MicroRNA-144 regulates cancer cell proliferation and cell-cycle transition in acute lymphoblastic leukemia through the interaction of FMN2
Abstract
Background
In the present study, we explored the functional roles of microRNA-144 (miR-144) upregulation and downregulation in human acute lymphoblastic leukemia (ALL).
Methods
Gene expression of miR-144 was examined using the quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) in both ALL cell lines and T-leukemic cells of ALL patients. In ALL cell lines Molt-3 and Jurkat cells, miR-144 was either upregulated or downregulated through lentiviral transduction. The subsequent effects of miR-144 upregulation or downregulation on ALL proliferation, cell-cycle transition and in vivo xenograft were assessed. A possible downstream target of miR-144, human formin-2 (FMN2), was assessed by a dual-luciferase activity assay, qRT-PCR and western blotting. FMN2 was then overexpressed in miR-144-upregulated Molt-3 and Jurkat cells to determine its effect on miR-144 induced tumor suppression on ALL proliferation and cell-cycle transition.
Results
MiR-144 was significantly downregulated in both ALL cell lines and T-leukemic cells of ALL patients. Lentiviral transfection successfully induced miR-144 upregulation or downregulation in Molt-3 and Jurkat cells without affecting cell viability. Functional assays demonstrated that miR-144 upregulation suppressed ALL proliferation and cell-cycle transition in vitro, as well as the growth of Jurkat xenograft in vivo, whereas miR-144 downregulation had no functional effect on ALL development. Multiple approaches confirmed that FMN2 was the downstream target of miR-144 in ALL. Finally, overexpressing FMN2 reversed the inhibitory effects of miR-144-upregulation on ALL proliferation and cell-cycle transition.
Conclusions
MiR-144 functions as a tumor suppressor in ALL, very likely through the inverse regulation of its target gene FMN2.
INTRODUCTION
Acute lymphoblastic leukemia (ALL) is a neoplastic malignancy that occurs in both children and adults 1-3. In the USA, more than 6000 patients are diagnosed with ALL, and almost 1500 patients die of ALL every year 4. In young patients, as a result of early diagnosis and advances in treatment strategies, the 5-year survival rate has been dramatically improved during the past decade from below 50% to 80–90% 1, 2, 4. However, for adult ALL patients, relapse often occurs and the 5-year survival rate remains relatively poor, at approximately 40% 1, 5, 6. Therefore, it is very important to understand the underlying mechanisms of ALL carcinogenesis and development to allow the identification of novel therapeutic reagents and the development of optimal treatment strategies for ALL patients at all ages.
MicroRNAs (miRNAs) are families of evolutionarily-conserved, short (19–22 nucleotides long), noncoding RNAs that transcriptionally suppress gene expression by binding to the complimentary DNA sequence on the 3′ untranslated region (3′-UTR) of downstream target genes [7–9]. In human ALL, miRNAs can either act as oncogenes to promote tumorigenesis and cancer progression, or function as tumor suppressors to inhibit cancer growth or induce cancer apoptosis 10-12. For example, microRNA-181a is an oncogene in ALL as a result of promoting cancer proliferation and cell-cycle transition through the downregulation of a growth response gene 1 13. On the other hand, tumor suppressor microRNA-204 inhibits ALL development through the inverse regulation of the oncogenic gene of sox4 14. Among many of the leukemia-associated miRNAs, microRNA-144-3p (miR-144) was found to be downregulated in both chronic and acute myeloid leukemia, and may also be associated with imatinib resistance in chronic myeloid leukemia cell line, K562R cells 15, 16. In addition, miR-144 was found to be abundantly expressed in normal human T-cells and also reduced T-cell proliferation by inhibiting inteferon-γ and tumor necrosis factor-α 17. However, little is known about the exact expression pattern or functional role of miR-144 in ALL.
The human formin-2 (FMN2) gene is a member of formin family that has a profound role in actin nucleation in central nervous system 18-20. In human ALL, FMN2 was found to be predominantly upregulated in 95% of pre-B acute lymphoblastic leukemias, thus indicating an oncogenic role in ALL 21. However, the exact role of FMN2 in regulating ALL development remains to be clarified.
In the present study, we firstly used the quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) to investigate the gene expression pattern of miR-144 in both ALL cell lines and ALL T-leukemic cells. We then used lentivirus to either upregulate or downregulate miR-144 in ALL cell lines Molt-3 and Jurkat cells, aiming to explore the functional roles of miR-144 in regulating ALL proliferation, cell-cycle transition in vitro and xenograft in vivo. We then used multiple functional assays, such as the luciferase activity assay, qRT-PCR and western blotting, to explore the correlation between miR-144 and FMN2 in ALL. Furthermore, we ectopically overexpressed FMN2 gene in miR-144-upregulated ALL cells to assess the functional roles of FMN2 in ALL.
MATERIALS AND METHODS
Ethic approval
Approval to conduct the present study was obtained from the Clinical Research and Ethic Committee at Daping Hospital, Third Military Medical University in Chongqing, China. All patients provided their written informed consent. Clinical and experimental procedures were conducted in accordance with the Declaration of Helsinki, federal laws and medical regulations in China.
ALL cell lines
ALL cell lines, Molt-3, Molt-4, Tall-104, Jurkat, CCRF-CEM, CEM/C1 and CEM/C2, were purchased from American Type Culture Collection (American Type Culture Collection, Manassas, VA, USA). A normal peripheral blood pan-T cell line (BPBPT) was purchased from AllCells (AllCells, Alameda, CA, USA). All cells were maintained in Dulbecco's modified Eagle's medium (Thermo Fisher Scientific Inc., Waltham, MA, USA) supplemented with 100 μg/mL streptomycin plus 100 units/mL penicillin (Sigma-Aldrich, St Louis, MO, USA) and 10% fetal bovine serum (Thermo Fisher Scientific Inc.) in a tissue-culturing environment at 37°C with 95% O2 and 5% CO2.
All human samples and T-cell isolation
Between 2011 and 2016, human samples of peripheral bloods from 59 ALL patients were included in the present study. Those patients were diagnosed with ALL based on combined testes of immunohistochemistry, morphological examination and flow cytometry using the French–American–British classification. In addition, peripheral bloods were drawn from 47 healthy control volunteers. T-cells were isolated from ALL patients and healthy control patients using a Pan T-Cell Isolation Kit (Miltenyi Biotec, Bergisch Gladbach, Germany) in accordance with the manufacturer's instructions. Once isolated, T-cells were immediately snap-frozen in liquid nitrogen and stored at −80°C.
RNA extraction and qRT-PCR
Total RNA was extracted from ALL cell lines or T-cells isolated from patients' peripheral blood using a Trizol kit (Thermo Fisher Scientific) in accordance with the manufacturer's instructions. Reverse transcription to generate cDNA was performed using a PrimeScript RT reagent Kit (Takara, Dalian, China). qRT-PCR was used to detect relative gene expression levels of mature hsa-miR-144-3p (miR-144) and human FMN2 gene on an ABI Prism 7900 sequence detection system (Thermo Fisher Scientific). For miR-144, a TaqMan miRNA assay (Applied Biosystems, Foster City, CA, USA) was used. For FMN2 gene, a SYBR qRT-PCR kit (Takara, Dalian, China) was used. All samples were measured against the expression levels of RNU48 small RNA, and then normalized to the expression levels in control samples using the 2-ΔΔCt method.
MiR-144 upregulation and downregulation in ALL
Lentiviruses containing human miR-144 mimics (miR-144-M), and its negative control miRNA (C-miRNA-m), as well as lentiviruses containing human miR-144 inhibitor (miR-144-I), and its positive control miRNA (C-miRNA-I), were all purchased from SunBio (SunBio Medical Biotechnology, Shangai, China). ALL cell lines, Molt-3 and Jurkat cells were then transduced with those four lentiviruses along with polybrene (8 μg/ml) at multiple of infections of 15–20. Forty-eight hours after transduction, healthy Molt-3 and Jurkat cells were suspended, centrifuged and cultured with fresh medium (without lentivirus) in 10-cm tissue-culture dishes at density of 1 × 104 cells/cm2. Cells were passaged at least three times to stabilize lentiviral transduction. qRT-PCR was used to examine the transduction efficiency.
Viability assay
Viability of lentiviral-transduced Molt-3 and Jurkat cells was assessed using a (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Sigma-Aldrich) in accordance with the manufacturer's instructions. Briefly, lentiviral-transduced Molt-3 and Jurkat cells were re-suspended and re-plated in 96-well plate at density of 5000 cells/well. Mitomycin (10 μg/mL; Sigma-Aldrich) was added to stop cell proliferation. Forty-eight hours later, MTT solution (0.5 mg/mL) was added into each well for 2 h, followed by the addition of 100 μL dimethyl sulfoxide (Sigma-Aldrich). Optical density (OD) at 570 nm was measured for each well. Relative viability was quantified by normalizing the OD of each well against the OD for the well containing Molt-3 or Jurkat cells transduced with C-miRNA-m.
Proliferation assay
Proliferation of lentiviral-transduced Molt-3 and Jurkat cells was also assessed using a MTT assay. Briefly, Molt-3 and Jurkat cells were plated in 96-well (5000 cells/well). Cells were allowed to proliferate for 5 days. At each 24-h interval, MTT solution was added into wells and the OD at 570 nm was measured.
Cell cycle assay
Molt-3 and Jurkat cells were fixed by 4% paraformaldehyde (Sigma-Aldrich) for 30 min at room temperature, then treated with propidium iodide (50 μg/mL; Sigma-Aldrich) for 30 min at 37°C. Cells were then collected and examined using a LSR II flow cytometer (Thermo Fisher Scientific Inc.) in accordance with the manufacturer's instructions. The percentages of cells at G0/G1, S or G2/M stages were then determined using a Multicycle AV software (Phoenix Flow Systems, San Diego, CA, USA) in accordance with the manufacturer's instructions.
In vivo xenograft assay
The in vivo xenograft of Jurkat cells was performed according to a previously described method 22. After lentiviral transduction, Jurkat cells were subcutaneously inoculated in abdominal flanks of adult female syngeneic BALB/c mice (6 weeks old) at a density of 1 million cells/mouse. The left flanks were injected with Jurkat cells transduced with c-miRNA-m and the right flanks were injected with Jurkat cells transduced with miR144-m. Then, 1, 2, 3 and 4 weeks after inoculation, widths (W; mm) and lengths (L; mm) of Jurkat xenografts were measured. In vivo tumor volume (V; mm3) was estimated as: V = L × W × W/2. At the end of xenograft assay, Jurkat explants were extracted from mice and examined under a microscope.
Luciferase reporter assay
Wild-type 3′-UTR of human FMN2 was amplified and cloned into the psiCHECK2 vector (Promega, Madison, WI, USA) to make firefly luciferase vector of Luc-FMN2(w). The putative miR-144 binding sequence on FMN2 3′-UTR was point-mutated. The mutant 3′-UTR was also cloned into psiCHECK2 to make firefly luciferase vector of Luc-FMN2(m). HEK 293 T cells were co-transfected with 30 ng of either Luc-FMN2(w) or Luc-FMN2(m), as well as 5 pmol of either C-miRNA-m or miR144-m. Forty-eight hours after co-transfection, Firefly/Renilla luciferase activities were assessed using a Dual-Luciferase Reporter Assay System (Promega) and normalized to the luciferase activity for cells co-transfected with C-miRNA-m and Luc-FMN2(w).
Western blot analysis
Protein was extracted from Molt-3 and Jurkat cells using a lysis buffer (Sigma-Aldrich) and checked by a protein assay (Bio-Rad, Hercules, CA, USA). Next, 30 ng of lysate protein of each sample was then separated by 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Bio-Rad) and transferred to a polyvinylidene fluoride membrane (DuPont, Wilmington, DE, USA). After 1 h of blocking of 5% nonfat dry milk in TBS-T buffer (Sigma-Aldrich) at room temperature, membrane was treated with a rabbit polyclonal FMN2 antibody (dilution 1:50; Cell Signaling Technology, Beverly, MA, USA) and a rabbit polyclonal β-actin antibody (dilution 1:10,000; Cell Signaling Technology) at 4°C over night, followed by incubation of horseradish peroxidase-conjugated secondary antibody at room temperature for 2 h. The blots were visualized using an enhanced chemiluminescence system (Thermo Fisher Scientific Inc.).
FMN2 overexpression assay
A Rapid DNA-ligation kit (Roche Applied Science, Madison, WI, USA) was used to insert the cDNA sequence of human FMN2 into a mammalian overexpressing vector pCDNA3/+ (Thermo Fisher Scientific Inc.), resulting in a FMN2 overexpressing vector pcDNA3.1/FMN2. Molt-3 and Jurkat cells were then transfected with 50 ng of pcDNA3.1/FMN2 or the empty overexpressing vector pCDNA3.1/+. Twenty-four hours after transfection, qRT-PCR was used to examine transfection efficiency.
Statistical analysis
In the present study, all results are reported as the mean ± SEM from at least three biological repeats. Data were compared using Student's t-test using Prism (GraphPad Software Inc., San Diego, CA, USA). p < 0.05 was considered statistically significant.
RESULTS
MiR-144 is downregulated in ALL cell lines and human T-leukemic cells
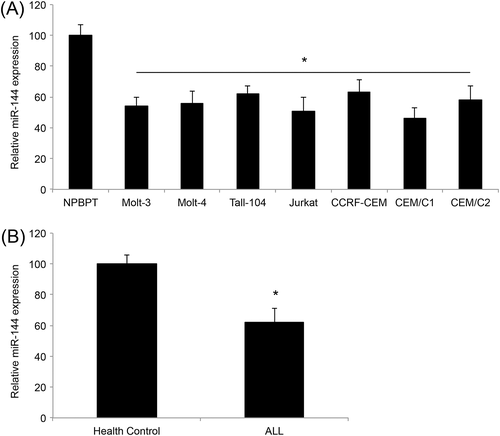
We first assessed the gene expression of miR-144 in ALL cell lines. Analysis of qRT-PCR demonstrated that miR-144 was significantly downregulated in ALL cell lines of Molt-3, Molt-4, Tall-104, Jurkat, CCRF-CEM, CEM/C1 and CEM/C2 compared to BPBPT cells, a normal peripheral blood pan-T cell line (p < 0.05) (Figure 1A). Second, we assessed miR-144 expression in T-cells between ALL patients and healthy control patients. The results of the qRT-PCR showed that miR-144 was significantly downregulated in T-leukemic cells compared to normal T cells (p < 0.05) (Figure 1B).
Creation of ALL cell lines with upregulated or downregulated miR-144
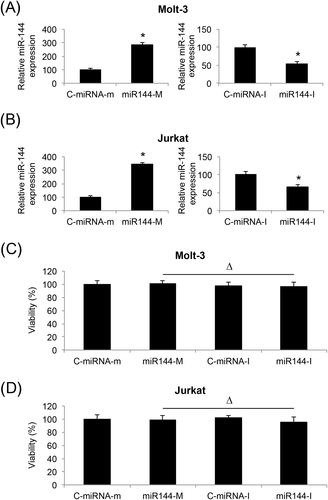
We used lentiviral technology to create ALL cell lines with stable miR-144 upregulation or downregulation. Analyses of qRT-PCR confirmed that lentivirus of miR-144 mimics (miR144-M) significantly upregulated endogenous miR-144, whereas lentivirus of miR-144 inhibitor (miR144-I) significantly downregulated endogenous miR-144, in both Molt-3 (p < 0.05) (Figure 2A) and Jurkat cells (p < 0.05) (Figure 2B). A viability assay also demonstrated that lentiviral transduction did not induce cell death in Molt-3 (Δp > 0.05) (Figure 2C) or Jurkat cells (Δp > 0.05) (Figure 2B).
MiR-144 upregulation reduced proliferation and induced cell-cycle arrest in ALL.
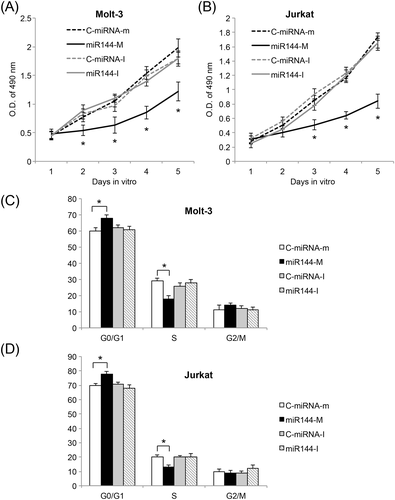
After lentiviral transduction was stabilized, Molt-3 and Jurkat cells were examined using a proliferation assay. miR-144 upregulation significantly reduced ALL proliferation in both Molt-3 and Jurkat cells, whereas miR-144 downregulation had no effect on cancer cell proliferation (p < 0.05) (Figure 3A and 3B). Lentivirus-transduced Molt-3 and Jurkat cells were also examined using a flow cytometry to assess their cell-cycle stages. In both Molt-3 and Jurkat cells, miR-144 upregulation significantly induced cell-cycle arrest at the G0/G1 stage, whereas miR-144 downregulation had no effect on cell cycle transition (p < 0.05) (Figure 3C and 3D). Therefore, our data demonstrated that miR-144, through its upregulation, acts as a tumor suppressor in ALL to suppress cancer cell proliferation and cell cycle transition. However, downregulation of miR-144 had no effect on ALL development.
MiR-144 upregulation inhibited in vivo growth of ALL xenograft
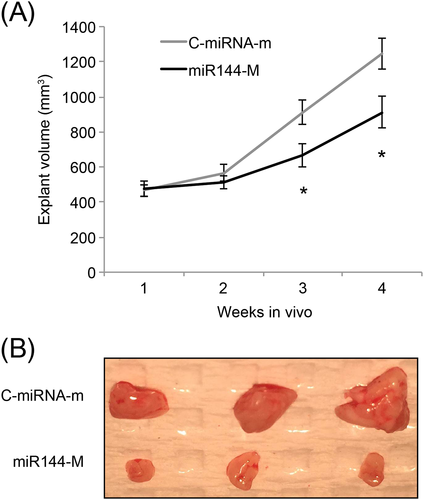
Jurkat cells, transduced with C-miRNA-m and miR144-m, were subcutaneously inoculated in abdominal flanks of adult female syngeneic BALB/c mice (6 weeks old) for 4 weeks. The volumes of Jurkat explants were compared weekly. Three and 4 weeks after grafting, miR-144 upregulation significantly inhibited the in vivo growth of ALL xenograft (p < 0.05) (Figure 4A). At the end of in vivo xenograft, Jurkat tumors were extracted. The imaging results showed that miR-144-upregulated Jurkat explants were significantly smaller than control explants (Figure 4B).
FMN2 is the downstream target gene of miR-144 in ALL
We then investigated what the downstream target gene of miR-144 might be in ALL. By searching through several online miRNA-targeting databases, it was noted that miR-144 may bind the complimentary DNA sequence on the 3′-UTR of the human FMN2 gene (Figure 5A). To explore this possibility, we co-transfected HEK293T cells with luciferase vector either expressing wild-type FMN2 3′-UTR (Luc-FMN2(W)) or expressing a mutated FMN2 3′-UTR without the miR-144 binding sequence (Luc-FMN2(W)), along with either C-miRNA-m or miR144-M. Forty-eight hours after co-transfection, analysis using a luciferase activity assay showed that FMN2 3′-UTR was indeed bound by miR-144 (p < 0.05) (Figure 5B).
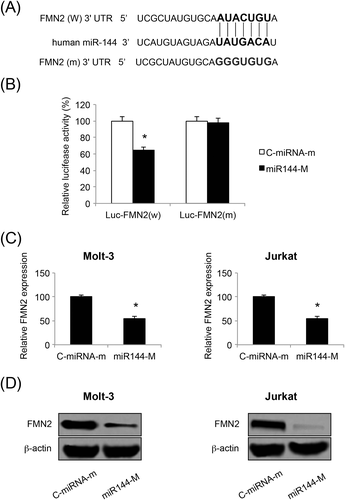
We then turned to ALL cell lines, assessing the effect of miR-144 upregulation on endogenous FMN2 expression in Molt-3 and Jurkat cells. Analysis of qRT-PCR demonstrated that FMN2 genes were significantly downregulated by miR-144 upregulation in both Molt-3 and Jurkat cells (p < 0.05) (Figure 5C). Moreover, analysis by western blotting confirmed our qRT-PCR data, showing that the production of FMN2 proteins was also significantly suppressed in ALL cells (Figure 5D).
FMN2 overexpression reversed tumor suppression in ALL induced by miR-144 upregulation
Finally, we asked whether FMN2 might modulate miR-144-induced tumor suppression in ALL. Molt-3 and Jurkat cells were transfected with a FMN2 overexpressing vector (pcDNA3.1/FMN2) for 24 h. Control ALL cells were transfected with the empty overexpressing vector of pcDNA3.1/+. Twenty-four hours after transfection, analysis of qRT-PCR showed that pcDNA3.1/FMN2 significantly upregulated the endogenous gene expression of FMN2 in both Molt-3 and Jurkat cells (p < 0.05) (Figure 6A). Forty-eight hours after transfection, western blot analysis confirmed our qRT-PCR results, showing that FMN2 proteins were subsequently upregulated in Molt-3 and Jurkat cells (Figure 6B).
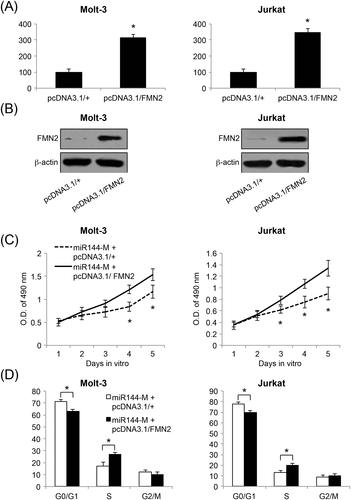
miR-144-upregulated Molt-3 and Jurkat cells (i.e. those transduced with miR144-M) were transfected with pcDNA3.1/FMN2 or pcDNA3.1/+ for 48 h. Subsequently, healthy cells were collected and re-seeded in 96-well plate (5000 cells/well) to be examined using a proliferation assay. The results showed that, 3 or 4 days after transfection, cancer proliferation was significantly increased by FMN2 overexpression (p < 0.05) (Figure 6C). Moreover, transfected Molt-3 and Jurkat cells were examined using a cell-cycle assay. The results showed that miR-144 upregulation-induced cell-cycle arrest at the G0/G1 stage was significantly relieved by FMN2 overexpression (p < 0.05) (Figure 6D). Therefore, our data demonstrated that FMN2 overexpression could reverse the tumor suppressive effects of miR-144 upregulation on ALL proliferation and cell-cycle transition, further confirming that FMN2 was the downstream target gene of miR-144 in ALL.
DISCUSSION
Previous studies have demonstrated that miR-144 is downregulated in several types of leukemia, such as chronic and acute myeloid leukemia, and also regulated imatinib chemosensitivity in chronic myeloid leukemia [15,16]. In the present study, we examined the expression of miR-144 in another type of leukemia, ALL, and found it to be significantly downregulated in both immortal ALL cell lines and T-leukemic cells of ALL patients. Therefore, it is very likely that miR-144 is predominantly under-expressed across various types of human leukemia.
We then used lentivirus to both upregulate and downregulate miR-144 in Molt-3 and Jurkat cells, aiming to exhaustedly explore the mechanistic roles of miR-144 in ALL. We found that miR-144 upregulation had tumor suppressive effect, inhibiting ALL proliferation and cell-cycle transition in vitro and Jurkat xenograft in vivo. On the other hand, we found that miR-144 downregulation had little effect on ALL development. In other human cancers, such as hepatocellular carcinoma, thyroid cancer, bladder cancer, colorectal cancer and nonsmall cell lung cancer, miRNA-144 was shown to mainly function as tumor suppressor with respect to inhibiting cancer proliferation, cell-cycle transition or inducing cancer cell apoptosis [23–27]. Therefore, our results are in line with previous studies, supporting the notion that miR-144 is predominantly acting as a tumor suppressor in various human cancers, including ALL, whereas downregulating or inhibiting miR-144 had no meaningful impact on cancer development.
Also in the present study, we demonstrated that the tumor suppressive effect of miR-144 acted via the inverse regulation on its downstream target gene, human FMN2. A dual-luciferase activity assay demonstrated that miR-144 could directly bind the 3′-UTR of FMN2. Then, in ALL cells directly, we showed that FMN2 was reversely regulated by miR-144 at both the gene and protein levels. Furthermore, although FMN2 was overexpressed in miR-144-upregulated Molt-3 and Jurkat cells, we demonstrated that miR-144-induced inhibition on ALL proliferation and cell-cycle transition was relieved. It is worth noting that the FMN2 gene can act as either an oncogene or tumor suppressor, depending on the type of carcinoma and the aberrant expression pattern of FMN2 in that particular type of carcinoma [28]. However, as in ALL, FMN2 has been demonstrated to be overwhelmingly upregulated in pre-B ALL [21]. Along with our data showing that FMN2 reversed the inhibition of miR-144 on ALL development, all of the evidence suggests that FMN2 is very likely acting as a oncogene in human ALL.
In conclusion, in the present study, we have provided strong evidence demonstrating a novel signaling pathway in human ALL, with miR-144 is functioning as a tumor suppressor, possibly through the inverse interaction on its target gene FMN2. These results would undoubtedly broaden our understanding of the fundamental mechanisms in human ALL, as well as help to develop novel and efficient therapeutic methods for treating patients with ALL.




