Prepregnancy Obesity Reprograms Offspring Skeletal Muscle Fibre Transition Through H3K9me3
Funding: This research was supported by the National Natural Science Foundation of China (No. 82001605) and the National Key Research and Development Program of China (No. 2023YFC2706300).
ABSTRACT
Background
Maternal prepregnancy obesity predisposes offspring to obesity and metabolic disorders, yet its impact on skeletal muscle fibre transition remains unclear. Given that skeletal muscle plays a crucial role in systemic metabolism, we investigated how maternal prepregnancy high-fat diet (HFD) influences muscle fibre composition and metabolic function in offspring.
Methods
We established mouse models with a prepregnancy chow diet (CD) and a prepregnancy high-fat diet (HFD) for 8 weeks to compare metabolic phenotypes in offspring. Skeletal muscles from offspring were analysed using RNA sequencing, quantitative reverse transcription polymerase chain reaction and western blot to understand the changes in metabolic and signalling pathways. siRNA knockdown and lentiviral-mediated overexpression experiments were conducted in vitro and in vivo to validate molecular mechanisms. Chromatin immunoprecipitation followed by qPCR (ChIP-qPCR) was used to assess histone modification levels at promoter regions.
Results
Male and female offspring of prepregnancy obese dams (mHFD) exhibited a significant reduction in slow-twitch oxidative fibres (p < 0.001) and an increase in fast-twitch glycolytic fibres compared with controls. This was accompanied by impaired glucose tolerance (AUC increased by 12.87%, p < 0.01), insulin resistance and mitochondrial dysfunction (mtDNA copy number reduced by 31%, p < 0.01). RNA sequencing identified IDH2 as the most significantly downregulated gene (29.67% decrease, p < 0.001), with protein levels further reduced in male (30.15%, p < 0.01) and female (46.02%, p < 0.0001) offspring. IDH2 knockdown in C2C12 cells impaired mitochondrial biogenesis and led to higher oxidative stress (NADP+/NADPH ratio elevated by 32%, p < 0.01), while IDH2 overexpression restored mitochondrial integrity, enhanced slow-twitch fibre proportion (26.43 ± 0.6936% in mHFD-LV-IDH2, p < 0.01) and improved glucose metabolism (fasting glucose reduced by 14.7%, p < 0.01). ChIP-qPCR revealed increased H3K9me3 enrichment at the IDH2 promoter (2.54-fold in males, 2.55-fold in females, p < 0.0001), suggesting transgenerational epigenetic regulation.
Conclusions
Maternal prepregnancy obesity induces a metabolic shift in offspring skeletal muscle by promoting a slow-to-fast fibre transition and impairing mitochondrial biogenesis. This effect is mediated by IDH2 suppression via H3K9me3 histone modification, contributing to systemic insulin resistance. Targeting IDH2 may represent a potential therapeutic strategy to mitigate metabolic dysfunction in offspring exposed to maternal prepregnancy obesity.
1 Introduction
The prevalence of obesity has increased substantially among women of reproductive age worldwide over the past two decades [1, 2]. Maternal overnutrition is a pivotal factor that shapes the developmental environment in early life and increases the risk of metabolic diseases in offspring, such as obesity and diabetes [3-12]. Maternal obesity, including prepregnancy obesity and excessive gestational weight gain (EGWG), impacts offspring metabolism differently. Prepregnancy obesity is more prevalent globally and affects early embryo development, whereas EGWG affects mid-to-late pregnancy. While gestational obesity has been extensively studied, fewer studies have focused on prepregnancy obesity.
Skeletal muscle is recognised as a key organ in energy metabolism. As the most abundant tissue in the human body, accounting for approximately 30%–40% of body mass, it serves as the primary site for insulin-mediated glucose uptake [13, 14]. The capacity of skeletal muscle to perform various functions, such as locomotion, thermogenesis and metabolism, is predominantly ascribed to the adaptability of muscle fibres [15]. Muscle fibres are primarily classified into three types based on different contraction speeds and metabolic activity: slow-twitch oxidative fibres, fast-twitch oxidative fibres and fast-twitch glycolytic fibres [16]. It has been well-documented that individuals with obesity and T2D exhibit a shift in muscle fibre composition, characterised by a reduction in oxidative Type I fibres and an increase in glycolytic fibres, which is closely linked to insulin resistance and mitochondrial dysfunction [17-19]. However, until now, there are no studies reporting muscle fibre type transition or skeletal muscle metabolism in offspring of mothers with prepregnancy obesity.
Epigenetic modifications have garnered significant attention in the metabolic diseases [12, 20-22]. Histone modifications, in particular, are believed to play a key role in the developmental programming of offspring in response to environmental exposures, which affect gene expression levels by regulating chromatin accessibility without altering the genomic sequence [21, 22]. Importantly, recent studies have highlighted the crucial roles of histone modifications in maintaining skeletal muscle function and regulating muscle fibre types [23, 24]. While no studies to date have explored the regulation of histone modifications in the muscles of offspring exposed to an adverse intrauterine environment, it is plausible to speculate that histone modifications may modulate muscle function and metabolism in offspring subjected to intrauterine overnutrition.
Therefore, clarifying the effects of prepregnancy obesity on offspring and the underlying mechanisms will help to develop novel preventive and therapeutic strategies. In this study, we constructed mouse models with prepregnancy chow diet (CD) and prepregnancy HFD to compare the metabolic phenotypes and muscle fibre types in the offspring of two groups and to investigate the potential role of epigenetic regulation in this process.
2 Methods
2.1 Animals
Four-week-old C57BL/6J mice were purchased from GemPharmatech Co. Ltd. (Jiangsu, China). Female mice were given either a standard diet or a high-fat diet (60%) before pregnancy, followed by a standard diet during pregnancy and lactation. Male mice used for breeding were continuously maintained on a chow diet. After 8 weeks of feeding, pregnancy was determined by the presence of a vaginal plug and assigned the embryonic age E0.5. All offspring were weaned onto a standard CD postnatally. Animals were anaesthetised with 1% pentobarbital before tissue collection. Samples were harvested at embryonic day 18.5 (E18.5) and at 8 weeks of age in offspring. Immediately after collection, tissues were flash-frozen in liquid nitrogen and stored at −80°C for further analysis. All animal procedures complied with the guidelines and regulations of the Chinese Laboratory Animal Guidelines and were approved by the Animal Care and Use Committee of Tongji Hospital (Approval No.: TJH-202209006).
2.2 Grip Strength Measurement and Treadmill Running
Grip strength was assessed in 8-week-old mice using a digital grip strength meter (BIOSEB, Vitrolles, France). To perform the test, the mice were placed on a grid with their bodies horizontal and their tails were gently pulled to determine the peak force, which was normalised by lean body mass. Each mouse was subjected to three trials at intervals greater than 15 min.
Mice were tested for exercise tolerance using a treadmill (Jeung Do Bio & Plant). To acclimatise the mice to the treadmill, they were run at 10 m/min for 5 min per day for 3 days. The mice were then tested by running on Day 4. The treadmill was run at 10 m/min for the first 5 min and then increased by 2 m/min every 2 min to 46 m/min until exhaustion. The running time of each mouse was recorded. Exhaustion was defined as the animal's inability to run on the treadmill for 10 s despite mechanical stimulation.
2.3 Additional Methods
Additional details regarding the methods and materials are provided in the Supporting Information.
2.4 Statistical Analysis
Data are presented as mean ± SEM. Statistical analyses and graphical presentations were performed using the GraphPad Prism 9.1 software. Normality and homogeneity of variance were tested before applying parametric tests. Two-group comparisons were conducted using an unpaired Student's t-test, while multiple group comparisons were performed using one-way ANOVA followed by Dunnett's post-hoc test. Significant differences are indicated as p < 0.05.
3 Results
3.1 Maternal Prepregnancy Obesity Induces Systemic and Muscular Insulin Resistance in Offspring
After 8 weeks of HFD feeding (60% calorie from fat), the maternal mice developed obesity, evidenced by a greater than 20% increase in body weight. Additionally, they developed hyperlipidaemia, higher hepatic lipid content, as well as elevated fasting blood glucose and insulin levels (Supplementary Figure 1A–E). The litter sizes and survival rates of offspring from different groups were comparable (Supplementary Figure 2A,B).
In the offspring, both male and female of mothers with prepregnancy obesity (mHFD) had significantly increased birth weights compared with those of mothers with a prepregnancy chow diet (mCD). These weight differences persisted until weaning but declined in female offspring during subsequent growth (Figure 1B and Supplementary Figure 3A). At 8 weeks of age, although no weight differences were observed in females, fasting glucose and fasting insulin were significantly elevated in both male and female mHFD (Figure 1C and Supplementary Figure 3B,C). Consistently, mHFD exhibited significantly higher blood glucose levels at 30 min (19.27 ± 0.74 vs. 15.84 ± 0.89 mmol/L, p < 0.01) and 60 min (13.84 ± 1.15 vs. 11.61 ± 0.42 mmol/L, p = 0.087) during the glucose tolerance test (GTT) compared with mCD (Figure 1D). Furthermore, the area under the curve (AUC) for glucose tolerance was 12.87% higher in mHFD (p < 0.01) (Figure 1D). During the insulin tolerance test (ITT), mHFD exhibited a slower decline in blood glucose levels, with levels remaining significantly higher at 30 min (4.36 ± 0.09 vs. 3.69 ± 0.21 mmol/L, p < 0.01) and 120 min (6.79 ± 0.29 vs. 5.64 ± 0.14 mmol/L, p < 0.01) compared with mCD offspring (Figure 1E). Similar results were observed in female offspring (Supplementary Figure 3D,E). These results indicate that mHFD exhibited impaired glucose tolerance and insulin resistance.
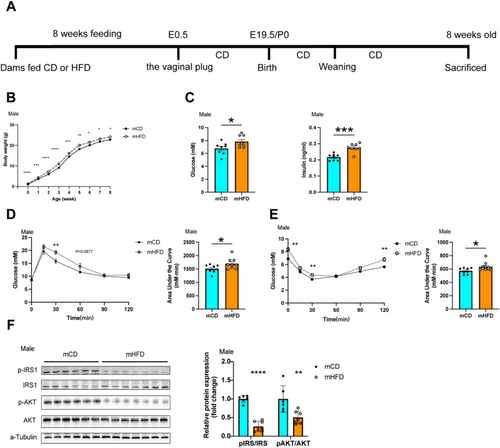
Considering the pivotal role of skeletal muscle in glucose utilisation, we further investigated the insulin signalling pathway in muscle tissue of the offspring. The results indicated disrupted insulin signalling in mHFD offspring. Serine phosphorylation of IRS1 (Ser636) was significantly reduced to 26.33% (p < 0.0001) in the skeletal muscle of mHFD offspring compared with mCD offspring (Figure 1F). Similarly, AKT (Ser473) phosphorylation reduced by 50.4% (p < 0.01) (Figure 1F). A similar downward trend was also observed in female offspring (Supplementary Figure 3F). These findings suggested that maternal prepregnancy obesity leads to systemic and muscular insulin resistance in the offspring, with similar effects observed in both sexes.
3.2 Maternal Prepregnancy Obesity Reduces Oxidative Muscle Fibre in Offspring
To further investigate alterations in muscle tissue of offspring, we assessed the exercise capacity of both groups. The group of mHFD showed a greater muscle grip strength, shorter running duration and reduced time to exhaustion during running tests (Figure 2A and Supplementary Figure 5A). These findings indicated that mHFD performed better in explosive exercises but had diminished endurance during prolonged physical activity, suggesting a reduction of oxidative muscle fibres.

We observed that both male and female mice in the mHFD group had a higher tibialis anterior (TA) muscle weight, while no significant differences were found in gastrocnemius (GAS) and quadriceps (QU) muscle weights between the two groups (Supplementary Figure 4A,B). To better assess relative skeletal muscle mass changes, we calculated the ratio of muscle weight to body weight (Figure 2B and Supplementary Figure 5B). Further analysis revealed that the GAS ratio was significantly lower, whereas the TA ratio was significantly higher in both male and female mHFD mice. In contrast, the QU ratio showed no statistically significant difference between the groups.
Immunofluorescence staining showed that the average cross-sectional area of individual muscle fibres was significantly larger in TA (1885 ± 183.9 μm2 vs. 1290 ± 149.3 μm2, p < 0.05), QU (1828 ± 110.4 μm2 vs. 1355 ± 99.75 μm2, p < 0.01), GAS (2043 ± 119.5 μm2 vs. 1655 ± 56.67 μm2, p < 0.01) in the mHFD group compared with the mCD group (Figure 2C and Supplementary Figure 5C). Furthermore, the staining for muscle fibre types showed that mHFD possessed a lower proportion of Type I fibres (8.66 ± 1.32% vs. 22.97 ± 1.31%, p < 0.001) and a higher percentage of Type IIb fibres (91.32 ± 1.32% vs. 77.37 ± 1.38%, p < 0.001) in the GAS in comparison with mCD (Figure 2D and Supplementary Figure 5D). In mHFD, the protein expression level of the slow-twitch oxidative fibre marker Myh7 was significantly reduced. Concurrently, the expression of the fast-twitch glycolytic fibre marker Myh4 was notably higher, while the expression of the fast-twitch oxidative marker Myh2 remained unchanged (Figure 2E and Supplementary Figure 5E). As expected, the activity of the oxidative enzyme succinate dehydrogenase (SDH) was lower, while the activity of the glycolytic enzyme lactate dehydrogenase (LDH) was significantly higher in mHFD (Figure 2F and Supplementary Figure 5F). These results strongly suggested that maternal prepregnancy HFD exposure induced the transition of muscle fibre types, characterised by a reduction in slow-twitch oxidative fibres and an increase in fast-twitch glycolytic fibres.
3.3 Maternal Prepregnancy Obesity Alters IDH2 Expression in the Muscle Tissue of Offspring
To explore the underlying molecular mechanisms, we performed RNA sequencing (RNA-seq) analysis on the GAS from both mCD and mHFD. Principal component analysis (PCA) revealed that PC1 and PC2 accounted for 51.09% and 15.07% of the total gene expression variance, respectively (Figure 3A). A total of 2170 differentially expressed genes were identified (Figure 3B). GO analysis showed that these genes were involved in multiple signalling pathways associated with biological processes and cellular component, such as muscle system process, mitochondrion, transition between fast and slow fibre and glucose homeostasis (Figure 3C). KEGG pathway analysis further indicated associations with pathways including ECM-receptor interaction and PI3K-Akt signalling pathway (Figure 3D). Because the skeletal muscle fibre characteristics of offspring from prepregnancy obese dams were primarily marked by a significant reduction in the proportion of slow-twitch oxidative fibres, we focused our investigation on the screening and validation of downregulated genes. Because GO analysis revealed significant enrichment of mitochondrial function-related gene sets, we ranked differentially expressed genes based on their log fold change and selected 10 highly altered candidate genes for validation using PCR. The selection criteria included genes that were both significantly downregulated (adjusted p < 0.05) and had the highest absolute log fold change values. Among these genes, mitochondrial NADP-dependent isocitrate dehydrogenase 2 (IDH2) exhibited the most pronounced reduction in mRNA expression, showing a 29.67% lower expression in the mHFD group compared with the control mCD group. (Figure 3E). Additionally, we also observed significant differences in the protein levels of IDH2 between the two groups, with a 30.15% reduction in male offspring (p < 0.01) and a 46.02% reduction in female offspring (p < 0.0001), further supporting its potential role in the skeletal muscle of mHFD offspring (Figure 3F).
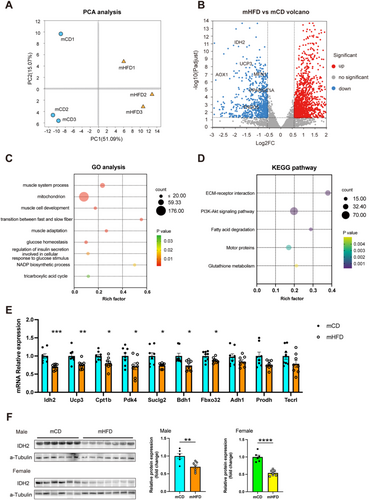
3.4 Muscle-Specific IDH2 Regulates Systemic Insulin Resistance in mHFD by Modulating Muscle Fibre Type Transition
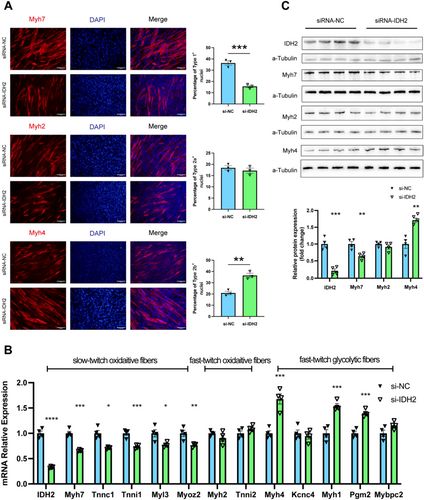
To investigate the role of IDH2 in muscle fibre type transition, we performed an in vitro siRNA-mediated knockdown of IDH2 (si-IDH2) in the C2C12 skeletal muscle cell line. Remarkably, compared with cells transfected with the negative control (si-NC), si-IDH2 triggered the transition of muscle fibre types, with immunostaining evidence showing a significant reduction of the slow-twitch oxidative fibres and a corresponding elevation of the fast-twitch glycolytic fibres (Figure 4A). Further molecular studies revealed that the mRNA expression of Type I (oxidative slow-twitch) fibre-specific genes was significantly lower, including Myh7 (0.66-fold, p < 0.001), Tnni1 (0.74-fold, p < 0.001), Tnnc1(0.71-fold, p < 0.05), Myl3 (0.77-fold, p < 0.05) and Myoz2 (0.76-fold, p < 0.01). The expression of Type IIa fibre-specific genes, including Myh2 and Tnni2, showed no significant changes. In contrast, Type IIb fibre-specific genes were significantly increased, including Myh4 (1.68-fold, p < 0.001), Myh1 (1.54-fold, p < 0.001) and Pgm2 (1.4-fold, p < 0.001) (Figure 4B,C). These results underscored the critical regulatory role of IDH2 in muscle fibre type transition.
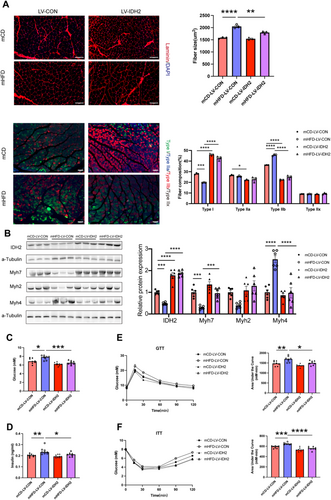
To further elucidate the role of IDH2 in muscle fibre transition and systemic metabolism in vivo, we utilised a tissue-specific IDH2 overexpression approach using lentiviral vectors. Specifically, at the age of 4 weeks, either a control lentivirus (LV-Control) or an IDH2 overexpressing lentivirus (LV-IDH2) was injected into the GAS, TA and QU muscles of offspring. Four weeks after injection, the overexpression of IDH2 was observed to reduce the cross-sectional area of muscle fibres in the GAS (Figure 5A). Notably, IDH2 overexpression partially restored the reduction of Type I fibres in mHFD, while simultaneously reducing the proportion of Type IIb fibres in the GAS (Figure 5A). Consistently, the overexpression of IDH2 significantly upregulated Myh7 expression (3.26-fold, p < 0.001) and suppressed Myh4 expression (0.39-fold, p < 0.0001) in mHFD (Figure 5B). More importantly, we found that muscle-specific IDH2 overexpression reduced fasting glucose levels (7.675 ± 0.177 mmol/L vs. 6.55 ± 0.157 mmol/L, p < 0.01) and fasting insulin levels (0.24 ± 0.01 ng/mL vs. 0.21 ± 0.005 ng/mL, p < 0.05) in mHFD offspring (Figure 5C,D) and alleviated impaired glucose tolerance and insulin sensitivity (Figure 5E,F). These data significantly implied that muscular IDH2 ameliorates systemic insulin sensitivity in offspring exposed to maternal prepregnancy obesity, by facilitating the transition of muscle fibres from fast-twitch glycolytic fibres to slow-twitch oxidative fibres.
3.5 IDH2 Promotes Mitochondrial Biogenesis Through the Maintenance of Redox Homeostasis
Given the strong link between mitochondrial biogenesis and muscle fibre composition, we hypothesised that IDH2 modulates muscle fibre transition by maintaining redox homeostasis and enhancing PGC-1α-mediated mitochondrial biogenesis in offspring exposed to maternal prepregnancy obesity.
To investigate mitochondrial abnormalities in mHFD offspring, we examined mitochondrial morphology, copy number and biogenesis-related gene expression. Electron microscopic analysis of GAS muscle revealed that mHFD exhibited mitochondrial swelling, disrupted cristae structure and reduced matrix density (Figure 6A). Compared with mCD, mitochondrial DNA (mtDNA) copy number was significantly reduced in mHFD offspring (0.69-fold, p < 0.01), suggesting impaired mitochondrial biogenesis (Supplementary Figure 6A). Furthermore, mRNA expression levels of key mitochondrial biogenesis markers were significantly lower in mHFD offspring, including PGC-1α (0.71-fold, p < 0.01), TFAM (0.79-fold, p < 0.05), TFB1m (0.77-fold, p < 0.05) and TFB2m (0.78-fold, p < 0.05), confirming impaired mitochondrial replication and transcription (Supplementary Figure 6B).
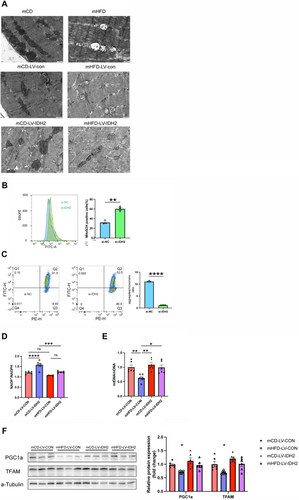
To further investigate the mechanisms by which IDH2 impacts mitochondrial function, we transfected C2C12 cells with si-IDH2. Flow cytometry revealed that compared with si-NC, C2C12 cells treated with si-IDH2 exhibited a significant increase in mitochondrial reactive oxygen species (ROS) levels (60.3 ± 2.65% in si-IDH2 vs. 32.0 ± 2.265% in si-NC, p < 0.01), coupled with a marked reduction in mitochondrial membrane potential, suggesting increased oxidative stress (Figure 6B,C). Consistently, we also observed that IDH2 knockdown led to an increase in the NADP+/NADPH ratio (1.62 ± 0.071 in si-IDH2 vs. 1.22 ± 0.121 in si-NC, p < 0.01) in cells (Supplementary Figure 6C). As expected, the expressions of the key mitochondrial biogenesis regulators, PGC1α and TFAM mRNA (Supplementary Figure 6D) and protein levels (Supplementary Figure 6E) were significantly decreased in si-IDH2-treated cells.
To confirm the role of IDH2 in mitochondrial biogenesis, we performed in vivo muscle-specific lentiviral overexpression of IDH2 in mHFD. IDH2 overexpression improved mitochondrial morphology, as evidenced by more compact cristae structure and enriched mitochondrial matrix density (Figure 6A). NADP+/NADPH levels were restored following IDH2 overexpression in skeletal muscle, confirming the role of IDH2 in maintaining redox homeostasis (Figure 6D). Consistently, IDH2 overexpression in mHFD significantly increased mitochondrial DNA content (Figure 6E) and restored the expression levels of PGC1α and TFAM (Figure 6F). These results suggest that IDH2 may promote mitochondrial biogenesis by regulating redox homeostasis, ultimately affecting fibre type composition in skeletal muscle.
To elucidate the underlying cause of reduced IDH2 levels in mHFD, we further analysed the expression levels of IDH2 and genes associated with mitochondrial biogenesis in foetal muscle. Compared with mCD, the mRNA expression of IDH2 was significantly lower in muscle tissue of mHFD (Supplementary Figure 6F). Consistently, mtDNA content (0.67-fold, p < 0.001) (Supplementary Figure 6G), as well as the expression levels of PGC1α (0.71-fold, p < 0.01), TFAM (0.72-fold, p < 0.05), TFB1m (0.75-fold, p < 0.05) and TFB2m (0.77-fold, p < 0.05) were reduced in the foetal muscle of mHFD (Supplementary Figure 6F). These results strongly implied an intra-uterine epigenetic modulation of IDH2 in offspring exposed to maternal prepregnancy obesity.
3.6 Prepregnancy Histone 3 Lysine 9 Trimethylation Modification in Oocyte Contributes to IDH2 Gene Downregulation in Muscle Tissue of mHFD
Histone modifications, particularly acetylation and methylation, are crucial for intra-uterine epigenetic control of protein expression. Acetylation of lysine 9 and lysine 27 on histone H3 (H3K9ac and H3K27ac) is associated with transcriptional activation, while tri-methylation of histone H3 on lysine 9, lysine 27 and lysine 36 (H3K9me3, H3K27me3 and H3K36me3) leads to transcriptional repression [25]. We conducted analysis of histone modifications in the skeletal muscle of mCD and mHFD. Compared with mCD, the mHFD exhibited a pronounced elevation in the levels of H3K9ac (1.78-fold, p < 0.05), H3K9me3 (2.41-fold, p < 0.0001) and H3K27ac (1.43-fold, p < 0.01), whereas the levels of H3K4me3, H3K36me3 and H3K36me3 remained unchanged (Figure 7A). Given that IDH2 expression was lower in mHFD mice, we focused on the investigation of H3K9me3, which is implicated in transcriptional silencing. ChIP-qPCR analysis revealed that H3K9me3 enrichment at the IDH2 promoter was higher in both male (2.54-fold, p < 0.0001) and female (2.55-fold, p < 0.0001) mHFD mice (Figure 7B).
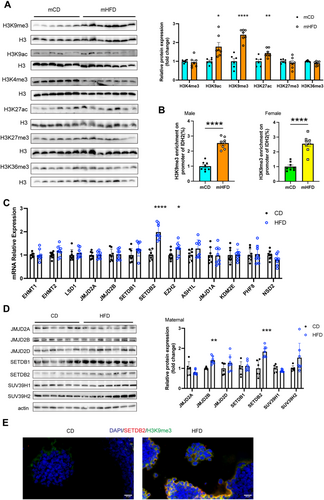
Given that maternal environmental exposures can alter epigenetic profiles of gametes and early embryos, we hypothesised that the origin of H3K9me3 modification might trace back to the gamete stage. We analysed the expression levels of several histone methyltransferases (EHMT1, EHMT2, JMJD2A, JMJD2B, SETDB1, SETDB2, EZH2, ASH1L, JMJD1A, NSD2) and demethylases (KDM5A and PHF8) in the ovaries of CD and HFD dams. Among these, the expression level of the histone methyltransferases SETDB2 was significantly higher in HFD dams (Figure 7C,D). Moreover, the increased expression of SETDB2 was co-localised with the H3K9me3 histone modification in ovaries of HFD dams (Figure 7E). Collectively, our results suggested that reduced IDH2 expression in muscle tissue of mHFD may be attributed to H3K9me3 histone modification, which was likely initiated by the upregulated expression of SETDB2 in the oocytes of HFD dams.
4 Discussion
Maternal overnutrition increases the risk of metabolic syndrome in the offspring [3-12]. The composition of muscle fibre types plays a critical role in both systemic and muscle-specific metabolism [26]. However, the impact of prepregnancy obesity alters muscle fibre transition in offspring remains largely unexplored. Here, we observed that maternal prepregnancy HFD exposure led to a reduction in slow-twitch oxidative muscle fibres in offspring without gender differentiation, accompanied with mitochondrial dysfunction in muscle tissue. Further investigation revealed that IDH2 was responsible for mitochondrial dysfunction, manipulating muscle fibre type transition. Restoring IDH2 expression in muscle tissue significantly enhanced the mitochondrial function, improved systemic and muscular insulin sensitivity and elevated the proportion of slow-twitch oxidative muscle fibres. Moreover, we discovered significant enrichment of H3K9me3 at the IDH2 promoter region in the muscle tissue of offspring exposed to prepregnancy obesity, which could be originated from the increased H3K9me3 levels in the oocytes from HFD-fed dams. Our findings highlight the intergenerational epigenetic mechanisms underlying the influence of prepregnancy obesity on muscle metabolism in offspring. To the best of our knowledge, this is the first study to report the impact of prepregnancy obesity on muscle fibre type transition muscle metabolism in offspring.
Offspring of mothers with prepregnancy obesity face an increased risk of obesity and type 2 diabetes (T2D), yet the underlying mechanisms remain elusive. Skeletal muscle insulin resistance, along with impaired glucose uptake, is a key factor driving the development of T2D in obesity, with alterations in muscle fibre composition playing a crucial role in this process [13, 14, 26, 27]. Slow-twitch oxidative fibres are rich in mitochondria, providing superior oxidative capacity and utilisation of glucose and fatty acids. In contrast, fast-twitch glycolytic fibres rely more on glycolytic metabolism with lower oxidative capacity [15, 16, 28]. Thus, muscles predominantly composed of slow-twitch fibres exhibit greater insulin sensitivity. Patients with T2D or obesity exhibit a reduction in slow-twitch oxidative fibres and a higher proportion of fast-twitch glycolytic fibres [17, 18]. We found that, compared with mCD, the net weight of the TA was increased in the mHFD group, while the cross-sectional area of individual muscle fibres was significantly larger in both male and female offspring of the mHFD group. Similarly, we observed fibre type transition (slow-to-fast shift) along with impaired systemic and muscular insulin sensitivity in offspring exposed to maternal prepregnancy obesity. Skeletal muscle is a highly heterogeneous and dynamic tissue, capable of adjusting fibre type composition and size in response to metabolic and functional demands [29]. Therefore, we propose that muscle fibre type transition in this model primarily represents an adaptive response to metabolic stress. Our findings highlight the reprogramming effects of prepregnancy obesity on muscle metabolism and muscle fibre type transitions in offspring.
RNA sequencing revealed reduced IDH2 expression. IDH2, a critical mitochondrial oxidase, catalyses the oxidative decarboxylation of isocitrate to α-ketoglutarate, thereby generating NADPH [30]. It has been shown to play an essential role in regulating mitochondrial redox homeostasis and mitigating oxidative stress-induced damage [30, 31]. The distinctively high mitochondrial density observed in oxidative muscle fibres underscored the potential significance of IDH2 in the regulation of muscle fibre type transition. Recent studies have identified an increasing number of mitochondria-associated proteins as key regulators of myofiber composition, including peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α), AMP-activated protein kinase (AMPK) and sirtuin 6 (Sirt6) [32-36]. Among these, PGC-1α serves as a master regulator of mitochondrial biogenesis and plays a critical role in promoting the formation of oxidative muscle fibres [28, 37]. Furthermore, a decline in intracellular redox homeostasis and an increase in ROS reduce PGC1α protein stability [38]. In line with this, IDH2 knockdown led to a reduction in slow-twitch oxidative fibres, accompanied by mitochondrial abnormalities, including disrupted morphology, redox homeostasis imbalance, impaired biogenesis and suppressed expression of key mitochondrial regulators PGC1α and TFAM—indicating that IDH2 deficiency disrupts mitochondrial biogenesis via oxidative stress-mediated PGC-1α suppression. More importantly, overexpression of IDH2 in mHFD not only restored the proportion of slow-twitch oxidative fibres but also significantly improved their glucose tolerance and insulin sensitivity. These findings highlight the crucial role of the IDH2-PGC1α signalling pathway in regulating skeletal muscle metabolism in the offspring of prepregnancy obese mothers.
Studies have shown that T2D is highly heritable [39, 40][S1]. However, genetic variants explain only a small portion of its heritability, emphasising the potential significance of epigenetics, particularly in the context of parental nutritional status correlates with offspring metabolic traits [3]. The harmful effects of maternal obesity may begin as early as the oocyte [S2, S3]. This early influence can affect both oocyte quality and its ability to support embryonic development [S4]. Notably, histone modifications are not completely erased during gametogenesis and fertilisation, particularly in regions of silent chromatin, where methylated histones can be retained through cell division [S5-S7]. In this study, we detected enrichment of H3K9me3 at the IDH2 promoter in mHFD skeletal muscle, leading to a reduction in IDH2 expression. Consistently, we observed a significant increase in H3K9me3 levels in oocytes of obese dams, along with elevated expression of the histone methyltransferase SETDB2. These results suggest that increased H3K9me3 levels induced by SETDB2 in oocytes of obese dams may be transmitted to offspring, leading to reduced IDH2 expression, which further impairs mitochondrial function, reduces slow-twitch oxidative muscle fibre composition and contributes to systemic insulin resistance. Our findings underscore the significance of transgenerational epigenetic inheritance of metabolic diseases through histone modifications.
This study has several limitations. First, we did not assess skeletal muscle function in F2 and F3 offspring, making it difficult to determine whether this epigenetic marker can be transmitted across multiple generations. Second, although we demonstrated the role of IDH2-PGC1α signalling in prepregnancy obese skeletal muscle metabolism, other pathways may also be involved. Additionally, we did not analyse other metabolic organs, such as the liver, adipose tissue and pancreas, which may collectively influence offspring health.
In conclusion, our study reveals that increased H3K9me3 levels in oocytes of obese mothers could be intergenerationally transmitted to offspring, inhibiting the post-transcriptional expression of IDH2, which further induces mitochondrial dysfunction, muscle fibre type transition and ultimately leads to impaired systemic insulin sensitivity. This work provides new perspectives on the development of metabolic diseases and identifies a potential therapeutic target for skeletal muscle insulin resistance, emphasising the critical importance of prepregnancy weight management for offspring health.
Acknowledgements
We are grateful to Experimental Medicine Center of Tongji Hospital for flow cytometry analysis. We would like to thank Professor Ling Hou from Tongji Hospital for her insightful suggestions regarding the manuscript preparation.
Conflicts of Interest
The authors declare no conflicts of interest.




