Intracellular efficacy of tumor-targeting group I intron-based trans-splicing ribozyme
Abstract
Background
Group I intron-based trans-splicing ribozyme, which can specifically reprogram human telomerase reverse transcriptase (hTERT) RNA, could be a useful tool for tumor-targeted gene therapy. In the present study, the therapeutic feasibility of this ribozyme was investigated by analyzing trans-splicing efficacy in vivo as well as in cells.
Methods
We assessed transgene activation, degree of ribozyme expression, targeted hTERT mRNA level, or the level of trans-splicing products in hTERT(+) cells or in human tumor nodules xenografted in animals after ribozyme administration.
Results
The activity and efficacy of the trans-splicing ribozyme in cells was dependent on the amount of endogenous hTERT mRNA and/or the accumulation of ribozyme RNA in cells. Intracellular activity of the ribozyme reached a plateau when no more targetable substrate mRNA was available or the ribozyme RNA level was fully saturated. In addition, the efficacy of ribozyme in xenografted tumor tissues was dependent on the dose of the delivered ribozyme-encoding adenoviral vector, indicating the potential of the ribozyme expression level as a determining factor for the in vivo efficacy of the trans-splicing ribozyme. On the basis of these results, we enhanced the intracellular ribozyme activity by increasing the ribozyme expression level transcriptionally and/or post-transcriptionally.
Conclusions
We analyzed ribozyme efficacy and determined the most influential factors of its trans-splicing reaction in mammalian cell lines as well as in vivo. The present study could provide insights into the optimization of the trans-splicing ribozyme-based RNA replacement approach to cancer treatment. Copyright © 2011 John Wiley & Sons, Ltd.
Introduction
A recently developed class of genetic tools known as RNA trans-splicing system can reprogram the sequences of endogenous or exogenous RNA to create a unique chimeric transcript whose translation results in de novo gene activation. The RNA trans-splicing system that mediates target-selectable and regulatable transgene expression has broad applications, and offers a number of significant advantages compared to conventional gene therapy approaches. Several RNA trans-splicing systems that involve trans-splicing ribozymes [1,2] spliceosome-mediated pre-mRNA trans-splicing [3,4], or tRNA-splicing endonuclease [5,6] have been assessed for gene therapy approaches against many incurable human diseases.
The Tetrahymena trans-splicing ribozyme is one of the most common and well-studied trans-splicing systems, which has been developed by modifying the self-splicing group I introns of Tetrahymena thermophila. The ribozyme trans-splices an exon joined to its 3′ end onto a separate substrate RNA that base pairs with the internal guide sequence of the ribozyme not only in the test tube [7], but also in Escherichia coli [1] and mammalian cells [8]. It requires only the coexistence of distinct target RNA and trans-splicing ribozymes, without the aid of other factors to perform trans-splicing reactions [9]. These characteristics of trans-splicing ribozymes are useful for diverse therapeutic applications, such as revising mutant RNA [10–12] or regulating potential transgene expression [13–16]. Therefore, trans-splicing ribozymes have great potential as a therapeutic modality in a range of gene-based treatments.
Previously, we reported a new gene therapy strategy against cancer with high specificity and efficacy, which is based on a human telomerase reverse transcriptase (hTERT) RNA-targeting trans-splicing ribozyme [17,18]. This approach induces therapeutic transgene activity selectively in telomerase-positive cancer cells and reduces the hTERT RNA level simultaneously with the expression of the target gene product, resulting in an additive, or perhaps a synergistic, anticancer effect. This approach may offer a means to overcome the potential limitations of the telomerase inhibition approach for cancer therapy, such as the requirement of a long lag phase for deleterious effects on cancer proliferation and/or a possible increase in genomic instability in the surviving cells [19,20]. Furthermore, we recently showed that, in hTERT-expressing tumor xenografts, an adenoviral vector harboring the hTERT-targeting ribozyme can both selectively mark tumor cells and selectively induce suicide gene activity in the tumors, thereby regressing the tumors via the prodrug treatment [21–24].
The primary concern for the successful application of this technology in clinical cancer treatment is the efficiency of the ribozyme in vivo for targeting and reprogramming pathogenic RNA [25]. It is necessary to clarify the factors that are most influential on ribozyme reaction efficiency so as to improve the efficiency of group I ribozyme-mediated RNA reprogramming. Especially for adenovirus vector-based gene therapy, a ribozyme that is maximally efficient is necessary to reduce the number of injected adenovirus particles because highly dangerous inflammatory responses can be inappropriately activated, especially when high doses of adenovirus vectors are used [26,27]. Enhancement of expression efficiency or therapeutic gene activity per viral particle unit is one possible method for alleviating immunogenic toxicity.
In the present study, to assess ribozyme efficacy and to reveal the most influential factors of its trans-splicing reaction in mammalian cell lines and in vivo, we analyzed the targeted transgene induction activity of the hTERT-specific trans-splicing ribozyme, which was previously developed to specifical target the 5′ untranslated region (UTR) of endogenous hTERT mRNA [17], in mice xenografted with hTERT(+) tumors, as well as in hTERT(+) cell cultures. Moreover, based on the results of the efficacy analysis, we attempted to improve the trans-splicing ribozyme efficiency in cells.
Materials and methods
Expression constructs
The pSV40-Rib-Fluc and pCMV-Rib-Fluc encoding hTERT-targeting trans-splicing ribozyme plus firefly luciferase gene (Fluc) as a 3′ exon under the control of the SV40 and CMV promoter, respectively, was constructed as described previously [17]. The pSV40-Fluc and pCMV-Fluc vectors encoding the Fluc gene controlled by the SV40 and CMV promoter, respectively, were generated as controls. To generate the pCMV-Rib-Fluc/WPRE fusion construct, the wild-type woodchuck hepatitis post-transcriptional regulatory element (WPRE) element was first obtained by digesting the pHR-CMV-GFP-WPRE vector (kindly supplied by Dr K. Oka, Baylor College of Medicine, Houston, TX, USA) with ClaI, and then cloned into the ClaI site of the 3′ UTR of the reporter Fluc gene of pCMV-Rib-Fluc.
Generation of recombinant adenoviruses expressing trans-splicing ribozyme
Recombinant adenoviral vectors were produced by homologous recombination and amplification in HEK293 cells as described previously [21–23]. Viral clones were purified by double cesium chloride gradient ultracentrifugation and titrated by serial dilution end point in HEK 293 cells [28]. The adenovirus derivative containing CMV promoter-driven ribozyme flanked by bacterial lacZ gene was designated as Ad-Ribo-LacZ. The control was adenovirus with lacZ driven by the CMV promoter (Ad-LacZ).
Cell cultures
Cell lines of human adenovirus-transformed embryonic kidney cells (293), liver cancer cells (SK-Hep-1), colon cancer cells (HT-29) and stomach cancer cells (AGS) were purchased from American Type Culture Collection (ATCC). Cells were cultured in Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS). Lung adenocarcinoma cells (SK-LU-1) and normal lung fibroblasts (IMR90) were cultured in Eagle's minimum essential medium (Invitrogen) supplemented with 10% FBS. Breast cancer cells (MCF7) acquired from ATCC were cultured in RPMI 1640 (Invitrogen) amended with 10% FBS. Cells were cultured in the presence of 100 U/ml penicillin and 100 µg/ml streptomycin (Sigma, St Louis, MO, USA) at 37 °C in an atmosphere of 5% CO2.
Telomerase activity assay
Telomerase activity was determined with a polymerase chain reaction (PCR)-based telomeric repeat amplification protocol (TRAP) enzyme-linked immunosorbent assay kit (Roche, Mannheim, Germany) in accordance with the manufacturer's instructions. In brief, cells were washed three times with phosphate-buffered saline (PBS), homogenized in 200 µl of cell lysis buffer, and incubated on ice for 30 min. Cell extract (2 µl) was added to 25 µl of reaction mixture and sterile water was added to a final volume of 50 µl. PCR was then performed as follows: prime elongation (20 min at 25 °C), telomerase inactivation (5 min at 94 °C) and product amplification (30 s at 94 °C, 30 s at 59 °C, 90 s at 72 °C for 33 cycles and 10 min at 72 °C). PCR products (5 µl) were bound to a streptavidin-coated 96-well plate and hybridized to a digoxigenin (DIG)-labeled telomeric repeat specific detection probe. The immobilized PCR products were detected with peroxidize conjugated anti-DIG antibody, and quantified on a plate reader at a wavelength of 450 nm.
Cellular studies
For the assessment of reporter gene activation, cells were seeded in 35-mm diameter plates at a density of 3.0 × 105 cells per plate 24 h before transfection. The pSV40-Rib-Fluc, pSV40-Fluc, pCMV-Rib-Fluc-WPRE, pCMV-Rib-Fluc or pCMV-Fluc constructs were transiently co-transfected into the hTERT(+) 293, AGS, HT-29, SK-Hep-1 or MCF7 cells, or hTERT(−) IMR90 or SK-LU-1 cells using Lipofectamine (Invitrogen), DMRIC-C (Invitrogen), or Exgen (MBI Fermentas, Glen Burnie, MD, USA) along with pTK-Rluc, which encodes Renilla luciferase (Rluc) under the control of the herpes simplex virus thymidine kinase (HSVtk) promoter, to normalize for transfection efficiency. Cell lysates were harvested 24 h after transfection, and the reporter Fluc gene activities were assayed by the measurement of light units and then normalized to the Rluc gene activity. Luminescence readings were obtained using a TD20/20 luminometer (Turner Designs Instrument, Sunnyvale, CA, USA). Reactions were carried out using the Dual-Luciferase Reporter Assay system (Promega, Madison, WI, USA). To compare the ribozyme efficiency between hTERT(+) cell lines, Fluc gene activities in the ribozyme transfected cells were expressed as a percentage of those in each cell transfected with pSV40-Fluc or pCMV-Fluc. The best transfectant reagent and the transfection efficiency into each cell line were determined using the GFP-encoding plasmid: 293 cells, Lipofectamine, 40%; SK-LU-1, DMRI/C-C, 7.5%; IMR90, Exgen, 20.6%; SK-Hep-1, Lipofectamine, 5%; MCF7, Exgen, 23%; HT29, Exgen, 2.6%; AGS, Exgen, 21%.
To determine whether new gene activity could be selectively induced in hTERT(+) cells by adenoviral vectors harboring the hTERT-targeting trans-splicing ribozymes with the downstream reporter gene (Ad-Ribo-LacZ), a β-galactosidase (β-gal) activity assay was performed. For β-gal activity, MCF7 cells were plated in 35-mm diameter plates at a density of 3.0 × 105 cells per plate 24 h before infection. One day later, MCF7 cells were infected with adenoviruses at various multiplicities of infection (MOI) (1, 20, 40, 80 and 160). After 48 h, cells were lysed in lysis buffer (Invitrogen) and protein concentrations were determined using a Bio-Rad protein assay kit (Bio-Rad, Hercules, CA, USA) in accordance with the manufacturer's instructions. β-gal activities were determined using a β-gal assay kit (Invitrogen). Specific activities of lysates were calculated as: Specific activity = nmol of O-nitrophenyl-β-D-galactopyranoside hydrolysed/min/mg protein.
Animal studies
Male BALB/cAnNCrI nude mice (4–5 weeks old; Orient Bio Inc., Seongnam, Korea) were utilized throughout the study. Animals were maintained under specific pathogen-free conditions, acclimated to laboratory conditions for at least 1 week before use, and were maintained in a Korean Food and Drug Administration (KFDA) animal facility in accordance with AAALAC International Animal Care policy (KFDA accredited unit number-000 996). For the subcutaneous tumor model in mice, HT-29 cells (2.5 × 107) were injected into the flanks of male nude mice. Two weeks after inoculation, tumor nodules achieved a size of 135–190 mm3. The randomly grouped mice were injected with Ad-Ribo-LacZ (n = 20) at a range of viral titers [1 × 108–1 × 1011 virus particle (vp)], or with Ad-LacZ (n = 5) at a high viral titer (1 × 1011 vp) directly into the growing tumors.
5-Bromo-4-chloro-3-indolyl-β-D-galactopyranoside staining
On day 2 after the final viral injection, mice were killed by cervical dislocation and tumors were harvested, sectioned, and frozen in a cryoprotective medium (Sakura Finetek, Zoeterwoude, The Netherlands). X-gal staining was performed in accordance with the manufacturer's instructions, using a β-gal staining kit (Invitrogen). The tumors were cut into 8-µm thick sections that were fixed with 2% paraformaldehyde in 100 mmol/l of PBS (pH 7.4) for 10 min at room temperature, washed twice in PBS, and stained with X-gal solution at 37 °C overnight. The sections were then counterstained with hematoxylin and eosin (H&E) and observed under light microscopy.
Quantitative RNA analysis
RNA was isolated from adenovirus infected cells or tissues from mice with Trizol reagent (Invitrogen) in accordance with the manufacturer's instructions. To determine the expression levels of the ribozyme RNA, endogenous hTERT mRNA, and trans-splicing molecules (TSMs) in vitro and in vivo, real-time PCR was performed with the total RNA obtained from MCF7 cells 2 days after infection with various MOIs (1–160) of Ad-Ribo-LacZ, or from tumors dissected from mice 2 days after direct adenoviral infection into the growing tumors with various viral titers of Ad-Ribo-LacZ (1 × 108 to 1 × 1011 vp). Total RNA (5 µg) was subjected to reverse transcription for ribozyme RNA and TSM RNA with the 3′-end of lacZ (5′-ACGCAACTCGCCGCACATCTGAA-3′) or for endogenous hTERT mRNA with an oligo(dT) primer in the presence of 10 mM L-argininamide. The cDNAs were then amplified for ribozyme RNA with lacZ-specific primers (5′-GGAATTCATGGTCGTTTTACAACGTCGTG-3′ and 5′-GGGAAGCTTCGGATTGACCGTAATGGGA-3′), for endogenous hTERT mRNA with specific primers (5′-CGGAAGAGTGTCTGGAGCAA-3′ and 5′-GGATGAAGCGG AGTCTGGA-3′), or for TSMs with a 5′ primer specific to the 5′ end of the hTERT RNA and with a 3′ primer specific to the 3′ exon lacZ sequence (5′-GGGAAGCTTCGGATTGACCGTAATGGGA-3′). To control for the expression level of hTERT in the reaction mix, we measured human GAPDH RNA level. The threshold levels obtained from the hTERT were adjusted to the threshold levels found in the GAPDH reaction to correct for minor variation in cDNA loading. All reagents with the exception of Taq polymerase (Takara, Otsu, Japan) were obtained for analysis from the SYBR-Green core reagent kit (Molecular Probes, Eugene, OR, USA). The protocol was conducted in accordance with the manufacturer's instructions for the real-time PCR kit.
Results
Determination of the key factor for trans-splicing ribozyme activity in vitro
The hTERT-targeting trans-splicing ribozyme specifically and accurately replaces the target transcript in vivo as well as in vitro, resulting in the selective and specific induction of therapeutic gene activities in hTERT-expressing cells [17]. However, significant differences in transgene activities were found between various hTERT(+) cells. To assess the differential transgene activities between various hTERT(+) cells by the specific trans-splicing ribozyme, a luciferase reporter assay was performed (Figure 1). A plasmid encoding the specific ribozyme that contained firefly luciferase (Fluc) as a 3′ exon under the control of the SV40 promoter (pSV40-Rib-Fluc) was transiently transfected into four hTERT(+) or two hTERT(−) cell lines. The degree of induced transgene activity was analyzed 24 h after transfection by the measurement of Fluc activities relative to Renilla luciferase (Rluc). Consistent with previous results [17], all the tested hTERT(+) cell lines showed active levels of Fluc expression, whereas Fluc remained poorly activated in both hTERT(−) SK-LU-1 and IMR90 cells (Figure 1A). However, the absolute level of Fluc gene expression by pSV40-Rib-Fluc significantly varied in each tested cell line: 424.33% for AGS, 34.87% for HT-29, 108.37% for SK-Hep-1 and 12.33% for MCF7 relative to the Fluc activity in 293 cells, respectively. These results suggest that the transient transfection of the pSV40-Rib-Fluc induces transgene activities selectively only in hTERT(+) cells lines, as described previously [17] but results in marked differences in the intracellular activity between the hTERT(+) inter-cell lines.
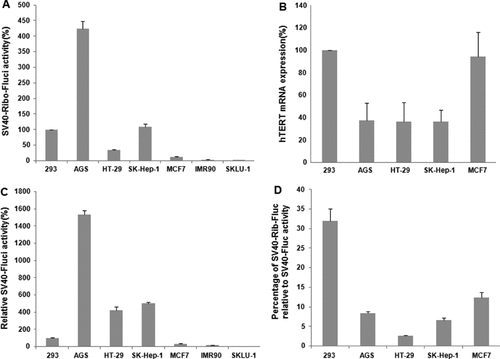
Assessment of the key factors for hTERT RNA-targeting specific trans-splicing ribozyme activity in human cell lines. (A) Four telomerase-positive or two telomerase-negative cell lines were transiently transfected with the plasmid pSV40-Rib-Fluc. The Fluc activities relative to the Rluc activities in each transfected cell line were measured 24 h after transfection and are presented as the percentage of those in 293 cells. (B) Expression level of endogenous hTERT mRNA was determined with real-time PCR in each hTERT(+) cell line and was quantified as a percentage of the amount in 293 cells. (C) Cells were transiently transfected with control plasmid pSV40-Fluc, and the Fluc activities relative to the Rluc activities were measured and represented as percentage of those in 293 cells to evaluate SV40 promoter activity in each cell line. (D) To compare the ribozyme efficiency between hTERT(+) cell lines, the Fluc activities triggered by the ribozymes were quantified as a percentage of those from the SV40 promoter
The reason for the observed differences in the activity of specific trans-splicing ribozyme in the cellular environment was determined by evaluating endogenous target hTERT mRNA by real time reverse transcriptase (RT)-PCR and SV40 promoter activity through the measurement of the SV40 promoter-derived Fluc gene activity. As shown in Figure 1B, the endogenous level of hTERT mRNA of 293 cells was similar to that of MCF7 cells. The hTERT mRNA levels in AGS, HT-29 and SK-Hep-1 cells were similar to each other but more than two-fold less than the level in 293 cells. The level of telomerase activity in human cancers has been shown to significantly correlate with that of the hTERT mRNA expression [29–32]. Consistent with this, the relative enzymatic activity of telomerase between the hTERT(+) cells corresponded well with the relative hTERT mRNA levels in the cells (Table 1). Of note, both the hTERT mRNA expression level and telomerase activity in each cell did not correspond to the ribozyme-triggered Fluc activity level shown in Figure 1A. By contrast, a markedly different level of Fluc gene expression under the control of the SV40 promoter was observed in the tested hTERT(+) cell lines (Figure 1C). This level of promoter activity correlated with transgene activity triggered by the ribozyme, at least in the AGS cell line. To compare the ribozyme efficiency levels between the hTERT(+) cell lines, the relative Fluc activities triggered by the ribozymes were quantified as a percentage of those from SV40 promoter in 293 cells. Although the absolute trans-splicing ribozyme activity was markedly highest in AGS cell lines, the relative ribozyme efficiency was significantly the highest in the 293 cell line (32.01%) (Figure 1D).
| Cell line | hTERT(+) 293 | AGS | HT-29 | SK-Hep-1 | MCF7 | hTERT(−) SKLU-1 |
|---|---|---|---|---|---|---|
| TRAP activity (%) | 100 | 29.4 | 33.5 | 22.0 | 79.0 | 0 |
- a Telomerase activity in each cell line was determined with a TRAP assay and was quantified as a percentage of the activity in 293 cells.
The expression of hTERT mRNA expression was higher in 293 cells than in SK-Hep1 and HT29 cell lines (2.6- and 2.8-fold, respectively), whereas SV40 promoter activity is lower in 293 cell lines than in SK-Hep1 and HT29 cell lines (five- and four-fold, respectively). Despite the higher differences in the trans-splicing ribozyme expression levels (SV40 promoter activity) than hTERT mRNA expression levels between the 293 cell line and the SK-Hep1 and HT29 cell lines (Figure 1C), ribozyme activity in 293 cells was similarly induced to the activity in SK-Hep1 cells (by approximately 97%), or induced by approximately three-fold greater than the activity in HT29 cells (Figure 1A and 1C), indicating that, if the level of ribozyme mRNA expression was not saturating in the transfected cells, the amount of endogenous target transcripts in the cells would become the main limiting factor for in vivo trans-splicing ribozyme activity.
When ribozyme efficiency was compared between cells expressing a similar level of endogenous hTERT mRNA, ribozyme activity was observed to be correlated with activity of the promoter used for the expression of the ribozyme. For example, in hTERT(+) cell lines such as AGS, HT29 and SK-Hep1, which displayed similar levels of endogenous hTERT mRNA (Figure 1B), the transient expression of trans-splicing ribozyme in AGS cells that had accumulated the highest levels (approximately 3.6- to five-fold more) of ribozyme among the tested three cell lines showed significantly higher levels of ribozyme activity, approximately 12-fold and three-fold more than HT29 and SK-Hep1 cells, respectively (Figures 1A and 1C). These results strongly supported the suggestion that, when the level of endogenous target mRNA expression is similar in different cells, the level of trans-splicing ribozyme activity is determined by the degree of ribozyme accumulation in those cells.
In AGS cells, the higher level of reporter activity induced by pSV40-Rib-Fluc (approximately four-fold more, relative to the activity in 293 cells) corresponded to the higher level of reporter activity under the control of the SV40 promoter (by approximately 15-fold, relative to the activity in 293 cells) (Figures 1A and 1C), although hTERT mRNA expression was approximately three-fold lower than in 293 cells (Figure 1B). Therefore, if trans-splicing ribozyme accumulated to a saturating level, maximum in vivo activity could be attained, which implies that the quantity of excess of trans-splicing ribozyme concentration over target transcripts in cell lines will be a principal limiting factor for in vivo trans-splicing ribozyme activity.
In vitro efficiency of trans-splicing ribozyme using adenoviral vectors
To evaluate the effect of expression level of hTERT-targeting ribozyme on in vitro catalytic efficiency of ribozyme, the intracellular levels of ribozyme, target hTERT and trans-spliced product RNA were quantified (Figure 2). To this end, an adenoviral vector encoding for the specific ribozyme fused to lacZ gene under the control of a CMV promoter (Ad-Ribo-LacZ) was constructed and used to infect hTERT(+) MCF7 cells. Ad-Ribo-LacZ efficiently induced β-gal activity in a dose-dependent manner, up to maximum levels of 39.92% at a MOI of 160, compared to Ad-LacZ (Figure 2A). Noticeably, β-gal activity triggered by the trans-splicing ribozyme reached a plateau at a MOI of 160 of the administered virus. The increase was only 4.88% compared to transgene activity induced by 80 MOI of virus. However, a dose-dependent increase in the expression level of ribozyme RNA was evident upon infection with an increasing MOI of Ad-Ribo-LacZ (a 2.2-fold increase at 160 MOI compared to ribozyme RNA at a MOI of 80) (Figure 2B).
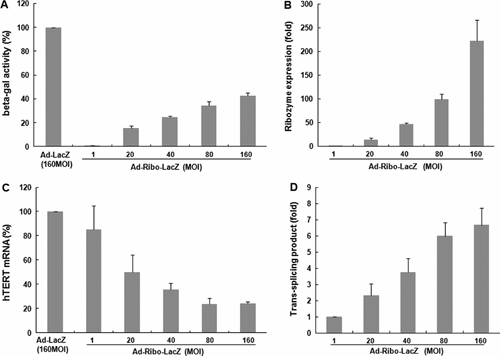
Assessment of the efficiency of hTERT RNA-targeting specific trans-splicing ribozyme in hTERT(+) MCF7 cells delivered by adenoviral vectors. MCF7 cells were transduced with Ad-Ribo-LacZ (MOI of 1–160), or infected with a MOI of 160 of Ad-LacZ as a negative trans-splicing control. (A) Forty-eight hours post-infection, specific β-gal activity in lysates of MCF7 cells infected by increasing MOIs of Ad-Ribo-LacZ was measured quantitatively by a chemilluminascent biological assay, and expressed as a percentage of the activity of Ad-LacZ. The mean ± SD values of measurements performed in triplicate are shown. To determine the expression levels of the ribozyme RNA (B), endogenous hTERT mRNA (C) and TSMs (D), real-time PCR was conducted with total RNA obtained from the MCF7 cells 2 days after adenoviral infection. As an internal control, human GAPDH RNA was amplified. The levels of ribozyme RNA and TSMs by increasing the MOI of Ad-Ribo-LacZ are represented as the fold-changes relative to those at a MOI of 1 of the virus. The level of hTERT mRNA reduced by Ad-Ribo-LacZ was expressed as percentage of the RNA level in cells infected by Ad-LacZ
To determine whether the plateau of transgene activity in cells at a MOI of 160 of administered Ad-Ribo-LacZ was the result of the almost complete depletion of target mRNA by the ribozyme-mediated trans-splicing reaction, the quantity of hTERT mRNA and TSMs generated from the virus-administered cells was examined. Ad-Ribo-LacZ reduced target hTERT mRNA in a dose-dependent manner up to a MOI of 80 (85%, 50%, 35.7% and 23.7% of the hTERT RNA amount at MOI of 1, 20, 40 and 80, respectively, compared to Ad-CMV) (Figure 2C). In addition, the corresponding increases of TSMs were also detected in the virus-administered cells (2.3-, 3.7- and 6.0-fold increase in the amount of TSMs at a MOI of 20, 40 and 80, respectively, compared to TSMs at a MOI of 1) (Figure 2D). However, no further knockdown of target mRNA by ribozyme-mediated trans-splicing reaction was detected with the maximal MOI of 160 of Ad-Ribo-LacZ, indicating that cellular ribozyme activity was almost saturated at this dose. Concurrently, production of TSMs also reached a plateau at a MOI of 160 of Ad-Ribo-LacZ.
In vivo efficiency of trans-splicing ribozyme using adenoviral vectors
The efficacy of trans-splicing ribozyme was assessed in vivo by establishing mice xenografted with hTERT(+) tumor cells. To this end, HT-29 cells were injected subcutaneously into the flanks of male nude mice. After 7 days of tumor xenograft induction, the tumor volumes had reached 135–190 mm3 and randomly grouped mice were injected with Ad-Ribo-LacZ (n = 5), Ad-LacZ (n = 5) or PBS (n = 3) directly into the growing tumors at a range of viral titers (1 × 108 to 1 × 1011 vp). All of the mice were killed and tumors were resected after 2 days of virus infection. Viral inverted terminal repeats were detected in tumor nodules infected with Ad-Ribo-LacZ and Ad-LacZ, using PCR analysis with vector specific primers, suggesting that the viruses efficiently dispersed within the tumor nodules (data not shown).
To determine whether ribozyme could induce transgene expression via the trans-splicing reaction in vivo, we first examined reporter gene expression patterns in frozen sections of tumor nodules by X-gal staining. At low viral titers (1 × 108 vp), Ad-Ribo-LacZ-induced activity was almost negligible. However, diffuse and extensive expression of β-gal, but a relatively reduced amount of the enzyme, was evident at high viral titers of Ad-Ribo-LacZ (1 × 1011 and 2 × 1011 vp) compared to the Ad-LacZ in the developed tumor nodules of mice (Figure 3A).
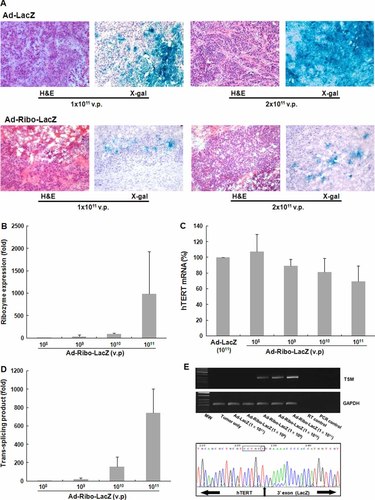
Assessment of the efficiency of hTERT RNA-targeting specific trans-splicing ribozyme in mouse cancer models delivered by adenoviral vectors. Tumor xenografts, approximately 135–190 mm3 in size and composed of hTERT(+) HT-29 cells, were injected with Ad-Ribo-LacZ (n = 5 for each viral titer) or Ad-LacZ (n = 5 for each viral titer) directly into the growing tumors at a range of viral titers (1 × 108 to 1 × 1011 vp). (A) Representative histochemical findings of dose-dependent increases of transgene expression patterns in developed tumor nodules. Histological findings (H&E, × 100) and β-gal expression (X-gal, × 100) are shown in tumor nodules from Ad-Ribo-LacZ or Ad-LacZ-injected tumor xenograft model mice. Quantitative analysis of ribozyme production (B), decreased expression of endogenous hTERT RNA (C) and the production of TSMs (D) were conducted in the Ad-Ribo-LacZ injected tumor nodules of mice at a range of viral titers, using real-time PCR. The levels of ribozyme RNA and TSMs by Ad-Ribo-LacZ are represented as the fold-changes relative to those by 1 × 108 vp of the virus. The level of hTERT mRNA reduced by Ad-Ribo-LacZ was expressed as a percentage of the RNA level in Ad-LacZ infected tumors of mice. (E) TSMs were generated by RT-PCR from Ad-Ribo-LacZ infected tumor nodules. A representative sequence of the trans-spliced transcripts (lanes 5, 6 or 7) is shown at the bottom. The correct splicing junction is indicated by an arrow.
The next experiment evaluated the efficacy of trans-splicing ribozyme in vivo by quantifying the amount of ribozyme expression level, target hTERT mRNA, and TSMs in Ad-Ribo-LacZ-injected tumor nodules of mice at a range of viral titers using real-time PCR. There was a viral dose-dependent increase in the expression level of ribozyme RNA in the Ad-Ribo-LacZ injected tumor nodules of mice (Figure 3B). A dose-dependent depletion of hTERT mRNA level was detected (100.6%, 90.0%, 79.9% and 68.0% hTERT RNA levels at viral titers of 1 × 108, 1 × 109, 1 × 1010 and 1 × 1011 vp, respectively, compared to those seen with Ad-LacZ). Moreover, TSMs in the Ad-Ribo-LacZ infected tumor nodules were detected in a dose-dependent manner (8.6-, 106.1- and 568.0-fold increase of TSMs at viral titers of 1 × 109, 1 × 1010 and 1 × 1011 vp, respectively, compared to those at 1 × 108 vp) (Figure 3D). Conclusively, increased quantity of produced TSMs correlated well with the increased quantity of inoculated Ad-Ribo-LacZ and the decreased quantity of tissue hTERT RNA. We confirmed that Ad-Ribo-LacZ had spliced its 3′ exon tag accurately onto targeted U21 of the hTERT RNA in vivo by sequence analysis of the trans-splicing junction (Figure 3E). Moreover, rapid amplification of cloned ends RT-PCR analysis revealed that all of the trans-splicing products generated in the tumor nodules of the Ad-Ribo-LacZ-injected mice resulted from reactions with only the targeted hTERT RNA, implying in vivo target specificity of the trans-splicing ribozyme (see Supporting information, Figure S1).
By contrast to the reduction level of hTERT mRNA in cell cultures, Ad-Ribo-LacZ infection reduced the endogenous level of hTERT RNA much less in an in vivo tumor nodule (only 32% reduction of the target RNA amount at highest titer, 1 × 1011 vp) and showed no plateau of reduction level at the highest dose of the virus (Figure 3C). Concurrently, production of TSMs also did not reach a plateau at the highest infectious dose of Ad-Ribo-LacZ in tumor nodules. These observations were consistent with an absence of a saturation effect of trans-splicing ribozyme at the viral titers 1 × 1011 vp, contrary to the observation shown in the human cell lines. These results suggest that the in vivo activity of hTERT RNA-targeting trans-splicing ribozyme yielded a significant, but still lesser degree, compared to the results in vitro because trans-splicing ribozyme did not accumulate to high levels to the extent of a saturating concentration in vivo at the highest viral titer of 1 × 1011 vp. This observation implied that the ribozyme expression level might be a limiting factor with respect to the activity of trans-splicing ribozyme in this in vivo system.
Enhancement of trans-splicing ribozyme activity by transcriptional or post-transcriptional regulation of gene expression
Previous results indicated that the accumulation level of the ribozyme expressed by the Ad-Ribo-LacZ was not enough to induce maximum activity in vivo, despite the use of the high viral titer of 1 × 1011 vp. On the basis of cell culture results showing that the level of trans-splicing ribozyme activity was determined by the degree of ribozyme accumulation level in cells if target RNA level is fixed, we tested whether intracellular ribozyme activity could be enhanced by transcriptionally increasing the ribozyme expression level (Figure 4). To this end, an experiment compared the ribozyme activity by two different ribozymes pSV40-Rib-Fluc and pCMV-Rib-Fluc, driven by the SV40 and CMV40 promoter, respectively, in a single hTERT(+) cell line, 293 cells. pSV40-Fluc and pCMV-Fluc were used as positive controls. The levels of reporter activity induced by ribozyme pCMV-Rib-Fluc (approximately 232-fold higher than pSV40-Rib-Fluc) corresponded to those of reporter activity under the control of the CMV promoter (approximately 512-fold higher than pSV40-Fluc) in 293 cells (Figures 4A and 4B), indicating enhancement of trans-splicing ribozyme activity in cells by the increase in the expression of the ribozyme.
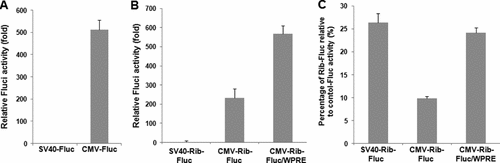
Enhancement of trans-splicing ribozyme activity by transcriptional or post-transcriptional regulation of gene expression. (A) Dual luciferase reporter assay was performed to assess CMV promoter activity relative to SV40 promoter activity in hTERT(+) 293 cells after transient transfection of pSV40-Fluc or pCMV-Fluc. (B) Ribozyme activities by pCMV-Rib-Fluc or pCMV-Rib-Fluc/WPRE were compared and expressed as the fold-changes relative to those by pSV40-Rib-Fluc. (C) To compare the efficiency of ribozymes, we quantified the Fluc activities triggered by the ribozymes as a percentage of those from each CMV or SV40 promoter in 293 cells
We also attempted to increase ribozyme activity by using a WPRE in the ribozyme expression cassette. The WPRE is a viral tripartite post-transcriptional element [33] that has been widely used to enhance transgene expression through an increase in mRNA processing and/or nucleocytoplasmic transport of RNA [34–36]. To this end, the WPRE sequence was incorporated in the 3′ UTR of the reporter gene. An approximately 2.4-fold increase was evident in the amount of luciferase expressed by the pCMV-Rib-Fluc/WPRE compared to pCMV-Rib-Fluc (Figure 4B).
To compare the ribozyme efficiency levels between the pCMV-Rib-Fluc and the pSV40-Rib-Fluc, the relative Fluc activities triggered by the ribozymes were quantified as a percentage of those from each CMV or SV40 promoter in 293 cells. Although the absolute trans-splicing ribozyme activity was 235-fold higher in pCMV-Rib-Fluc than in pSV40-Rib-Fluc, the relative ribozyme efficiency driven by the SV40 promoter was significantly higher (26.40%) than that driven by the CMV promoter (9.86%; Figure 4C). However, the relatively low efficiency of the pCMV-Rib-Fluc was increased by up to 24.2% of that from CMV promoter in 293 cells by the insertion of the WPRE into the 3′ exon of trans-splicing ribozyme. Taken together, these results indicate that WPRE inserted into the 3′ exon of trans-splicing ribozyme enhanced the ribozyme activity and efficacy in the cellular environment.
Discussion
Subsequent to the development of the trans-splicing ribozyme, considerable progress has been made to improve the specificity and efficiency of the ribozyme. When ribozyme is expressed by the pol II promoter in mammalian cells, both the efficiency and specificity of the trans-splicing reaction can be increased by modification of the ribozyme so as to contain an extension of P1 helix, and the addition of P10 helix and antisense sequences complementary to the downstream region of the targeted RNA [37,38]. A recent study has demonstrated a detailed understanding of Tetrahymena group I intron trans-splicing efficiency in mammalian cells [39]. In the latter study, cotransfection of the modified ribozyme driven by the pol II promoter with the sickle β-globin expression plasmid into 293 cells resulted in an increase in repair efficiency (10% of sickle β-globin mRNAs when the ribozyme expression cassette was introduced into cells at a 20-fold molar excess over the substrate construct). However, it is unclear whether the in vitro data from the cotransfection experiments, which used an exogenous mRNA as the substrate, represent the circumstances with respect to endogenous genes as substrate in vivo.
In the present study, to evaluate efficacy of the trans-splicing ribozyme in natural circumstances, we used ribozyme targeting endogenous hTERT RNA, which highly specifically induces therapeutic activity in hTERT(+) cancer cells [17,18,21–23] and assessed the efficiency of the ribozyme in vivo as well as in cell cultures. In the experiments involving transient transfection with a ribozyme-encoding vector, limitation of ribozyme expression led to ribozyme efficacy being determined by the endogenous target RNA level. In other words, the more target RNA, the higher the ribozyme efficacy. By contrast, if the endogenous target RNA level is similar between different cells, the level of ribozyme accumulation in each cell is a critical factor for determining intracellular ribozyme activity. Consistently, in the experiments investigating the infection of ribozyme-encoding adenoviral vector, doses of introduced virus that represent the expression level of ribozyme RNA were important for the increased activity of the cellular ribozyme. However, ribozyme activity reached a plateau at the highest infectious dose of adenovirus. The plateau did not reflect either a limitation of ribozyme RNA level or an absence of further hTERT RNA. This is because, although the expression level of ribozyme RNA was increased in a dose-dependent manner by an increased MOI of Ad-Ribo-LacZ in cells, the target RNA level was not completely destroyed and so was not further affected at the maximum dose of infected virus. The reason for the saturating efficacy of trans-splicing ribozyme despite the excess of trans-splicing ribozyme concentration is unclear. Possible explanations include the inaccessibility of the ribozyme for the target RNA as a result of tight binding of proteins onto the target RNA, the different localization between target and ribozyme [40] or the presence of inaccessible target RNA structures [41,42].
By contrast to the in vitro results, ribozyme activity in vivo did not reach a plateau even at highest dose of injected ribozyme-encoding adenovirus. This result suggests that the level of ribozyme in the adenovirus-infected cells was not sufficient to target and reprogram all the targetable endogenous target RNA, even at highest dose of adenovirus. The highest viral titers (1 × 1011 vp) resulted in only a 32% reduction of the hTERT mRNA level in vivo. Accordingly, such a high dose of Ad-Ribo-LacZ injection could not significantly regress tumor growth in vivo (data not shown), suggesting that hTERT inhibition levels by the ribozyme may not be sufficient to produce in vivo anti-tumor effects. Alternatively, or additively, cell subpopulations, which are capable of escaping from a short-term growth inhibition response caused by telomerase inhibition, would grow out. A long lag phase will be needed for bulk telomere shortening before any harmful effects are observed on those cells, which could limit an anticancer approach employing only the inhibition of the hTERT level [43]. It should be noted that the hTERT-targeting trans-splicing ribozyme reduced the target gene inhibition simultaneously with a target-dependent induction of therapeutic gene product, including suicide genes such as HSVtk gene, which can overcome such a lag phase [17,18]. We have shown that the adenoviral vectors harboring the hTERT-targeting ribozyme with the HSVtk gene selectively regressed growth of hTERT-expressing human tumors in vivo with the prodrug treatment [21–24]. Improvement of ribozyme activity could enhance its anticancer effect by increasing both a reduction in the level of hTERT mRNA and an induction of the amount of trans-spliced therapeutic gene. On the basis of the results obtained in the present study, we tested whether it could be possible to enhance ribozyme activity by increasing the ribozyme expression level in cells. Indeed, we could enhance the ribozyme activity in cells by using a much stronger promoter system to express the ribozyme or by inserting a factor that could post-transcriptionally increase the level of RNA in the ribozyme. Improvement of ribozyme efficacy in vivo through an increase in ribozyme levels will reduce the injection dose of the ribozyme delivery vehicle such as adenovirus, alleviating the immunological toxicities engendered by the introduction of high amounts of virus. This optimization of the ribozyme with respect to its efficacy will be one of the most important determining factors for the realization of the trans-splicing ribozyme-based RNA replacement strategy as an approach for clinical cancer treatment.
Acknowledgements
This work was supported by grants from the National Research Foundation of Korea (No. 2010-0002123 and 2010-0028177 to S.W.L.); from KOSEF by MEST (No. R13-2002-044-05001-0 to J.S.J.); and from the National R&D Program for Cancer Control by Korean Ministry for Health, Welfare and Family Affairs (0720520). Y.S.W. and C.H.L. are recipients of Brain Korea 21 Fellowship.
Supporting information
Figure S1. Specificity in vivo trans-splicing reaction by specific ribozyme. Trans-spliced RNA in the tumor nodules from Ad-Ribo-LacZ-injected mice was reverse transcribed with random primer. The 3′ ends of cDNAs were tailed with dATP with terminal transferase (Roche) and adaptor oligonucleotides (Takara) were added to the end. Then, the cDNAs were amplified with PCR reaction using 10 pmol adaptor 5′ primer (Takara) and lacZ 3′ promer (Takara) (5′-GGGAAGCTTCGGATTGACCGTAATGGGA-3′). The amplified products from trans-splicing reaction between ribozyme and unknown RNA were cloned and 20 different clones were sequenced. All of the clones showed expected sequence harboring the splicing junction site, which is indicated by an arrow, with the ribozyme recognition sequence in target hTERT RNA (boxed) and the uridine at position 21 (circled).




