Relationship Between Patient Demographics and Biologic Therapy Use in Inflammatory Bowel Disease. A Single Center Cross-Sectional Study
Funding: The authors received no specific funding for this work.
ABSTRACT
Introduction
Biologic therapies treat patients with moderate to severe inflammatory bowel disease (IBD). This study aims to investigate the demographics of biologic therapy use and its association with patient characteristics, a topic that has not yet been thoroughly assessed in our region.
Methods
Electronic health record data from June 1, 2021, to May 31, 2023, were collected at a tertiary care IBD center in Kuwait. The primary outcome of this single-center cross-sectional study was to assess the demographics of use of various biologic therapies among patients with IBD. The secondary outcome was to assess whether the type of biologic therapy differed based on gender, age, and IBD type.
Results
Among the 513 patients using biologic therapy in this study, there were 210 (40.9%) on adalimumab (ADL), 154 (30.0%) on infliximab (IFX), 112 (21.9%) on ustekinumab (UST), and 33 (6.4%) on vedolizumab (VDZ). Patients taking VDZ were more likely to have ulcerative colitis (UC) (p < 0.001) and were more likely to be over 30 years old (p < 0.001). In contrast, patients on UST were less likely to be over 30 (p = 0.011) and more likely to have Crohn's disease (CD) (p < 0.001). In addition, patients on ADL were more likely to have Crohn's disease (p = 0.003), as were patients on IFX (p < 0.001).
Conclusion
Patients taking VDZ were more likely to have UC and be over 30 years of age, while those on UST were more likely to be under 30 years of age and to have CD. Additionally, patients on ADL and IFX were more likely to have CD. This study highlighted the need for further research evaluating physicians' preferences and the effectiveness of different biological therapies in patients with IBD.
1 Introduction
Inflammatory bowel disease (IBD) is a disorder under the umbrella of autoimmune diseases and is often divided into two common conditions, ulcerative colitis (UC) and Crohn's disease (CD). IBD is widely believed to result from a complicated interplay of hereditary and environmental variables. Consequently, different classes of medications are used in the management of IBD, such as, but not limited to, aminosalicylates, corticosteroids, immunomodulators, and biologic therapies [1, 2]. Factors such as severity, cost, age, and clinical response are all recognizable influential markers for allocating specific regimens [3].
The demographics of patients diagnosed with IBD vary slightly by region; however, they follow the same trend of increasing prevalence worldwide. In regions like Europe and the United States, gender distribution is relatively equal for UC, with slightly greater female prevalence in reported cases of CD [4, 5]. In Asia, however, IBD affects males more frequently, particularly CD [5, 6]. Some variance can be remarked in specific countries, but generally, the average onset of IBD occurs around the early-mid 30s in Western and Asian countries [6]. The demographics of patients with IBD are evolving in Asia and the Middle East, requiring a regular evaluation of the use of therapies in the management of IBD [7].
While some research has been conducted to assess patterns and trends in the general management of IBD among different demographics, little has been done to uncover the relationship between demographics and specific biologic therapies in our region. Thus, this study aims to evaluate the relationships between the use of various biologic therapies and patient demographics in IBD in the Middle East.
2 Methods
2.1 Study Design and Recruitment
A single-center prospective cross-sectional study was conducted at Mubarak Al-Kabeer University Hospital, a tertiary care IBD center in Kuwait, covering approximately 700 000 people in its catchment area. Data from electronic health records of patients with IBD were retrieved from June 1, 2021, to May 31, 2023. Inclusion criteria for eligible patients included a confirmed diagnosis of IBD before the start of the study, treatment with a biologic agent for at least 6 weeks, and patient age of at least 18 years. Exclusion criteria were patient records with incomplete demographic or biologic therapy data, and patients who were receiving concomitant biologic or small-molecule therapy for other conditions, for example, rheumatological disease.
This study was performed and reported in accordance with Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [8]. The international classification of diseases (ICD-10 Version:2016) was used to make the diagnosis of IBD. Patients were considered to have IBD (CD) when they had ICD-10 classifications of K50, K50.1, K50.8, or K50.9, and IBD (UC) for classifications of K51, K51.0, K51.2, K51.3, K51.5, K51.8, or K51.9 [9].
The following baseline patient data were extracted: gender, age, ethnicity, IBD type, type of biologic therapy, body weight, duration of disease, smoking status, location, and ICD-10 IBD classification. The primary outcome was the demographics of biologic use among patients with IBD. The secondary outcome was to assess whether the type of biologic therapy differed based on gender, age, and type of IBD (UC or CD).
2.2 Statistical Analysis
Statistical analyses were executed with IBM SPSS Statistics 25.0 (IBM Corp, Armonk, NY). Descriptive statistics were used to calculate frequencies and central tendency, expressed as means and percentages. Descriptive analyses were conducted to calculate frequencies and proportions of categorical variables. χ2 tests were used to assess whether the type of biologic therapy differed based on age, gender, and type of IBD (UC or CD).
3 Results
3.1 Patient Demographics
In total, 513 patients with IBD on biologic therapies were included in this study. Out of the 513 patients, there were 210 (40.9%) on adalimumab (ADL), 154 (30.0%) on infliximab (IFX), 112 (21.9%) on ustekinumab (UST), and 33 (6.4%) on vedolizumab (VDZ). There were 158 (30.9%) patients who concomitantly used immunomodulators, including azathioprine, 6-mercaptopurine, or methotrexate. Moreover, 56 (10.9%) patients had concomitant corticosteroid use. The median age observed among the patient population was 23.4 years. With regards to gender, 199 (38.8%) patients were female and 314 (61.2%) were male. Additionally, disease distribution showed that 391 (76.2%) patients were diagnosed with CD, and 122 (23.8%) were diagnosed with UC (Table 1).
| Patient characteristics | (n = 513) |
|---|---|
| Median age (years) | 23.4 |
| Male n (%) | 314 (61.2) |
| Female n (%) | 199 (38.8) |
| Type of biologic or small molecule used | n (%) |
| Adalimumab | 210 (40.9) |
| Infliximab | 154 (30.0) |
| Ustekinumab | 112 (21.9) |
| Vedolizumab | 33 (6.4) |
| Tofacitinib | 2 (0.4) |
| Golimumab | 2 (0.4) |
| Ulcerative colitis n (%) | 122 (23.8) |
| Ulcerative colitis (UC) type | n (%) |
| E1: Proctitis | 39 (32.0) |
| E2: Left-sided | 33 (27.0) |
| E3: Extensive | 50 (41.0) |
| Crohn's disease n (%) | 391 (76.2) |
| Crohn's disease (CD) location | n (%) |
| L1: Ileal | 207 (52.9) |
| L2: colonic | 64 (16.4) |
| L3: ileocolonic | 117 (29.9) |
| L4: Upper gastrointestinal | 3 (0.8) |
| Crohn's disease (CD) phenotype | n (%) |
| B1: Non-stricturing, non-penetrating | 198 (50.6) |
| B2: Stricturing | 58 (14.8) |
| B3: Penetrating | 38 (9.7) |
| Missing | 97 (24.8) |
| Crohn's disease (CD) perianal disease | n (%) |
| Yes | 82 (21.0) |
| No | 309 (79.0) |
| Smoking | n (%) |
| Yes | 86 (16.7) |
| No | 427 (83.3) |
| Ethnicity | n (%) |
| Middle-Eastern | 484 (94.3) |
| Others | 29 (5.7) |
| Body Mass Index (BMI), mean | 25 |
| Biologic use (median) in months | |
| Adalimumab | 26 |
| Infliximab | 32 |
| Ustekinumab | 13 |
| Vedolizumab | 12 |
| Concomitant immunomodulator usea | 158 (30.9%) |
| Concomitant corticosteroid use | 56 (10.9%) |
- a Immunomodulator use included azathioprine, 6-mercaptopurine and methotrexate.
Out of 154 patients who were on IFX, 85 (55.2%) were on concomitant immunomodulators, while only 14 (9.1%) were on steroids. In addition, out of 210 patients on ADL, 73 (34.8%) were also on an immunomodulator, while only 25 (11.9%) were on steroids. In contrast, none of the patients who were on UST or VDZ used an immunomodulator concomitantly with the biologics. However, 11 (9.8%) out of 112 patients who were on UST, and 6 (18.2%) out of 33 patients who were on VDZ, were also on steroids (Table 2).
| Biologic type | Immunomodulator use n (%) | Steroid use n (%) |
|---|---|---|
| Total number of patients | 158 | 56 |
| Infliximab | 85 (55.2%) | 14 (9.1%) |
| Adalimumab | 73 (34.8%) | 25 (11.9%) |
| Ustekinumab | 0 | 11 (9.8%) |
| Vedolizumab | 0 | 6 (18.2%) |
| (%) out of the total number of patients on each biologic | ||
3.2 Relationship Between Biologic Use and Patient Demographics
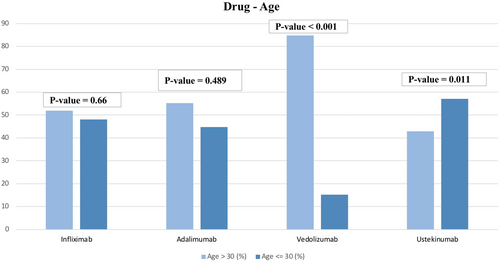
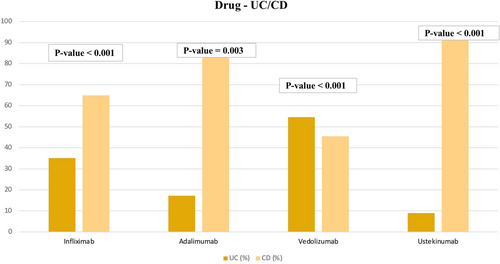
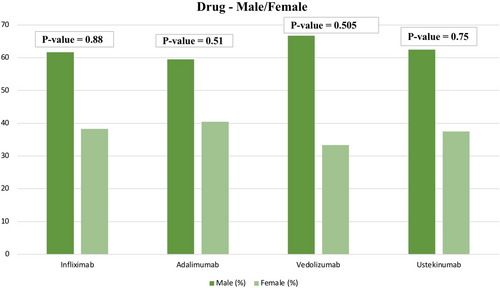
Patients on IFX did not differ based on gender or age (p = 0.88 and p = 0.66). However, patients on IFX were more likely to have CD (p < 0.001), with 100 patients having CD and 54 only having UC. Regarding age, 80 of these patients were over 30 years of age and 74 were 30 or younger. Likewise, patients on ADL did not differ based on gender and age (p = 0.51 and p = 0.489). However, use of ADL was significantly associated with having CD (p = 0.003). A total of 116 of these patients were over 30 years of age and 94 were 30 or younger. Unlike IFX and ADL, patients taking VDZ were more likely to have UC (18 out of 33 patients) (p < 0.001). Additionally, patients were more likely to be over 30 years of age (28 out of 33) (p < 0.001). In contrast, patients on UST were less likely to be over 30 (48 out of 112) (p = 0.011) and more likely to have CD (102 out of 112). (See Table 3 and Figures 1, 2, and 3).
| Drug | Variable | Study group (n = 513) | p | ||
|---|---|---|---|---|---|
| Infliximab | Male | Female | 95 | 59 | 0.88 |
| Age > 30 | Age ≤ 30 | 80 | 74 | 0.66 | |
| UC | CD | 54 | 100 | < 0.001 | |
| Adalimumab | Male | Female | 125 | 85 | 0.51 |
| Age > 30 | Age ≤ 30 | 116 | 94 | 0.489 | |
| UC | CD | 36 | 174 | 0.003 | |
| Vedolizumab | Male | Female | 22 | 11 | 0.505 |
| Age > 30 | Age ≤ 30 | 28 | 5 | < 0.001 | |
| UC | CD | 18 | 15 | < 0.001 | |
| Ustekinumab | Male | Female | 70 | 42 | 0.75 |
| Age > 30 | Age ≤ 30 | 48 | 64 | 0.011 | |
| UC | CD | 10 | 102 | < 0.001 | |
3.3 Relationship Between Current and Previous Biologic Therapy
The study examined the prevalence of using previous biologic drugs among patients with CD and UC. The results revealed that a substantial fraction of patients had previously taken biologic medicines (Table 4).
| Current infliximab treatment | ||
|---|---|---|
| Ulcerative colitis (n = 54) | Crohn's disease (n = 100) | |
| Naïve n (%) | 39 (72.2) | 76 (76) |
| Experience n (%) |
Vedolizumab 10 (18.5) Ustekinumab 5 (9.2) |
Vedolizumab 3 (3) Ustekinumab 21 (21) |
| Current adalimumab treatment | ||
|---|---|---|
| Ulcerative colitis (n = 36) | Crohn's disease (n = 174) | |
| Naïve n (%) | 25 (69.4) | 133 (76.4) |
| Experience n (%) |
Vedolizumab 7 (19.4) Ustekinumab 4 (11.1) |
Vedolizumab 9 (5.1) Ustekinumab 32 (18.3) |
| Current vedolizumab treatment | ||
|---|---|---|
| Ulcerative colitis (n = 18) | Crohn's disease (n = 15) | |
| Naïve n (%) | 14 (77.7) | 8 (53.3) |
| Experience n (%) | Infliximab 4 (22.2) |
Infliximab 3 (20) Adalimumab 3 (20) Ustekinumab 1 (6.6) |
| Current ustekinumab treatment | ||
|---|---|---|
| Ulcerative colitis (n = 10) | Crohn's disease (n = 102) | |
| Naïve n (%) | 6 (60) | 71 (69.6) |
| Experience n (%) |
Infliximab 3 (30) Vedolizumab 1 (10) |
Infliximab 14 (13.7) Adalimumab 17 (16.7) |
Among patients with UC who were currently being treated with IFX, 18.5% had previously used VDZ, and 9.2% had used UST. On the other hand, among patients with CD who were being treated with IFX, 3% had previously used VDZ, and 21% had used UST.
The study also looked at the medication history of patients currently being treated with ADL. Among these patients, 19.4% had previously used VDZ, and 11.1% had used UST. Furthermore, among patients with CD treated with ADL, 5% had previously used VDZ, and 18.3% had used UST.
The study also investigated the prevalence of patients currently being treated with VDZ who had previously taken other biologic agents. Among patients with UC currently using VDZ, 22.2% had previously taken IFX. Similarly, patients with CD currently using VDZ had a history of taking IFX (20%), ADL (20%), and UST (6.6%).
Finally, among patients with UC currently being treated with UST, some had previously taken IFX (30%) or VDZ (10%). Among patients with CD currently using UST, some had previously taken IFX (13.7%) or ADL (16.7%).
4 Discussion
This study analyzed patient demographics, including age, gender, and type of IBD, to identify correlations with biologic use among Kuwaiti patients and compare these findings with global patterns, aiming to uncover trends in biologic therapy usage. As such, the clinical and epidemiological profiles of individuals with IBD can be used to recommend successful interventions targeted at improving the quality of life.
As mentioned in the results, the median age observed among patients with IBD was 23.4 years, demonstrating results similar to other studies [10-12]. Furthermore, another topic of interest within our results was the distribution of age among patients taking VDZ. Most patients in our cohort taking VDZ were over 30 years old, likely because its safety profile makes physicians more comfortable prescribing it to older patients. Despite this, the GEMINI 1 and GEMINI 2 studies, which stratified the efficacy of VDZ based on age, concluded that there was no difference in effectiveness across all age groups in patients with CD and UC [13-15]. More research assessing demographic trends in IBD patients receiving VDZ is needed.
Our results revealed a slightly preferential usage of UST among patients at or below 30 years of age. One of the more striking results in our study was the sheer number of CD patients taking UST compared to VDZ. In the cohort of patients taking UST, the majority were diagnosed with CD, with only a few having UC. In our experience, physicians tend to prefer UST for CD due to its efficacy and mechanism of action, as clinical observations suggest it works more effectively than VDZ. However, this has not been confirmed by clinical trials, as direct head-to-head comparisons between UST and VDZ are limited. On the other hand, the preference for prescribing VDZ over UST for UC may be due to UST being more recently approved for UC than for CD by local and international regulatory bodies such as the FDA (2019 for UC vs. 2016 for CD) [16]. Nonetheless, it is important to note that UST has shown success in inducing maintenance and remission in both CD and UC, with equal variance of success [17-21]. Future follow-up regarding these trends is needed to assess whether this disparity in usage between CD and UC patients persists over time. Most earlier cohort studies have suggested superior effectiveness for UST over VDZ in those who have refractory CD [22-24]. For that reason, UST was mainly used for patients with CD, despite recent studies illustrating a significant difference in effectiveness between UST and VDZ in regard to failure of anti-TNF therapy [25, 26].
In our cohort, there was a male predominance in total number of patients with IBD among all age groups. However, different studies have shown differing results in this regard. In one study of 841 patients over 60 years of age, CD showed a female predominance, while more males had UC [27]. In contrast, in younger patients, incidence of IBD is equal for both genders [28-30].
Kuwait's local health protocols follow both ECCO and AGA guidelines, which recommends that patients with moderate to severe UC or CD adopt any biologic regimen. Thus, physicians have the option of using anti-TNF, VDZ, or UST as a first choice without limitations. However, in our study, ADL, followed by IFX, were the most commonly prescribed drugs across all patients. Similar findings were highlighted by Brady et al. [31] who studied the numbers of patients with UC and CD separately and found IFX and ADL were preferred, respectively. This preference for ADL and IFX is in line with global management trends seen in the United States and the European Union [32-34]. This may be attributed to the abundance of evidence and research done on the efficacy of these agents. The relative unfamiliarity of VDZ, UST, and tofacitinib may make their use less prevalent than IFX and ADL in IBD patients. This has been confirmed by other research showing that in clinical practice in Kuwait, IFX and ADL were widely utilized because physicians were more confident in using them [35-38].
Medication safety profile is another factor in the process of prescribing biologics. When treating individuals with IBD who are elderly or have co-morbidities, VDZ is considered a safe and effective option. It is also the preferred biologic for combination therapy with another biologic agent or with concurrent steroid use, due to the drug's benefits, particularly in the area of safety [39, 40]. Supporting this approach, another study showed that the co-administration of VDZ with corticosteroids led to greater rates of clinical remission, compared with either the VDZ-only group or the corticosteroid and placebo combination therapy group, while rates of adverse effects were comparable across groups [41]. Therefore, VDZ and steroid combination therapy appears to enhance clinical remission. In general, it is common practice in Kuwait to induce sick patients with steroids, as therapies such as VDZ and UST can take time to demonstrate their efficacy. In contrast, IFX and ADL patients are less likely to be induced with steroids.
From a related perspective, biologic sequencing has been presented in the literature as a crucial point of focus that also has an impact on clinical practice. Bressler shed light on biologic sequencing in patients with UC and CD, showing the outcome of clinical remission based on pooled data from multiple studies [42]. Results showed that clinical remission occurred at a lower rate in patients with UC and CD when VDZ and ADL were used for patients who had prior anti-TNF treatment. Therefore, VDZ and ADL are more effective as first-line agents but may be less effective when used as second-line agents following anti-TNF treatment. Effectiveness of UST and tofacitinib did not differ much with prior exposure to anti-TNF therapy in CD and UC [42]. IFX, meanwhile, remains the drug of choice for fistulizing CD [43].
Another consideration when choosing a biologic agent for patients was the cost-effectiveness of a drug. Studies showed that IFX is often the most cost-effective compared to other biologics, such as ADL, certolizumab pegol, natalizumab, and VDZ [44, 45]. A study conducted in Saudi Arabia illustrated that VDZ had the highest annual cost across all biologics [46].
Finally, based on current practice, we speculate that local gastroenterologists in Kuwait, as well as others in the region, prefer VDZ for older patients due to its safety profile, and favor its use as a first-line treatment for UC, despite its comparable efficacy in CD. On the other hand, physicians favor UST for CD possibly due to its more efficacious nature among patients in comparison to VDZ, although more studies need to be conducted in this regard.
Our study has several strengths. It is the first study in our region to estimate the prevalence of biologic use in patients with IBD. In addition, the study encompassed a reasonably large sample size from a population that has not yet been studied, and it included the most important biologics commonly used in IBD. Our inclusion and exclusion criteria were selected carefully to eliminate bias. We believe that, due to incorporation of a reasonable sample size, our research should help to describe the clinical and epidemiological profile of patients in Kuwait. However, one of the limitations of our study is that it is a single-center study. Finally, possible confounders and biases could exist due to the observational nature of the study. To understand the clinical and epidemiological data better, multi-center population-based studies may be required.
5 Conclusion
Patients taking VDZ were more likely to have ulcerative colitis and be over 30 years of age, while those on UST were more likely to be under 30 years of age and to have Crohn's disease. In addition, patients on ADL and IFX were more likely to have CD. This study highlights the need for further research evaluating physicians' preferences and the effectiveness of different biologic therapies in IBD patients with varying demographics.
Acknowledgments
The authors have nothing to report.
Ethics Statement
This study was reviewed and approved by the Ethical Review Board of the Ministry of Health of Kuwait (reference: 3526, protocol number 2145/2022).
Consent
Patient consent was waived by the Ethical Review Board.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data are available from the corresponding author upon reasonable request.




