Safety and Efficacy of a 6-Month Placement of a Fully Covered Self-Expanding Metallic Stent for Refractory Benign Biliary Stricture: A Multicenter Prospective Study
Funding: The authors received no specific funding for this work.
ABSTRACT
Background and Aim
Managing benign biliary stricture endoscopically is complicated and challenging. This study aimed to evaluate the safety and efficacy of a 6-month placement of a fully covered self-expanding metallic stent for refractory benign biliary stricture.
Methods
Twenty-two patients with refractory benign biliary stricture (13 with chronic pancreatitis and 9 without) were recruited from five higher tertiary care centers. All patients received a planned 6-month fully covered self-expanding metallic stent placement. Primary outcomes included clinical success, technical success of stent removal, adverse events, and stricture recurrence.
Results
Of the 21 cases (one case was excluded owing to malignant findings), fully covered self-expanding metallic stent placement was successful in all cases, with contralateral bile duct plastic stents used in three patients and stents remaining in place for 6 months in 16 of 21 patients. Distal stent migration occurred in three cases, two of which had resolved strictures. Adverse events were observed in 19.0% of patients: one case of severe cholangitis, two cases of mild cholangitis, and one case of hyperplasia formation. No stent-induced pancreatitis or cholecystitis occurred. All stents were removed successfully, and the treatment success rate was 85%. One patient experienced recurrent stricture 6.5 months post-stent removal.
Conclusions
A six-month placement of a modified fully covered self-expanding metallic stent effectively improved strictures and minimized stent-induced Adverse events in patients with refractory benign biliary stricture.
Trial Registration
UMIN ID: UMIN000025027
1 Introduction
Benign biliary stricture (BBS) has various causes, including chronic pancreatitis (CP), post-operative biliary tract or liver surgery, and chronic cholangitis. Advanced CP is associated with symptomatic BBS in 10%–30% of cases [1], anastomotic strictures develop in 4%–9% of orthotopic liver transplant patients [2], and postoperative strictures occur in 0.3%–0.7% of laparoscopic cholecystectomy patients [1]. Bile duct stricture with alkaline phosphatase (ALP) and bilirubin elevations 2–3 times above normal for > 4 weeks indicate treatment [3, 4]. Although plastic stent (PS) placement is recommended as the initial endoscopic treatment for BBS [4], a single PS of approximately 10 Fr offers only short-term benefit, with a long-term stricture resolution rate of 12%–38%, which is insufficient for effective treatment. Adverse events, including stent occlusion and migration, are high and occur in 34%–72% of cases [5-8]. Consequently, multiple PS placements for > 1 year are attempted to enhance long-term therapeutic outcomes, improving the bile duct stricture resolution rate to 44%–60% [9, 10].
In recent years, fully covered self-expandable metallic stents (FCSEMS), with a larger diameter than PS, have been successfully applied and are considered superior to PS, reducing the number of endoscopic procedures, although its therapeutic effect is equivalent to that of multiple PS [11-13]. Therefore, both multiple PS and FCSEMS treatments are equally recommended in the 2018 European Society of Gastrointestinal Endoscopy (ESGE) guidelines [4].
FCSEMS generally provides > 6 months of patency once placed; however, long-term placement may complicate removal owing to hyperplasia caused by stimulation of the bile duct or tearing of the cover; thus, an FCSEMS is generally retained for a short period, such as 3 months [14]. In recent years, a modified FCSEMS has been developed [15] to reduce the risk of bile duct damage and facilitate safe removal; however, its effectiveness remains uncertain. This study aimed to evaluate the safety and efficacy of a 6-month placement of a modified FCSEMS for refractory BBS.
2 Methods
2.1 Patients
A total of 22 patients were enrolled in this study between February 2017 and January 2021 at five tertiary care centers in Japan. All patients initially underwent conventional endoscopic management using 7 or 10F PS placement. Inclusion criteria were as follows: age 18 years or older, negative histopathologic and radiologic evaluations for malignant biliary strictures before enrollment, and clinical symptoms of obstructive jaundice due to refractory BBS. Refractory BBS was defined as clinically symptomatic with a persistent stricture on cholangiogram after at least 6 months of PS placement. Exclusion criteria were as follows: suspected malignant stricture, cases treatable with medication (such as IgG4-related diseases), progressive disease (such as primary sclerosing cholangitis), or refusal to participate. Written informed consent was obtained from all enrolled patients. This clinical prospective study was reviewed and approved by the Institutional Ethics Committee of Kitasato University (No. C16-963).
2.2 The Modified FCSEMS and Procedures
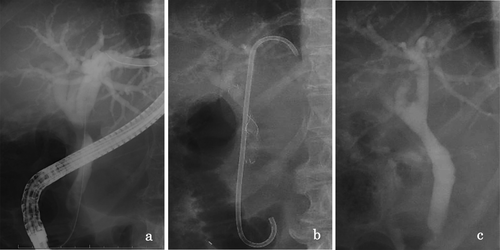
The FCSEMS (BONASTENT M-Intraductal; Standard SciTech Inc., Seoul, South Korea) is composed of nitinol wire and fully covered with a silicone membrane on both sides. It features a convex margin at each end to prevent tissue hyperplasia and a narrowed waist in the central segment (1–2 cm in length) to reduce migration risk. An effective retrieval lasso is attached to the distal end of the stent to aid removal (Figure 1). This stent utilizes an 8F introduction system and is offered in diameters of 8, 10, and 12 mm, with lengths ranging from 3 to 7 cm.

Endoscopic sphincterotomy or endoscopic papillary balloon dilatation was basically performed when a bile duct biopsy was performed to rule out malignancy or multiple plastic stent placement. For distal BBS, the FCSEMS was deployed across the papilla under fluoroscopic and endoscopic guidance, with the center portion positioned at the stricture site. For hilar BBS, an additional PS was inserted into the contralateral intrahepatic duct by FCSEMS to prevent contraductal obstruction. Patients with CP and an indwelling pancreatic ductal stent underwent stent replacement every 3 months. Plain abdominal radiographs were obtained following successful stent placement the next day, 2 weeks, 3 months, and 6 months later to evaluate stent migration. Six months after initial placement, the stent was removed, and a balloon-occluded cholangiogram was obtained to evaluate stricture resolution and detect any de novo strictures.
2.3 Definition of Events
Technical success was defined as the accurate positioning of the stent along the entire length of the stricture. Stricture improvement was determined after FCSEMS removal or migration, based on improvement in the stricture's waist on a balloon-occluded cholangiogram and improved liver function test results 2 weeks after stent removal. Clinical success was defined as the absence of additional stent placement after FCSEMS removal or migration during the follow-up period. Stent migration was defined as the migration of the entire FCSEMS proximal or distal to the stricture segment; if the stricture remained unresolved at the time, a new FCSEMS was placed for 6 months. Stent dysfunction was defined per the Tokyo criteria [16], and in such cases, the stent was cleaned or replaced. A recurrent stricture was defined as the recurrence of a stricture, clinically and on cholangiogram, after successful treatment.
2.4 Statistical Analysis
Continuous variables were expressed as medians and ranges. Stricture recurrence was calculated using Kaplan–Meier analysis. Statistical analyses were performed using EZR version 1.54 (free software for medical statistics, available at https://www.jichi.ac.jp/saitama-sct/SaitamaHP.files/file/EZRsetup.exe) [17]. Sample size calculation was not performed in this study because of its single-arm observational nature.
3 Results
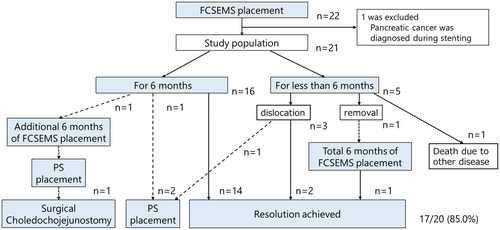
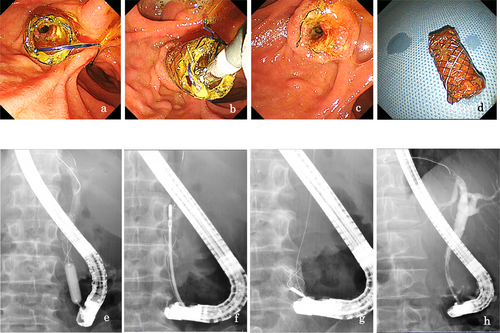
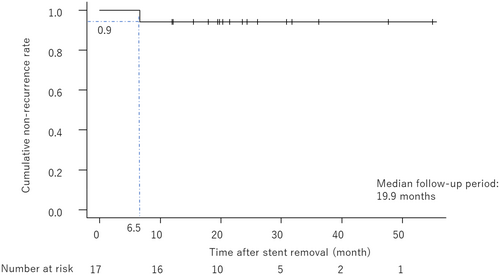
Baseline demographic and clinical data are shown in Tables 1 and 2. A total of 22 patients were enrolled, including 13 with CP (8 alcoholics and 5 non-alcoholics) and 9 with non-CP. One patient with CP, diagnosed with pancreatic cancer during stenting, was excluded, leaving 21 patients for analysis. The technical success rate of FCSEMS placement was 100%. Stents remained in place for 6 months as planned in 16 of 21 patients (76.2%). Stent migration occurred in 3 cases (14.3%), all to the distal side. Two of them had strictures resolved, while one did not, necessitating the placement of a PS because the stricture was judged to be soft, and the risk of migration was considered to be high. The treatment flow for all cases is shown in Figure 2. All stents were removed successfully, and marked improvement or resolution of the biliary stricture was observed on cholangiograms after stenting in 85% of cases (17 of 20). The median diameter of the stricture improved from 1 [0–2.9] mm to 6.0 [1−9] mm (Figure 3). However, one case required a reversal removal procedure owing to hyperplasia caused by tearing of the cover around the papilla Vater (Figure 4). During a median follow-up period of 19.9 [12.0–55.0] months post-FCSEMS removal or dislocation, one patient with alcoholic CP, who continued drinking, developed jaundice because of a recurrent stricture at 6.5 months after stent removal (Figure 5). Adverse events during stent placement were observed in 19.0% of patients (4/21): three cases of cholangitis and one case of hyperplasia formation. Of the three cases of cholangitis, one was severe due to stent obstruction caused by food impaction 4 months after stent placement. So, FCSEMS was removed, and a naso-biliary tube was temporarily inserted, and FCSEMS was re-placed again for 2 months after the infection was controlled. The remaining two patients had non-obstructive cholangitis, and they were treated conservatively with antibiotics for about a week. No episodes of post-stenting pancreatitis or stent-induced cholecystitis were observed (Table 2).
| Number of patients | 22 |
|---|---|
| Age, median [range] | 64 [34–86] |
| Sex, male/female, n | 20/2 |
| Etiology, n (%) | |
| Chronic pancreatitis | 13 (59.1) |
| Alcoholic | 8 (62) |
| Non-alcoholic | 5 (38) |
| Non-chronic pancreatitis | 9 (40.9) |
| Bile duct stone | 5 (55.6) |
| Cholecystectomy | 4 (44.4) |
| Stricture site, n (%) | |
| Distal bile duct | 16 (72.7) |
| Proximal bile duct | 6 (27.3) |
| Previous treatment period with PS, n (%) | |
| < 6 months | 9 (40.9) |
| 6 months ≤, < 12 months | 5 (22.7) |
| ≥ 12 months | 8 (36.4) |
| Stricture length, mm [range] | 15 [5–45] |
| Diameter of FCSEMS, n 8/10/12 mm | 3/17/2 |
| Bile duct stone removal before FCSEMSa placement, n (%) | 5 (22.7) |
- a FCSEMS: fully covered self-expanding metallic stent; PS: plastic stent.
| Number | 21a |
| Technical success, n (%) | 21 (100) |
| Across/above the papilla, n | 16/5 |
| Combination with contralateral bile duct plastic stents, n (%) | 3 (14.3) |
| Combination with pancreatic duct plastic stents, n (%) | 2 (9.5) |
| Placement for 6 months as planned, n (%) | 16/21 (76.2) |
| Reason for the failure | |
| Migration (distal side), n | 3b |
| Extracted owing to cholangitis, n | 1 |
| Death due to heart disease, n | 1 |
| Success of extraction, n (%) | 17 (100)c |
| Resolution of the stricture, n (%) | |
| Yes | 17 (85.0)d |
| No | 3 (15.0) |
| Diameter of the stricture part (before treatment), median, mm [range] | 1 [0–2.9] |
| Diameter of the stricture part (after treatment), median, mm [range] | 6.0 [1–9] |
| Need for stone removal, n (%) | 3 (14.3) |
| Adverse events (during stent placement), n (%) | 4 (19.0) |
| Cholangitis | 3 (14.3) |
| Severe (due to food impaction) | 1 |
| Mild (non obstructive) | 2 |
| Hyperplasia (tearing of the cover) | 1 (4.8) |
| After stent removal | |
| Re-stenosis, n (%) | 1 (5.9)e |
| Recurrence period after stent removal, month | 6.5 |
| Observation period, median, month [range] | 19.9 [12.0–55.0] |
- a One case diagnosed with pancreatic cancer during stenting was excluded, n = 21.
- b 2 of 3 cases were between 3 and 6 months, and the other was 2 days later.
- c Cases placed for 6 months n = 17.
- d Excluded one case, who died from heart disease and included 2 early dislocation cases, n = 20.
- e Among the cases in which stricture was resolved, n = 17.
4 Discussion
This long-term prospective observational study evaluated the 6-month placement of modified FCSEMS in patients with refractory BBS. Despite considerable advances in endoscopic techniques and devices, BBS management remains challenging. Contrary to patients with malignant biliary strictures, patients with BBS have normal life expectancies. Therefore, ensuring safe and reliable stent removal and subsequent permanent stricture resolution is required. Although no established solution for resolving strictures and preventing restenosis after stent removal exists, securing the largest possible stent diameter is considered a potential approach. The stricture resolution rate with a single PS of approximately 10 Fr is inadequate (12%–38%), with high adverse events such as stent occlusion and dislocation (34%–72%) [5-8]. In contrast, the bile duct stricture resolution rate improved to 44%–60% when multiple PSs were placed > 1 year [9, 10]. In a systematic review by Boeckel et al. [18] comparing single PS placement with multiple PS placement, they reported a higher clinical improvement rate with multiple stenting (59.6% vs. 94.3%) and a lower rate of adverse events (36% vs. 20.3%).
Recently, FCSEMS has been used to treat BBS owing to its prolonged patency and the absence of the need for periodic replacement. In the previous reports comparing PS and FCSEMS for BBS, no significant difference was observed in treatment success rates; however, FCSEMS offers advantages such as fewer procedures [11-13], fewer complications [12, 13], and cost benefit [13]. Haapamäki et al. [19] conducted a randomized controlled trial in 60 patients with chronic CP and BBS, comparing six 10Fr PSs placements with FCSEMS during a 6-month treatment period. They reported that the 2-year, stricture-free success rate was 90% versus 92%, and the stent migration rate was similar at 10% versus 7%. Based on these results, treatment with multiple PSs and FCSEMS is equally recommended. The choice between the two strategies depends on the etiology of the stricture, its location, the bile duct diameter, and the endoscopist experience in the ESGE guidelines 2018 [4].
Regarding the indwelling period of FCSEMS, several studies reported a direct relationship between stricture resolution rate and stent placement duration. Kahaleh et al. [20] reported significantly better stricture resolution with stent indwelling duration > 3 months compared with < 3 months. Saxena et al. [21] reported a predictor of stricture resolution and found 6 months of treatment to be superior to 3 months. Long-term stent placement may increase the risk of stent-related adverse events, such as stent migration, −induced stricture, and occlusion. Usually, the ends of the FCSEMS are typically flared to provide an anchoring function. The FCSEMS used in this study features a convex margin at each end to prevent tissue hyperplasia, while the central segment has a narrowed waist to reduce migration risk. In this study, 2 of 3 cases of distal migration had already resolved the stricture and did not require additional treatment. In the remaining case, additional PSs were placed; however, the stenosis was not resolved. As a result of stent-induced damage, hyperplasia due to cover tearing occurred in one case. Covering materials for metal stents can be categorized into two: silicone and polytetrafluoroethylene (PTFE). Silicon membrane is widely used as it is considered less sludge forming inside the stent owing to its smooth surface; however, it is less durable than PTFE and may suffer cover damage after long-term placement. The FCSEMS used in this study had a silicone membrane, and as shown in Table 1, no additional cases of stricture resolutions were observed even after > 6 months of stent placement. Furthermore, three cases of cholangitis occurred, and longer-term placement might increase the number of adverse events. Therefore, the duration of FCSEMS placement is recommended to be 6 months.
In the previous studies, this modified FCSEMS was applied to patients with intraductal placement for various etiology for 3–5 months [15], living donor liver transplantation for 3–4 months [21], and perihilar stricture for 4–6 months [22-24]. The reported stricture resolution rate ranged from 81.2%–100%, with stent migration occurring in 5.2%–15.6% of cases, and the recurrence rate after stent removal was 0%–16%. No cases of stent removal failure or stent-induced bile duct stricture were reported, but the number of subjects of these studies was small, around 20 cases. It is not still obvious which stent to place and for how long, as previous studies using conventional FCSEMS have used different types of FCSEMS [19, 21] and the duration of stenting has been inconsistent [21, 25]. We believe that the strength of the present study is that we have demonstrated the efficacy and safety of stent treatment in a uniform setting for both the type of FCSEMS and the duration of stenting.
Lower stricture resolution rates have been observed in patients with CP compared with other groups in several FCSEMS studies [25-27]. Park et al. [25] reported that CP was significantly associated with recurrence. Conversely, Devier et al. [28] reported that treatment success was more frequent in the CP group (80.5%) than in the liver transplantation (63.4%) or cholecystectomy (61.1%) groups. Moreover, the migration and cholangitis rates also were substantially lower than those of the other groups. In our study, 12 of 21 patients had CP (57.1%), and the stricture release rate was 83.3%, similar to the 87.5% in the non-CP group. In addition, 2 of the 12 patients in the CP group continued to drink, one of whom suffered a recurrence. Successful treatment of patients with CP largely depends on whether or not the patient continues drinking; therefore, a larger sample size with careful consideration of their backgrounds is needed. In the present study, surgical choledochojejunostomy was performed in one patient who had been refractory to FCSEMS treatment for more than 1 year. We previously reported [29] a case of endoscopic ultrasound (EUS)-guided treatment for refractory BBS that was unsuccessful after 3 years of stenting. A new drainage route was created by EUS-guided choledochoduodenostomy (EUS-CDS) using this modified FCSEMS and the stent was removed after 6 months with successful creation of a permanent anastomosis. However, EUS-CDS for BBS is not a well-established option, so surgery should be considered if the stricture does not resolve after one or two years of endoscopic treatment.
This study had some limitations. It was a single-arm prospective study without a control group for comparisons with other types of FCSEMS. Although we included various etiologies of BBS, the sample size was insufficient to evaluate the effectiveness of each etiology as the cases were relatively uncommon. Therefore, larger sample size studies and comparative studies are needed. Despite these limitations, the 85% stricture resolution rate, 19% adverse event rate, and 5.9% recurrence rate suggest good feasibility and promising outcomes; therefore, this treatment strategy could be a viable option for refractory BBS.
In conclusion, the 6-month placement of an FCSEMS was effective in resolving refractory BBS and demonstrated a favorable safety profile. Further studies with larger sample sizes are needed to assess the long-term safety and efficacy of temporary metal stent placement for refractory BBS.
Ethics Statement
This prospective study was reviewed and approved by the Institutional Ethics Committee of Kitasato University (No. C16-963).
Consent
All participants signed an informed consent statement prior to participation in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.




