Pirh2 modulates amyloid-β aggregation through the regulation of glucose-regulated protein 78 and chaperone-mediated signaling
Abstract
Amyloid-β (Aβ) protein aggregation in the brain is a pathological hallmark of Alzheimer's disease (AD) however, the underlying molecular mechanisms regulating amyloid aggregation are not well understood. Here, we studied the propitious role of E3 ubiquitin ligase Pirh2 in Aβ protein aggregation in view of its regulatory ligase activity in the ubiquitin-proteasome system employing both cellular and sporadic rodent models of AD. Pirh2 protein abundance was significantly increased during Streptozotocin (STZ) induced AD conditions, and transient silencing of Pirh2 significantly inhibited the Aβ aggregation and modified the dendrite morphology along with the substantial decrease in choline level in the differentiated neurons. MALDI-TOF/TOF, coimmunoprecipitation, and UbcH7-linked in vitro ubiquitylation analysis confirmed the high interaction of Pirh2 with chaperone GRP78. Furthermore, Pirh2 silencing inhibits the STZ induced altered level of endoplasmic reticulum stress and intracellular Ca2+ levels in neuronal N2a cells. Pirh2 silencing also inhibited the AD conditions related to the altered protein abundance of HSP90 and its co-chaperones which may collectively involve in the reduced burden of amyloid aggregates in neuronal cells. Pirh2 silencing further stabilized the nuclear translocation of phospho-Nrf2 and inhibited the altered level of autophagy factors. Taken together, our data indicated that Pirh2 is critically involved in STZ induced AD pathogenesis through its interaction with ER-chaperone GRP78, improves the neuronal connectivity, affects the altered level of chaperones, co-chaperones, & autophagic markers, and collectively inhibits the Aβ aggregation.
Abbreviations
-
- ABC
-
- ammonium bicarbonate
-
- ACN
-
- acetonitrile
-
- ACSF
-
- artificial cerebrospinal fluid
-
- AD
-
- Alzheimer's disease
-
- APP
-
- amyloid precursor protein
-
- CHIP
-
- C-terminus of HSC-70
-
- Co-IP
-
- coimmunoprecipitation
-
- CR
-
- cortex
-
- ER
-
- endoplasmic reticulum
-
- ERAD
-
- endoplasmic reticulum-associated degradation
-
- GRP78
-
- glucose-regulated protein 78
-
- HP
-
- hippocampus
-
- HSP70
-
- heat shock protein 70
-
- HSP90
-
- heat shock protein 90
-
- IgG
-
- immunoglobulin G
-
- MALDI-TOF
-
- matrix assisted laser desorption ionization-time of flight
-
- MS
-
- mass spectrometry
-
- N2a
-
- mouse neuroblastoma Neuro2a
-
- PIRH2
-
- p-53-induced Ring-H2 protein
-
- PQC
-
- protein quality control
-
- p-Tau
-
- phosphorylated tau
-
- RA
-
- retinoic acid
-
- Scrambled
-
- control siRNA
-
- STZ
-
- streptozotocin
-
- TFA
-
- trifluro acetic acid
-
- Th-S
-
- thioflavin-S
-
- UPR
-
- unfolded protein responses
-
- UPS
-
- ubiquitin proteasome system
1 INTRODUCTION
Presence of amyloid plaque is one of the major pathological hallmarks in Alzheimer's disease (AD) patients since decades, and today also this is one of the relevant diagnostic criteria. These plaques are primarily composed of amyloid-β (Aβ) formed through its precursor protein amyloid precursor protein (APP) involving β-secretase (Selkoe, 2001). In spite of its clinical relevance, we still do not know whether it is the causative or consequential agent of disease, and therefore, further investigations are required. In view of observed protein aggregation during diseased conditions, it could be hypothesized that protein quality control (PQC) must be impaired in AD conditions. In agreement, various reports are available and supporting this hypothesis (Chiti & Dobson, 2017; Ghemrawi & Khair, 2020; Liu et al., 2022) however, PQC itself is complex machinery and all the components must be explored to understand their role in AD pathology. If we look at sequential events, the endoplasmic reticulum (ER) stress is one of the upstream critical events in AD pathology, as reported by us and others (Ajoolabady et al., 2022; Biswas et al., 2018). It has been shown that the level of ER-specific chaperone GRP78 is significantly augmented in diseased conditions, suggesting the imperative role of ER stress in AD conditions (Biswas et al., 2018; Gorbatyuk & Gorbatyuk, 2013). Reciprocally ER stress could be further aggravate due to the accumulation of unfolded proteins in ER, primarily provoked by an imbalance in ER homeostasis and depleted proteasome activity during cell degeneration and differentiation (Ajoolabady et al., 2022; Friedlander et al., 2000). ER stress signaling pathways act through transmembrane kinases IRE1, PERK, & ATF6 and are collectively known as unfolded protein responses (Almanza et al., 2019). These transmembrane kinases and ATF6 remain associated with GRP78 in physiological conditions and induction of ER stress takes place after the dissociation of GRP78 from these ER membrane-located kinases and ATF6. GRP78-induced prolonged ER stress can further turn on the cell death pathway predominantly through the activation of CHOP/GADD153 and caspase12 (Oyadomari & Mori, 2003; Szegezdi et al., 2006). In fact, ER chaperone GRP78 has been shown to physically interact with APP (Yang et al., 1998). Moreover, the accumulation of GRP78 in senile plaques and the colocalization of ER chaperones with Aβ suggested its probable role in the modulation of Aβ aggregation (Wilhelmus et al., 2007). The previous study has shown that mitochondrial enzymes and ER-calcium stores significantly contribute in disease-related oxidative stress in neurodegeneration (Gibson & Huang, 2004). Such oxidative stress could be regulated by the Nrf2-keap1 system during physiological conditions however, the translocation of Nrf2 contributes to autophagy and apoptotic death of neurons (Ma, 2013; Singh et al., 2021). Findings have also suggested that Nrf2 knockout mice are more susceptible to Aβ-induced AD pathology and neuronal death accompanying oxidative damage (Chen et al., 2009).
Besides, the colocalization of amyloid plaques has been reported with professional chaperones (heat shock proteins [HSP]), which may be involved in conformational changes of Aβ and also in its clearance from the brain through phagocytosis or active transport across the blood-brain-barrier (Wilhelmus et al., 2007). These HSPs may also be involved in AD-related neurodegenerative mechanisms like inflammation and may serve as therapeutic interventions (Adachi et al., 2009; Lackie et al., 2017; Marino Gammazza et al., 2016). Imperative role of HSPs has been suggested in cellular protein turnover through regulating ER-associated degradation, the ubiquitin-proteasome system (UPS), and autophagy (Bozaykut et al., 2014). Among the numbers of HSPs, the HSP90, 70, 40, 100, and some of the small HSPs are suggested to be involved in protein degradation-related mechanisms (Bozaykut et al., 2014; Fernández-Fernández et al., 2017; Vos et al., 2011). In fact, these HSPs are considered as machines for protein folding, unfolding, and disaggregation, unlike enzyme functions (Saibil, 2013) and, therefore, may have implications in AD pathology. Particularly in the context of AD, the critical role of the HSP70 and HSP90 network has been suggested in underlying protein misfolding (Lackie et al., 2017; Rosenzweig et al., 2019). In AD, the association of deregulated chaperones and UPS has also been suggested (Sulistio & Heese, 2016). Recently the role of UPS has also been reported in underlying tau accumulation (Weng & He, 2021), suggesting the role of UPS in AD pathogenesis.
In UPS, the attachment of ubiquitin to substrate protein through E1, E2, and E3 enzymes is essentially required to carry the substrate protein to the proteasome core for degradation. During AD pathogenesis, the exploitation of ubiquitin signaling to understand the crosstalk of UPS-enzymes and toxic protein tau and Aβ is suggested to be as one of the approach for next-generation therapeutics (Gadhave et al., 2016; Harris et al., 2020; Schmidt et al., 2021). Along with ubiquitin attachment, the enzyme E3 ligases play a regulatory role in UPS functions and may have diagnostic or therapeutic implications in AD. In this regard, reports are available showing the role of E3 ubiquitin ligases in AD pathology (Potjewyd & Axtman, 2021). The presence of ubiquitin residue in tangles and Aβ also suggest the coherence of ubiquitin and related modifications on the disease-related signaling proteins which conjointly contribute in phosphorylation of tau, Aβ, and consequent neuronal apoptosis (Potjewyd & Axtman, 2021).
Ubiquitylation also controls various steps of autophagy. In fact, the ubiquitylation of the autophagy-regulatory component is essential to positively or negatively regulate autophagic flux in both selective and nonselective pathways (Grumati & Dikic, 2018). Ubiquitin signals are required for the selective incorporation of cargoes like proteins, organelles, and microbes into autophagosomes involving LC3 (Watanabe et al., 2020). During AD pathology, the association of UPS and autophagy has been reported (Zhang et al., 2017).
Our study is focused on elucidating the molecular pathways involved in AD targeting amyloid aggregation therefore, utilized the neurotoxin streptozotocin (STZ) induced experimental models of AD (Lannert et al., 1998; Singh et al., 2022). STZ is known for having broad spectrum effect on molecular pathways of AD involving ER stress, mitochondrial dysfunction, neuroinflammation, oxidative stress, nitrative stress, glucose metabolism, and biochemical alterations (Biswas et al., 2016, 2017, 2018; Gupta et al., 2021; Singh et al., 2022). The present study was conducted to investigate the mechanistic role of Pirh2 in the reported AD related neurodegenerative signaling along with its effects on neuronal connectivity employing both cellular and sporadic rodent model of AD. Also, previously we and others have reported the significant role of DNA damage in AD pathogenesis, and E3 ligase Pirh2 has a central role in the regulation of DNA damage responses (Halaby et al., 2013) therefore, it's worth investigating the role of Pirh2 in AD-related pathological mechanisms.
2 MATERIALS AND METHODS
2.1 Cell culture and treatment
The mouse neuroblastoma Neuro2a (N2a) was procured from the National Centre of Cell Science, and maintained at CDRI. Culture medium DMEM/F12 supplemented with 10% FBS was changed on an alternate day to assert the healthy stipulation of cells. All the experiments were done at cell passage range between 3 and 10. STZ treatment was given at a concentration of 1 mM for 48 h (Biswas et al., 2016). MG132 (a potent, cell-permeable proteasome inhibitor) treatment was given in N2a cells at 5 mM concentration preceding 6 h from respective experiment initiation to inhibit the endogenous proteasome activity. N2a cells were differentiated with retinoic acid (RA) 5 µM for 5 days followed by transfection (for 24 h) and STZ treatment for 48 h (Narasimhan et al., 2020).
2.2 Animals and stereotaxy
Male Sprague−Dawley rats weighing 200−220 g and aged 7−8 weeks were procured from the “National Laboratory Animals Centre” of CSIR-CDRI. The experiments were conducted in accordance to ARRIVE guidelines, following international ethical standard protocols after approval from the CSIR-CDRI animal research ethics committee (IAEC/2018/89). Animals were divided into two groups and kept in a polyacrylic cage with food and water ad libitum. Standard ambient conditions with 12 h light and dark cycle, room temperature 22 ± 1°C, and humidity 60%−65% were provided. The rat was anesthetized using a mixture of xylazine (10 mg/kg) and ketamine (80 mg/kg) and kept on the stereotaxic apparatus (Stoelting), measured the coordinates from the bregma (George & Watson, 1982) and STZ dissolved in ACSF 3 mg/kg was administered in rat brain through intracerebroventricular rout bilaterally to induce sporadic experimental rodent model of AD (Biswas et al., 2018; Lannert et al., 1998; Singh et al., 2022) Appropriate care for rats was taken to prevent mortality and after 21 days of STZ administration, the rats were anesthetized, followed by intracardiac perfusion with 0.9% saline and further decapitated to remove the brain. The cortex and HP (hippocampus) regions were isolated and processed for the different assays.
The list of chemicals and antibodies used for immunofluorescence and immunoblot analysis with their appropriate dilutions and the observed molecular weight is mentioned in Supporting Information: Table 1.
2.3 siRNA mediated knockdown
The predesigned siRNA constructs used for RNA interference were purchased from Invitrogen (Cat#: AM16708; Assay ID 102888) along with negative control (Cat#: 4390843). Transient silencing of Pirh2 was done using LipofectamineTM RNAiMAX transfection reagent (Catalog no. 13778075).
2.4 Western blot analysis
Following transient transfection with scrambled siRNA and siRNA-Pirh2 followed by STZ treatment, the cells were scrapped, collected in culture medium and centrifuged at 3000xg for 10 min at 4°C. The pellets were rinsed with PBS and lysed in Lysis buffer and centrifuged at 15,000xg for 20 min at 4°C. Similarly, cortex and HP brain region of SD rats were isolated and lysed in RIPA lysis buffer followed by centrifugation at 20,000xg for 30 min at 4°C. Western blot analysis was performed according to the published protocol (Singh et al., 2022). Membranes were incubated with respective antibodies, followed by visualization of signals with Chemi DOC XRS+ (biored). The mean intensity of bands was analyzed and normalized by β-actin using ImageJ software (NIH).
2.5 Quantification of neurite length and neurite-bearing cells
Briefly, randomly selected fields containing 50−100 cells per field were chosen and neurite-bearing cells were measured using Fiji (ImageJ; NCBI). The number of neurite outgrowths, defined as axon extensions, that were more than double from cell body were counted and recorded. The percentage of neurite-bearing cells is calculated by measuring the cell that contains neurite and the total number of cells.
2.6 Choline level
Choline level was estimated in scrambled and/or siRNA-Pirh2 transfected differentiated neuronal N2a cells with and without STZ treatment for 48 h using a commercially available kit (Biovision) following given instructions. Fluorescence intensity was measured using Fluorimeter (Varian Cary Eclipse) at excitation and emission of 535/587 nm wavelength, respectively.
2.7 Calcium level
Intracellular calcium level was estimated in scrambled and siRNA-Pirh2 transfected N2a cells treated with or without STZ according to protocol from (Biswas et al., 2016). The reaction mixture was incubated with 1 µM Fluo-3-AM dye for 1 h in the dark at 37°C. The fluorescence was detected using a FACS calibur at excitation and emission spectra of 506 and 530 nm, respectively.
2.8 Immunolabelling and immunofluorescence/confocal microscopy
Immunolabelling to assess the protein level and localization was done in cells based on published protocol (Tiwari et al., 2022). Images were captured using fluorescent microscopy (Nikon) using ×40 objective.
Confocal imaging was performed in N2a cells to visualize the fluorescence and colocalization of GRP78, Pirh2 with mitotracker red using a similar procedure of immunofluorescence and mounting with DAPI-containing mounting medium. The slides were observed under ×100 objective in confocal microscopy (Leica).
2.9 Immunohistochemistry
Immunolabelling of β-amyloid was done after deparaffinisation and dehydration of sections. Further the sections were permeabilized with PBS-0.01% Triton X-100 for 5 min and incubated in blocking buffer (5% BSA in PBS-Triton X-100) for 1 h, wash the sections with PBS for three times and incubated overnight at 4°C with β-amyloid antibody. Following day, the sections was rinsed with PBS-Triton X-100 thrice and incubated for 1 h in anti-rabbit secondary antibody followed by washing with PBS-Triton X-100 for 5 min each three times. The section was mounted with anti-fade DAPI containing mounting medium and images were captured by fluorescent microscope (Tiwari et al., 2022).
2.10 Coimmunoprecipitation (Co-IP)
After transfection and treatment cell and tissue lysate were lysed in lysis buffer and protein were estimated of each sample. Co-IP, 1−2 mg lysate from each sample was incubated overnight at 4°C with anti-Pirh2-protein-G-Sepharose bead complex. The nonspecific proteins were removed by washing with lysis buffer and bound protein complex were mixed with 2X Laemmli buffer and boiled for 5 min to denature the proteins. The proteins were resolved on 4%−20% gradient SDS-PAGE gel and immunoblotted with respective antibodies for visualizing the protein using chemiluminescence substrate and observed by Chem Doc XRS+ Biorad (Tiwari et al., 2022).
2.11 Thioflavin-S (Th-S) assay
The assay was performed to assess the amyloid aggregate formation (Tiwari et al., 2022). The cells were seeded on a poly-l-lysine coated coverslip, followed by differentiated with RA (5 µM) for 5 days, followed by silencing of the set of cells with siRNA-Pirh2 with and without STZ treatment. For staining, the cells were incubated with Th-S (0.05% prepared in ethanol) for 10 min at room temperature in the dark. Similarly, brain sections were deparaffinised in xylene and then dehydrated through the series of alcohol wash and kept in water for few seconds and incubated in 0.05% Th-S solution in 70% ethanol for 15 min at RT. The cells/sections were washed thrice with 70% ethanol then once rinse with PBS and mounted on the slides using an anti-fade medium with DAPI and visualized under a fluorescent microscope (Nikon Eclipse E200).
2.12 Mass spectrometry
The sample were resolved on SDS-PAGE and dipped in Coomassie blue (G-250) for 2 h followed by destaining (50:40:10: dH20: Methanol: Glacial acetic acid solution), after partial destaining the separated bands were sliced with blade and stored in microcentrifuge tubes, To remove the stain completely, the gel slices were immersed in 100 µL of 25 mm ammonium bicarbonate (ABC) followed by dehydrating the gels slices in 50 µL of solution (2:1 mixture of acetonitrile: 50 mM ABC) for 5 min at RT, this step was repeated till the gel slices became transparent. The gel slices were vortex for 5 min in 100% acetonitrile and gel slices were dehydrated in speed vacuum for 20 min. After that 150 ng trypsin was added in the gel slices and incubated for 45 min on ice followed by addition of 100 µL ABC solution in the tube for overnight incubation at 37°C. Following day, the supernatant was collected in new tube and gel was again dipped in 20−30 µL of 50% Acetonitrile (ACN) and 2% tri-fluro acetic acid (TFA) for 5 min followed by vortexing the tube and collecting the supernatant in the new tube. Finally, the supernatant was evaporated on a speed vac till completely dry. The lyophilized samples were resuspended in 30% ACN and 0.1%TFA (5 µL) and sample was vortex for 15 min. The 1 µL of sample mixed with matrix (α-cyano-4-hydroxycinnamic acid [α-CHCA], 1:1), spotted on the MALDI plate and analyzed by AB Sciex 4800 matrix-assisted laser desorption ionization-time of flight (MALDI-TOF)/TOF mass spectrometer (Tiwari et al., 2022).
2.13 Ubiquitylation assay
In vitro ubiquitylation of GRP78, first Co-IP was performed using overnight incubation of cell and tissue lysates and anti-Pirh2 antibody-protein-G beads at 4°C. The immunocomplexes were rinsed four to five times with lysis buffer and added to the mixture, comprising of reaction components provided in the in vitro ubiquitylation assay kit from Enzo life sciences. The final mixture was incubated for 1 h at 37°C and the reaction was terminated by adding 2X nonreducing Laemmli buffer provided in assay kit (Tiwari et al., 2022). The protein was resolved on 10% SDS-PAGE, immunoblotted with GRP78 antibodies followed by their visualization using chemiluminescence to confirm ubiquitylation of GRP78.
2.14 Statistical analysis
Data were analyzed from at least three independent experiments represented as n = 3 using student's unpaired Students t-tests, one-way or two-way analysis of variance (ANOVA) and the difference between control and treated sets was analyzed by post hoc Dunnett's multiple comparison test or Tukey's multiple comparison test. Values are expressed as the mean ± SEM and the p-values less than 0.05 was considered statistically significant.
3 RESULT
3.1 Transient silencing of Pirh2 attenuates the AD-related loss in dendrite length, neuronal morphology, and amyloid β aggregation in differentiated neuronal N2a cell lines
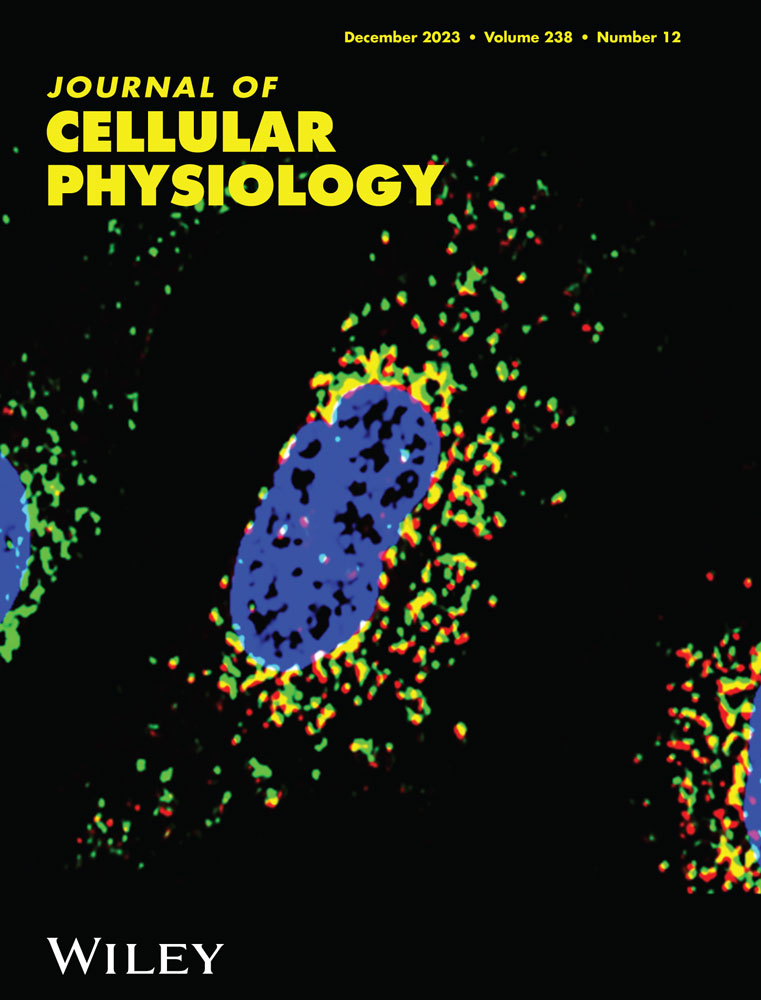
The investigation was done to assess the effect of Pirh2 on neuronal synaptic connections. The neuronal N2a cells were differentiated with RA, and the effect of Pirh2 on neurite length was assessed. Neurite outgrowths were seen on Day 1 and maturation of neurons/axons began on Day 3 in RA-treated N2a cells. Fully grown neurons with axonal projections, dendrites, and synaptic clefts were apparent on Day 5 (Figure 1a). The quantitative analysis showing the neurite bearing cells at different days and analyzed by one-way ANOVA followed by Dunnett's post hoc test (Figure 1b). Further, transient transfection of Pirh2 for 24 h was performed (Figure 1c), followed by STZ treatment for 48 h (Day 8) (Figure 1d). The morphometric and morphologic analyses showed less number of cells bearing neurite outgrowth and reduced neurite length in STZ treated wild type cells in comparison to control wild type cells. While the Pirh2 silenced, cells did not show STZ-induced decreased neurite outgrowth and reduced neurite length (Figure 1d,e). Further, the differentiated neurons were immunolabelled for MAP2 and Tuj1. The observed immunolabelling suggested that STZ treatment causes decreased labeling of both proteins, which may be due to STZ-induced neurotoxicity and decreased neuronal viability (Biswas et al., 2016) which will interfere in the differentiation process and thus inhibit the immunolabelling of MAP2 and Tuj1. Whereas the Pirh2 silenced differentiated neurons showed a comparable less decrease in labeling of both MAP2 and Tuj1 in comparison to wild-type-STZ treated cells (Figure 1f). This observed finding is in concordance to data of cell viability (MTT) (Supporting Information: Figure 1).
Further the level of choline, a precursor for acetylcholine neurotransmitter, was estimated. Both total and free choline was measured in wild type and Pirh2 silenced cells with and without STZ treatment. Interestingly, we found that in siRNA-Pirh2 transfected cells choline content was more in comparison to wild type STZ treated cells (Figure 1g,h). This observation suggests that the Pirh2 level contributes in the synaptic dysfunction in AD pathology.
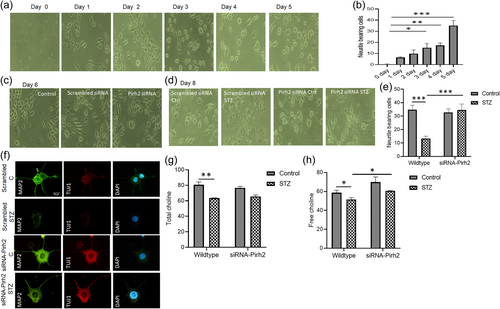
In the undifferentiated N2a cells, we have observed the particular effect of Pirh2 on AD-specific pathological markers like protein level of p-tau and Aβ (Supporting Information: Figure 2a,c,d) and neuronal death marker cleaved caspase 3 (Supporting Information: Figure 2a,e). Additionally, we also study the RA-induced differentiated neurons and assess the effect of Pirh2 on neuronal connectivity and its effect on Aβ aggregation via involvement of chaperones. A significantly decreased level of Aβ, APP, and cleaved caspase 3 was observed in siRNA-Pirh2 transfected cells in comparison to scrambled-siRNA transfected cells with STZ treatment (Figure 2a). Moreover, we checked the Aβ aggregation in STZ induced AD model through immunolabelling of β-amyloid and Th-S staining in rat brain section. We observed significant increase in immunolabelling of β-amyloid and Aβ aggregation in STZ administered rat brain sections in comparison to control rat brain section (Supporting Information: Figure 3a,b). In parallel, we also confirmed aggregation of Aβ through immunofluorescence for Aβ and Th-S staining in both wild type and siRNA-Pirh2 transfected differentiated N2a cells with or without STZ treatment (Figure 2b). In Pirh2 silenced neurons Aβ aggregation was less in comparison to wild type STZ treated neurons, suggesting that Pirh2 might play a role in the processing of APP during STZ-induced Alzheimer's disease (AD) model (Figure 2b−d). Findings suggested that Pirh2 interferes in AD conditions specific synaptic dysfunction, Aβ aggregation, and choline level.
3.2 Pirh2 interacts and ubiquitinates ER chaperone GRP78
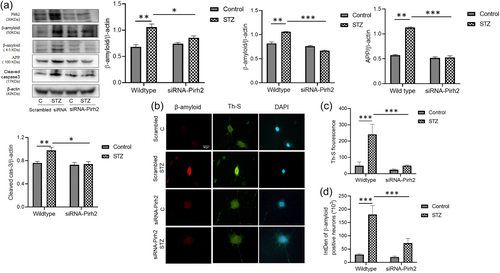
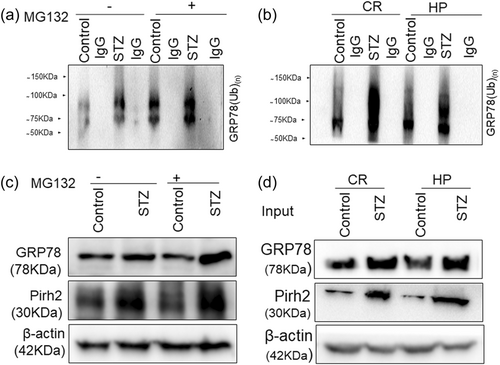
Here we intended to investigate the mechanistic role of Pirh2 in AD pathology. We first screened the interacting partners of Pirh2 by MALDI-TOF. The experiment was done in control and STZ-treated undifferentiated N2A cells and STZ-induced AD model in the cortex and HP rats brain region, and Co-IP was performed using G-Sepharose beads bound to anti-Pirh2 antibody to check for its interaction with these cellular/tissue homogenates and the sample was processed for MS to get a broad idea for interacting protein. The obtained hits that interacted with Pirh2 were sorted based on physiological functions. We have shown the data of STZ-treated N2a cells in the pie chart (Figure 3a). Interestingly in the series of hits obtained from mass spectrometry data, GRP78 has more score (241) in comparison to other hits. To verify the MS data, further, Co-IP was performed with Pirh2 monoclonal antibodies and IgG antibodies, and immune complexes were analyzed by western blot analysis for GRP78 along with IgG as negative control. The observation was in agreement with mass data and showed the interaction of Pirh2 with GRP78 in both control and STZ induced AD model. In view of this observation and previous finding of the augmented level of GRP78 during AD pathology and being a key chaperone to regulate the ER stress signaling and proteostasis disease (Singh et al., 2022) further study was focused on evaluating the coherence of Pirh2 and GRP78 in AD pathology. In STZ treated cells and cortex and HP rat brain region the interaction was considerably more in comparison to control conditions (Figure 3b,c) which may be due to the augmented level of cytosolic GRP78 in STZ treated conditions in both cellular and rodent models (Figure 3b,c). Moreover, we have also performed Co-IP in siRNA-Pirh2 transfected N2a cells with or without STZ treatment. Interestingly, we observed that interaction between Pirh2 and GRP78 was lesser in siRNA-Pirh2 cells compared to scrambled-siRNA-treated sets (Figure 3d). The finding suggested the participation of Pirh2 in AD pathology through the involvement of GRP78. Both approaches confirmed the high interaction of Pirh2 with ER chaperone GRP78 in STZ induced AD model.
Pirh2 is an E3 ligase and it attached ubiquitin to substrate protein therefore, we further intended to check its involvement in the ubiquitylation of GRP78 utilizing in vitro ubiquitylation assay. Using various E2 enzymes in the assay, we obtained more profound ubiquitylation of GRP78 with E2 UbcH7 enzyme (Figure 4a,b). Incoherence to immunoprecipitation results, the intensity of ubiquitylation was higher in the STZ-treated group compared to the respective control sets (Figure 4a,b). Protein abundance of GRP78 and Pirh2 with or without STZ treatment in cells and cortex and HP rat brain region was shown as input for ubiquitination assay (Figure 4c,d).
3.3 Silencing of Pirh2 reduces ER stress caused by STZ in N2a cells
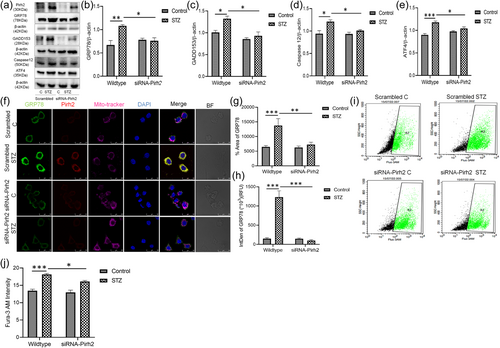
Under pathological conditions, GRP78 dissociates from the ER membrane located kinases IRE1, PERK and ATF6 and therefore the cytosolic GRP78 level increased in STZ induced AD conditions as reported by us previously (Biswas et al., 2017). To elucidate the particular effect of Pirh2 on GRP78 in STZ induced AD conditions, the cells were silenced for Pirh2 through siRNA and treatment of STZ was given to induce the diseased conditions. The assessment for protein level of ER stress markers was done in both scrambled and siRNA-Pirh2 transfected cells. Data showed that STZ treatment caused the augmented level of ER stress markers (GRP78, GADD153, Caspase12, and ATF4) in neuronal cells in comparison to control cells which was significantly inhibited in siRNA-Pirh2 transfected N2a cells (Figure 5a−e). Immunofluorescence images and analysis suggested the increased immunolabelling of GRP78 in STZ treated N2a cells compare to control cells. However, the immunolabelling was less in siRNA-Pirh2 transfected N2a cells with or without STZ treatment (Figure 5f−h). Further, the intracellular calcium level was measured and previously we have reported that altered calcium level disturb the homeostatic of ER in STZ induced AD model therefore we estimated its level in both scrambled and siRNA-Pirh2 transfected neuronal N2a cells. Data observed showed the significantly increased level of intracellular calcium in STZ treated N2a cells which was inhibited in siRNA-Pirh2 transfected N2a cells with or without STZ treatment (Figure 5i,j), suggesting the participation of Pirh2 in ER stress-induced neuronal death.
3.4 Silencing of Pirh2 regulates the level of HSPs and co-chaperones and reduces the Aβ aggregation
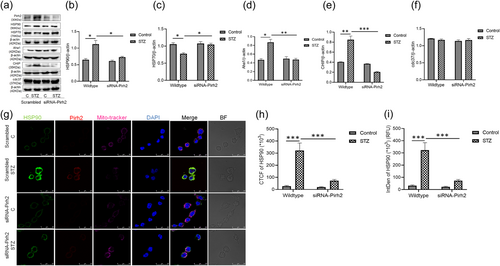
A significantly increased level of chaperone HSP90 was observed in STZ treated N2a cells, which was decreased in Pirh2 silenced N2a cells with STZ treatment (Figure 6a,b). Immunolabelling, corrected total cell fluorescence and integrated density of Hsp90 in neuronal N2a cells suggested that HSP90 immunofluorescence was more in scrambled STZ treated cell, while in siRNA-Pirh2 transfected with STZ treatment, the immunofluorescence was significantly less, advocating that Pirh2 is inducing the increased level of HSP90 in STZ treated N2a cells (Figure 6g−i). It has been reported that chaperone HSP90 forms macromolecular complexes with co-chaperones and regulate the Aβ processing (Blair et al., 2014). The co-chaperones involved in such macromolecules are Aha1, cdc37, and CHIP. It has also been reported that HSP90 inhibitors could redirect protein aggregation and protect the neurons through the activation of HSP70 in AD conditions (Ou et al., 2014). In agreement, the investigation was further proceeded to assess the effect of Pirh2 on these co-chaperones. Observation suggested that the protein level of HSP70, Aha1, and CHIP was significantly altered in STZ induced AD conditions. It has been reported that HSP70 has neuroprotective effects through the destabilization of misfolded proteins, promoting protein refolding, and preventing protein aggregation (Wentink et al., 2020). In agreement, we have also found the decreased level of HSP70 in STZ treated cells which were significantly inhibited in siRNA-Pirh2 transfected cells treated with STZ (Figure 6a,c). Further, the level of co-chaperones Aha1 and CHIP was significantly upregulated in AD conditions and inhibited in siRNA-Pirh2 transfected neuronal cells (Figure 6a,d,e). However, the protein level of cdc37 was unaltered with or without STZ treatment in N2a cells (Figure 6a,f). In total, findings suggested that Pirh2 interacts with HSP90 and regulate the protein level of HSP90, HSP70 and co-chaperones Aha1 and CHIP, thus collectively contributing to suppress the Aβ aggregation.
3.5 Pirh2 alters the autophagy markers
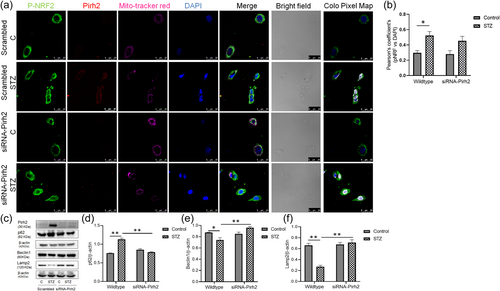
In view of observed oxidative stress in AD conditions (Biswas et al., 2018; Chen & Zhong, 2014) and the critical role of Nrf2 in age-related oxidative stress (Hybertson et al., 2011; Zhang et al., 2015), the effect of Pirh2 on nuclear translocation of Nrf2 was investigated. Immunofluorescence images showed the significant nuclear translocation of Nrf2 in STZ treated cells, and in siRNA-Pirh2 transfected cells with STZ treatment, the Nrf2 translocation was not inhibited, may be due to participation of Nrf2 in activation of neuroprotective gene (Figure 7a). The Pearson's coefficients analysis of pNRF2 and DAPI also advocating that in STZ treated N2a cells significant translocation of pNRF2 was observed, however, translocation was not inhibited in siRNA-Pirh2 transfected N2a cell with STZ treatment (Figure 7b). In physiological conditions, the keap1 remains bound to Nrf2 and keeps the Nrf2 in inactive conditions while in pathological conditions this complex gets activated by free radicals and Nrf2 enters the nucleus (He & Sun, 2021). Report has suggested the crosstalk between Nrf2 pathway and autophagy and could be considered to search for the novel target for treating AD patients (Zhang et al., 2021). In addition, it has been reported that autophagy marker p62 stabilizes Nrf2 through competitive binding with keap1 (Komatsu et al., 2010) therefore, we further measured the level of p62 in STZ treated N2a cells and assess the effect of Pirh2 on p62 level. Data suggested that the p62 level was significantly augmented in STZ treated N2a cells, and in siRNA-Pirh2 transfected cells with or without STZ treatment, p62 protein abundance was significantly decrease (Figure 7c,d). In addition, the autophagy protein beclin1 and Lamp2 were also decreased in STZ treated N2a cells, which were inhibited in Pirh2 transfected cells (Figure 7c,e,f). Observations suggested that Pirh2 significantly affects the STZ induced altered protein level of Nrf2, p62, beclin1, and Lamp2 in neuronal N2a cells.
4 DISCUSSION
Despite the crucial role of protein aggregation in AD pathology and the regulatory role of E3 ubiquitin ligases in protein degradation mechanisms like UPS, the information on the role of E3 ligases in AD conditions is limited. Previously we have reported DNA damage in AD conditions (Biswas et al., 2017), and in other study it (Krishna-K et al., 2020) has also been suggested that balancing of protein can be done by regulating UPS components during AD conditions, and their utilization may be worthwhile for development of therapeutic interventions.
In view of the considerable contribution of DNA damage and inhibited proteasome activity in AD pathology (Biswas et al., 2017; Tseng et al., 2009) and the central role of Pirh2 in the regulation of DNA damage, the present study was conducted to investigate the role of ubiquitin E3 ligase Pirh2 in amyloid beta aggregation employing STZ induced experimental models of AD. The effect of Pirh2 was assessed on RA—induced differentiated neurons to understand the effect of Pirh2 on neuronal connectivity through the synapse. Differentiation was confirmed through immunolabelling of MAP2 and Tuj1. STZ-induced diseased conditions exhibited a less number and length of dendrites in differentiated neurons compared to wild-type control differentiated neuronal cells. Moreover, choline, a precursor of acetylcholine was also measured in both wild-type, and Pirh2 silenced neurons. Data suggested that Pirh2 silencing showed a comparatively slighter decrease in choline level in diseased conditions. This observation suggested the role of Pirh2 in modulating neuronal connections and choline level in AD conditions. Our findings in proliferative cells also showed the increased protein abundance of Pirh2 in STZ-induced cellular models and suggested that silencing of Pirh2 offered protection against AD-specific disease markers like, level of tau/p-tau and level of Aβ and data obtained from the proliferative cells are in concordance to findings obtained in differentiated neurons, suggesting that Pirh2 has significant implications in neuronal connectivity during STZ induced diseased conditions. The protein abundance of β-amyloid and APP was significantly decreased in Pirh2 transfected N2a cells with STZ treatment compared to scrambled transfected cells in differentiated neurons. The Th-S staining also showed the significant inhibition of Aβ aggregation in siRNA-Pirh2 silenced neuronal cells along with a decreased level of cleaved caspase-3 suggesting the contribution of Pirh2 in reduced Aβ aggregation and neuronal death in STZ treated neuronal cells. The interaction of Pirh2 has been reported with various proteins of RNA metabolism (Daks et al., 2021) particularly with protein ELAVL1 which is involved in regulation of protein splicing and stability. It has also been reported that Pirh2 ubiquitylates the ELAVL1 and facilitates the proteasome-mediated protein degradation. Observed findings suggested that Pirh2 may be involved in the processing of APP through a further detailed investigation is required to make conclusive statement.
It has been reported that E3 ubiquitin ligases are closely associated to ER stress (Ajoolabady et al., 2022; Qu et al., 2021) which significantly involved in AD pathology (Biswas et al., 2017), therefore, further investigation was done to find out whether Pirh2 has implications in ER stress pathway or not with a focus on chaperones as these are critical in protein folding and proteostasis (Hartl et al., 2011; Kim et al., 2013). Mass spectrometric and Co-IP data suggested that Pirh2 significantly interacts with chaperones, particularly with the master chaperone of ER GRP78 and various HSPs. The study further proceeded with a focus on GRP78, being a master regulator of ER functions (Pobre et al., 2019) and also interact with APP and modulates the intracellular APP maturation and processing (Yang et al., 1998). In physiological conditions, GRP78 remain bound to transmembrane kinases and ATF6 to keep them inactive however, pathological conditions cause it's dissociation and further induction of ER stress signaling pathways (Shen et al., 2002). In concordance, we have observed the increased cytosolic GRP78 in STZ treated N2a cells, which was significantly inhibited in siRNA-Pirh2 transfected cells with STZ treatment suggesting the role of Pirh2 in the regulation of ER stress-induced neurodegenerative signaling. Colocalization data also suggested the interaction of both Pirh2 and GRP78. Further considering the E3 ligase activity of Pirh2 (Halaby et al., 2013), it's role in the ubiquitylation of GRP78 was estimated. Findings suggested that Pirh2 contributes in the ubiquitylation of GRP78 and this might be a cellular defense mechanism to act, as the level of both GRP78 and Pirh2 were increased in diseased conditions. However, Pirh2 is not the only ubiquitin ligase in a cell therefore the ubiquitylation of GRP78 must not solely dependent on Pirh2 and further studies can be done with a focus on other ligases. Nonetheless, findings of decreased Aβ aggregation in siRNA-Pirh2 transfected cells suggested the imperative role of Pirh2 in the PQC system during AD conditions. Further, the augmented intracellular calcium level was observed in STZ treated cells, which was significantly inhibited in siRNA-Pirh2 transfected cells treated with STZ, and data further support the impairment in ER functions. Along with ER-chaperone GRP78, the role of HSP70, HSP90, and its co-chaperones has also been investigated. In agreement to the protective functions of HSP70 (Repalli & Meruelo, 2015), its decreased level was observed in diseased conditions, which was attenuated in siRNA-Pirh2 transfected neuronal cells. In comparison, the level of HSP90 and its co-chaperones was significantly augmented in STZ treated N2a cells, which was reverted in siRNA-Pirh2 transfected neuronal cells. Together these findings suggested the chaperone-dependent facilitation of Pirh2 in regulating the PQC system and further study may be done to decipher its particular contribution.
It has been reported that appropriate calcium signals from ER facilitate mitochondrion-mediated essential functions like metabolism, energy production and apoptosis (Jin et al., 2021; Marchi et al., 2018). The observed augmented calcium level suggested the probable impairment in mitochondrial functions and observation is in agreement to previous findings that showed the significant role of oxidative stress in AD conditions (Smith et al., 2000). Downstream, such oxidative stress may cause the translocation of Nrf2 therefore, further the role of Nrf2 was estimated. Significant nuclear translocation of phosphorylated Nrf2 was found in STZ treated N2a cells, and in siRNA-Pirh2 transfected neuronal cells, Nrf2 remain in the nucleus to induce cytoprotective gene and suggesting the implication of Pirh2 in the regulation of oxidative stress-induced neurodegenerative pathways in AD pathogenesis. The oxidative stress may also execute the autophagic responses (Filomeni et al., 2015; Gao, 2019), and particularly the role of p62 has been reported to act as a link between autophagy and Nrf2 signaling (Bartolini et al., 2018). Therefore, further the level of p62 and autophagic markers Beclin1 and Lamp2 was estimated. Findings suggested that STZ treated N2a cells exhibited the significant increase in level of p62, which were inhibited in siRNA-Pirh2 transfected cells suggesting the participation of Pirh2 in Nrf2:p62 mediated signaling. Further, the diseased conditions specific depleted levels of Beclin1 and Lamp2 were reverted in siRNA-Pirh2 transfected cells intimating the participation of Pirh2 in autophagic responses during AD conditions. Since Pirh2 has considerable interaction with GRP78 and regulates the level of other HSPs and co-chaperones, it may have implications in chaperone-mediated autophagy. In conclusion (graphical abstract), here we demonstrated the crucial role of Pirh2 in the maintenance of neuronal connections and protein processing during STZ induced AD conditions involving its regulatory role in chaperone levels, calcium levels, apoptosis, and autophagic responses.
AUTHOR CONTRIBUTIONS
Abhishek Singh conceptualized, designed & performed the experiments and contributed in preparation of the draft manuscript. Shubhangini Tiwari performed the experiments. Sarika Singh conceptualized, designed and supervised the study as well as reviewed and edited the final manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
We thank Dr Ramesh Sharma, Ms Anupma Saxena, Ms Rima Ray Sarkar, Mr Toofan Rout, and SAIF facility (CSIR-CDRI) for technical assistance during experiments. We further thank Dr Sharad Sharma for auditing our data and providing critical reviews. Web-based tool Canva was used for Pie-chart illustration. Web-server Biorender was used for graphical illustration. We also acknowledge AcSIR and Department of Biotechnology (DBT), India for providing research opportunity and one-time research grant, respectively. The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of the article: Indian Council of Medical Research (2016-0264/CMB/ADHOC-BMS) and Science and Engineering Research Board (SPG/2021/004545) for providing financial support (SPG/2021/004545).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Animals were used after approval by the Institutional animal ethics committee of CSIR-Central Drug Research Institute (IAEC/2018/F-52).