors have confirmed clinical trial registration is not needed for this submission.
Open Research
Data Availability Statement
Data are available in the Supporting Information, including details of algorithm development and use, variable deviations, additional demographic, and longitudinal pathway data. Data are owned by the NHS and were accessed by IQVIA through Health Data Insights (HDI) via a partnership agreement with the National Disease Registration Service (NDRS).




