CircANKRD17 promotes glycolysis by inhibiting miR-143 in breast cancer cells
Hong Chen and Ling-Fei Zhang contributed equally to this work.
Abstract
Glucose metabolic reprogramming, known as the Warburg effect, is one of the metabolic hallmarks of tumor cells. Cancer cells preferentially metabolize glucose by glycolysis rather than mitochondrial oxidative phosphorylation regardless of oxygen availability, but the regulatory mechanism underlying this switch has been incompletely understood. Here, we report that the circular RNA circ ankyrin repeat domain 17 (ANKRD17) functions as a key regulator for glycolysis to promote cell growth, migration, invasion, and cell-cycle progression in breast cancer (BC) cells. We further show that circANKRD17 acts to accelerate glycolysis in BC cells by acting as a sponge for miR-143 and in turn overrides the repressive effect of miR-143, a well-documented glycolytic repressor, on hexokinase 2 in BC cells, thus resulting in enhanced glycolysis in BC cells. These data suggest the circANKRD17-miR-143 cascade as a novel mechanism in controlling glucose metabolic reprogramming in BC cells and suggest circANKRD17 as a promising therapeutic target to interrupt cancerous glycolysis.
1 INTRODUCTION
Breast cancer (BC) is the most common malignancy in women and a leading cause of cancer-related deaths across the world (Siegel et al., 2017). Even though some studies have led to a better understanding of BC, such as microRNAs (miRNAs) which have been linked to the occurrence and development of BC (Tang et al., 2014). The early diagnosis and treatment of BC is still a significant challenge due to the lack of understanding of its complex pathogenesis. Therefore, exploring more effective therapeutic strategies based on the molecular mechanism is required.
Metabolic reprogramming is a hallmark of cancer (Hanahan & Weinberg, 2011; Pavlova et al., 2022), referring to the prioritization of aerobic glycolysis in tumor cells. This metabolic pattern which differs from mitochondrial oxidative phosphorylation in normal tissue cells is known as the Warburg effect (Hanahan & Weinberg, 2011). Currently, 18F-fluoro-2-deoxy-d-glucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) is the best technique for assessing the glucose metabolism of tumor cells in vivo. The standardized uptake value (SUV) acquired from 18F-FDG PET/CT was considered to indicate tumor aggressiveness (Berghmans et al., 2008; Guo et al., 2021). Hexokinase 2 (HK2), a primary isoenzyme that contributes to aerobic glycolysis, is a crucial player in the Warburg effect (Mathupala et al., 2009). Our group and others have demonstrated in previous works that miR-143 is a potent glycolysis inhibitor and a promising treatment target for BC (Avalle et al., 2017; Jiang et al., 2012; Miao et al., 2016; Wang et al., 2017) and that miR-143 exerts its function by repressing the protein level of HK2 (Jiang et al., 2012; Miao et al., 2016).
Circular RNAs (circRNAs) are endogenous noncoding RNAs with covalent closed-loop structures (Sanger et al., 1976). CircRNAs have been implicated in several critical steps during tumor progression (Jiang et al., 2022; Li et al., 2022). CircRNAs regulate tumor cells through various biological processes, including serving as miRNA sponges, regulating gene transcription and translation, and binding to RNA-binding proteins (Gao et al., 2021; Li et al., 2018; Wang et al., 2018; Wong et al., 2020). CircRNA can act as competing endogenous RNA (ceRNA) to repress miRNA activity and thus upregulate the expression of miRNA-related target genes (Hansen et al., 2013; Yu et al., 2019; Zeng et al., 2021). Argonaute 2 (Ago2) can bind to circRNAs and miRNAs, mediating the ceRNA mechanism (Hansen et al., 2013). Ago2 acted as a vital component of miRNA-mediated silencing complexes (Chen et al., 2019). The ceRNA hypothesis is important for understanding posttranscriptional regulatory networks.
In this article, we validated that a specific circRNA, circ ankyrin repeat domain 17 (ANKRD17), acts as a critical glycolysis regulator by interacting with miR-143, and in turn, overrides the repressive effect of miR-143 on HK2 in BC cells. Metabolic assays showed that circANKRD17 controlled glycolysis by accelerating 18F-FDG uptake in BC cells and xenograft tumors. These results suggest the circANKRD17-miR-143 cascade as a novel mechanism in controlling glucose metabolic reprogramming in BC cells and circANKRD17 as a promising therapeutic target to interrupt cancerous glycolysis.
2 MATERIALS AND METHODS
Detailed materials and methods are provided in the Supporting Information.
3 RESULTS
3.1 Prediction of circRNAs with miR-143 binding sites
The ceRNA mechanism hypothesis reveals that circRNAs could regulate miRNAs through an interaction mechanism between them (Chen, 2020). We first aimed to screen circRNAs potentially regulating miR-143 by following the process shown in Figure 1a. According to the screening strategies in the method, we identified a total of 50 circRNAs that contained the miR-143 binding site. The endogenous levels of these circRNAs were evaluated by quantitative real-time polymerase chain reaction (qRT-PCR) with specific primers and verified by Sanger sequencing in SK-BR-3 cells. Based on the screening criteria in Figure 1b, 19 circRNAs were excluded due to the low-level endogenous expression. We obtained 31 higher abundant circRNAs whose head-to-tail splicing sites were confirmed by Sanger sequencing. Next, we performed the biotin miR-143 pulldown assay. Enrichment of circRNAs by biotinylated miR-143 probe immunoprecipitation was measured by qRT-PCR. A total of 27 circRNAs were excluded as Ct value > 28, four circRNAs were included as high-abundant circRNAs (Ct value ≤ 28). Two of these circRNAs were enriched by the miR-143 probe (the fold change of miR-143 capture/NC capture ≥ 2.5, Figure 1c). We observed an increase in the expression level of miR-143 and a decrease in HK2 with hsa_circ_0001417 knockdown in SK-BR-3 cells, respectively (Supporting Information: Figure S1A–B). Additionally, hsa_circ_0007883 did not act as a sponge for miR-143 in SK-BR-3 cells (Supporting Information: Figure S1C–D). Finally, circular ANKRD17 (hsa_circ_0001417, termed as circANKRD17) was identified as a potential sponge for miR-143.
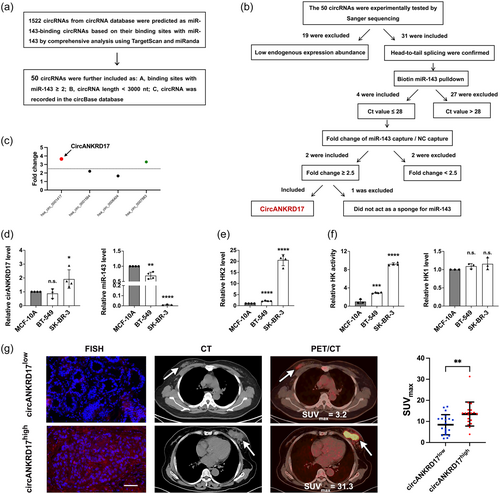
In BC cell lines, circANKRD17 was significantly upregulated in the SK-BR-3 cells compared with the human breast epithelial cell line MCF-10A (Figure 1d, left), and there was an inverse correlation between miR-143 and circANKRD17 levels (Figure 1d, right) in these cells. In addition, the mRNA level of HK2 was upregulated in BC cells with circANKRD17 highly expressing compared with that of MCF-10A cells (Figure 1e).
We performed the in vitro HK activity assay in BC cells. As shown in Figure 1f (left), the HK activity was dramatically upregulated in BC cell lines compared with that in MCF-10A cells. The highest HK activity was observed in SK-BR-3 cells with highest levels of both endogenous HK2 and circANKRD17. As HK1 is ubiquitously expressed in many tissues (Roberts et al., 2014), we determined whether HK1 also acts as a key contributor to the uncontrolled glycolysis of BC cells. Our results showed that the mRNA level of HK1 did not significantly elevate in BC cells compared with normal breast epithelial cell line MCF-10A (Figure 1f, right). These results implied that high HK activity was maintained mainly by the increase of HK2 levels in BC cells.
A fluorescent in situ hybridization (FISH) experiment was performed on BC tissues to detect the expression level of circANKRD17. As shown in Figure 1g, 18F-FDG PET/CT imaging showed that the SUVmax in the circANKRD17high patients were significantly higher than that in circANKRD17low patients. Collectively, these results suggested that the upregulated circANKRD17 could block the function of miR-143 through the sponge mechanism in BC.
3.2 The circularity and cytoplasmic localization of circANKRD17
The structure of circANKRD17 was derived from exons 29 to 30 of ANKRD17 with a length of 1832 nt. The back-spliced junction of circANKRD17 was amplified and confirmed by Sanger sequencing by using divergent primers (Figure 2a). The half-life of circANKRD17 transcript was more stable than that of linear ANKRD17 mRNA in SK-BR-3 cells treated with actinomycin D (Figure 2b).
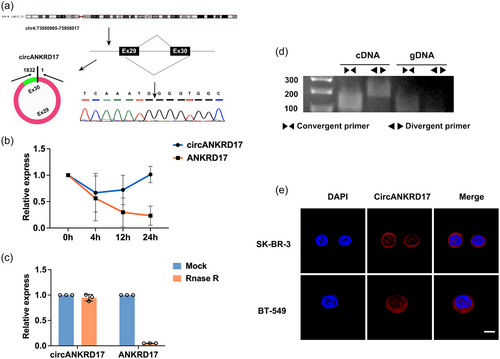
Furthermore, circANKRD17, rather than linear ANKRD17 mRNA, was resistant to RNase R digestion (Figure 2c). PCR analysis demonstrated that both divergent primers for circANKRD17 and convergent primers for linear ANKRD17 amplified products of expected sizes from cDNA isolated from SK-BR-3 cells. In contrast, only linear ANKRD17 mRNA-specific convergent primers conferred an amplification from gDNA (Figure 2d), suggesting the circularity of circANKRD17. FISH assay showed that circANKRD17 was preferentially localized in the cytoplasm of BC cells (Figure 2e).
3.3 CircANKRD17 acts as a sponge for miR-143
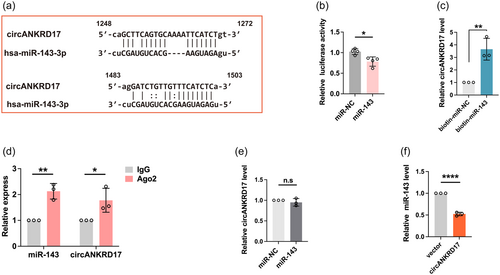
Given that circANKRD17 harbors two binding sites of miR-143 (Figure 3a), reporter assay was performed to verify the interaction between them by using a luciferase reporter with circANKRD17 sequence cloned downstream of the firefly luciferase gene. As shown in Figure 3b, the luciferase activity was reduced in miR-143 mimics-transfected cells compared with miR-NC group. Moreover, miRNA pulldown assay demonstrated that circANKRD17 could be enriched by biotin-labeled miR-143 mimics, but not by miR-NC (Figure 3c). Given that the Ago2 binding to both miRNAs and circRNAs as a common phenomenon based on the ceRNA hypothesis, we then investigate whether circANKRD17-miR-143 complex could bind to Ago2 protein. By performing RNA immunoprecipitation (RIP) analysis, we showed that compared with the IgG control, circANKRD17 and miR-143 were enriched in Ago2 precipitate (Figure 3d). We observed that transfection of miR-143 mimics did not change circANKRD17 expression (Figure 3e). We overexpressed circANKRD17 in BT-549 cells and observed that the expression level of miR-143 was downregulated (Figure 3f). Collectively, these results indicated that the interaction between circANKRD17 and miR-143 through ceRNA competition posttranscriptionally contributes to the high level of HK2 in BC cells.
3.4 CircANKRD17 promotes glycolysis in BC cells
We selected SK-BR-3 cells for loss-of-function and BT-549 cells for gain-of-function analyses for circANKRD17 expression being highest in SK-BR-3 cells and lowest in BT-549 cells (Figure 1d). SK-BR-3 cells were transfected with a siRNA specifically targeting the back-splicing junction sequence of circANKRD17 without altering the expression of ANKRD17 mRNA (Supporting Information: Figure S2A, S2B); Similarly, overexpression of circANKRD17 in BT-549 cells did not affect endogenous ANKRD17 mRNA expression (Supporting Information: Figure S2C, S2D).
The expression level of miR-143 was increased in SK-BR-3 cells with circANKRD17 knockdown (Supporting Information: Figure S1A); in contrast, miR-143 was downregulated in BT-549 cells overexpressed by circANKRD17 (Figure 3f). Along with that miR-143 directly represses HK2 (Jiang et al., 2012; Miao et al., 2016, 2019), we observed that HK2 protein was downregulated in BC cells with circANKRD17 knockdown (Figure 4a). In contrast, circANKRD17 overexpression could upregulate HK2 (Figure 4f). Histology and immunohistochemistry (IHC) showed that circANKRD17 could increase HK2 protein level in xenografts (Figures 4b,g). Additionally, circANKRD17 inhibition reduced the rates of glucose consumption (left) and lactate production (right) (Figure 4c), whereas circANKRD17 overexpression stimulated these processes (Figure 4h).
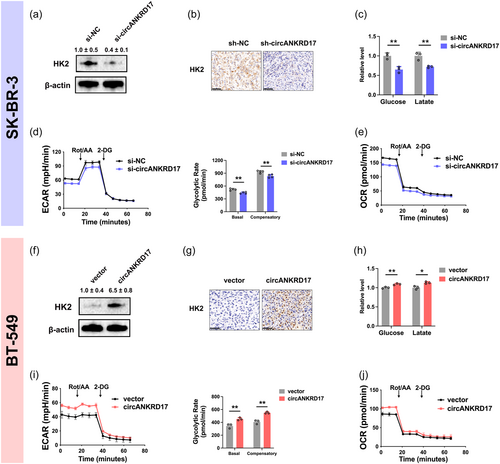
Consistently, the extracellular acidification rate (ECAR) levels (left) (Figure 4d), basal and compensatory glycolytic rates (right) (Figure 4d), and oxygen consumption rate (OCR) levels (Figure 4e) were decreased in BC cells with circANKRD17 knockdown. Meanwhile, the ECAR levels (left) (Figure 4i), basal and compensatory glycolytic rates (right) (Figure 4i), and OCR levels (Figure 4j) were increased with circANKRD17 overexpression.
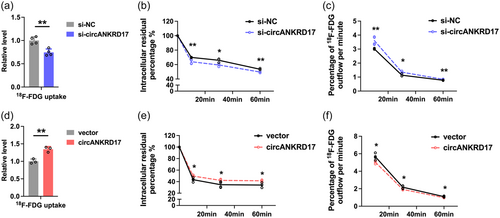
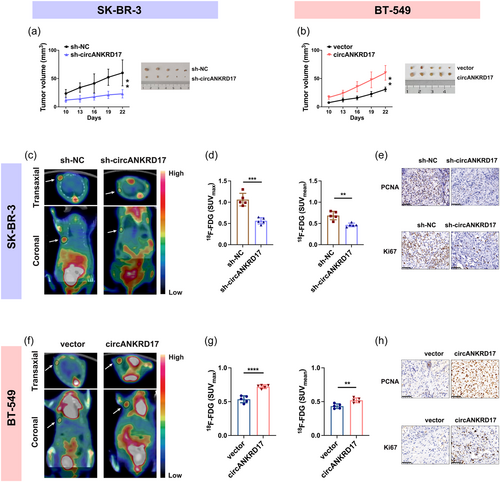
We observed that circANKRD17-knockdown cells exhibited lower levels of 18F-FDG uptake (Figure 5a), and cells overexpressing circANKRD17 exhibited significantly higher 18F-FDG uptake levels (Figure 5d). Tumor malignancy is associated with elevated glucose metabolism, particularly the ability to retain glucose (Xi et al., 2020), suggesting that cellular concentration of 18F-FDG may represent the glycolytic activity and malignant biological behavior of viable tumor cells. Further 18F-FDG flow tests revealed that circANKRD17 inhibition decreased the intracellular residual 18F-FDG level (Figure 5b) and significantly accelerated 18F-FDG outflow in BC cells (Figure 5c). Meanwhile, more 18F-FDG was enriched in circANKRD17 overexpression cells over time (Figure 5e), with the outflow rates significantly reduced (Figure 5f). In conclusion, our findings suggest that circANKRD17 plays a crucial role in regulating glycolysis in BC cells.
3.5 CircANKRD17 promotes cell proliferation, migration, invasion, and cell cycle progression in BC cells
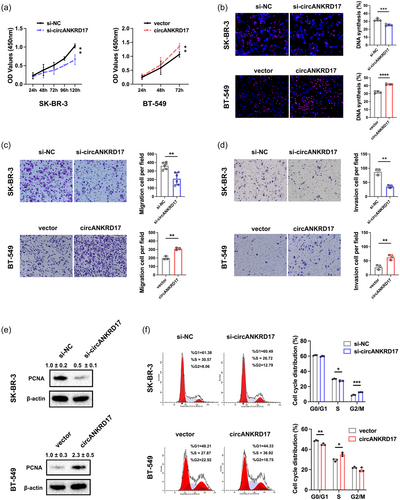
Then, we investigated the role of circANKRD17 in promoting malignant phenotypes caused by dysregulation of glycolysis in BC. CCK8 and EDU assays consistently indicated that circANKRD17 knockdown inhibited, whereas circANKRD17 overexpression promoted BC cell growth (Figure 6a-b). Moreover, for cell migration (Figure 6c) and invasion (Figure 6d), knockdown of circANKRD17 inhibited while circANKRD17 overexpression promoted these phenotypes. The protein expression level of proliferating cell nuclear antigen (PCNA) was consistent with circANKRD17, with coordinated up- or downregulation (Figure 6e). Further cell cycle analysis showed that circANKRD17 knockdown decreased the number of cells in the S phase, and circANKRD17 overexpression significantly accelerated cell-cycle progression (Figure 6f). Together, these results demonstrated the involvement of circANKRD17 in promoting the malignant biological behaviors of BC cells.
3.6 CircANKRD17 promoted glycolysis via interacting with miR-143 in BC cells
Given the important role of Ago2 in mediating ceRNAs, first, we determined Ago2 in the circANKRD17-binding protein and explored an MS2-based circRNA pulldown assay (Figure 7a), in which circANKRD17 was tagged with MS2 RNA stem-loop structures (named MS2bs-circANKRD17) and co-transfected with the MS2BP-yellow fluorescent protein (YFP)-HA construct (containing MS2 binding protein that recognizes MS2 RNA sequence and YFP for cellular localization signal). The YFP indicated a nuclear localization signal when MS2BP-YFP-HA was transfected alone (Figure 7b). However, when MS2bs-circANKRD17 and MS2BP-YFP-HA were co-transfected, the YFP tag showed a cytoplasmic localization signal (Figure 7b), consistent with the observation from the FISH assay (Figure 2e). These results demonstrated that MS2BP-YFP-HA could efficiently capture MS2bs-circANKRD17 in BC cells. Mass spectrometry (MS) analysis showed the enrichment of Ago2 protein in the MS2BP-MS2-circANKRD17 complex (Figure 7c), confirming the role of circANKRD17 as a miRNA sponge.
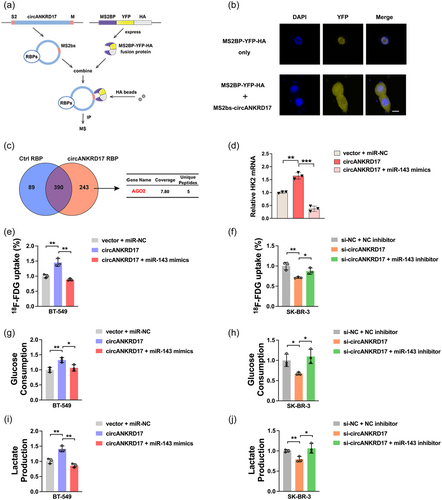
To assess the relationship among circANKRD17, miR-143, and HK2 in BC cells, rescue experiments were performed. We transfected BT-549 cells with vector + miR-NC, circANKRD17, circANKRD17 + miR-143 mimics, and examined the expression of HK2 mRNA. As showed in Figure 7d, overexpression of circANKRD17 upregulated the mRNA expression of HK2, which could be abolished by circANKRD17-miR-143 mimics co-transfection. In addition, circANKRD17 overexpression increased 18F-FDG uptake (Figure 7e), glucose consumption (Figure 7g), and lactate production (Figure 7i) in BC cells, which could be also abolished by circANKRD17-miR-143 mimics co-transfection.
Conversely, SK-BR-3 cells were introduced with si-circANKRD17 or/and miR-143 inhibitor. Results showed that circANKRD17 silencing reduced glycolysis through sponging miR-143. CircANKRD17 inhibition attenuated 18F-FDG uptake (Figure 7f), glucose consumption (Figure 7h), and lactate production (Figure 7j), which could also be reversed by miR-143 inhibitor. Taken together, these results demonstrated that circANKRD17 promoted glycolysis via sponging miR-143.
3.7 The role of circANKRD17 in regulating glycolysis can be evaluated by 18F-FDG microPET/CT
We finally investigated the effects of circANKRD17 dysregulation on BC progression in vivo. We observed decreased tumor sizes in mice bearing xenografts formed by circANKRD17-downregulated cells (Figure 8a). In contrast, increased tumor sizes were observed in mice bearing BT-549 xenografts overexpressing circANKRD17 (Figure 8b). 18F-FDG microPET/CT scanning was performed and SUV values were measured to identify the character of circANKRD17 in regulating glycolysis of BC in vivo. As shown in Figure 8c, xenografts with repressed circANKRD17 expression exhibited significantly lower levels of 18F-FDG uptake than controls. SUV measurements show that SUVmax and SUVmean levels of tumor uptake were significantly reduced by 47% and 32% upon circANKRD17 knockdown, respectively (Figure 8d). Reciprocally, circANKRD17-overexpressing xenografts exhibited higher levels of 18F-FDG uptake (Figure 8f). As shown in Figure 8g, SUVmax and SUVmean were increased by 35% and 17%, respectively, in circANKRD17-overexpressing xenografts. Furthermore, IHC staining showed that the level of PCNA and Ki67, two proliferation markers, were decreased in the circANKRD17-knockdown group (Figure 8e) and increased in the circANKRD17-overexpressing group (Figure 8h). Together, these results demonstrated that circANKRD17 plays a critical role in modulating glycolysis of BC and that 18F-FDG PET/CT imaging is an effective technique for assessing abnormal glucose metabolism induced by circANKRD17 in tumor cells.
4 DISCUSSION
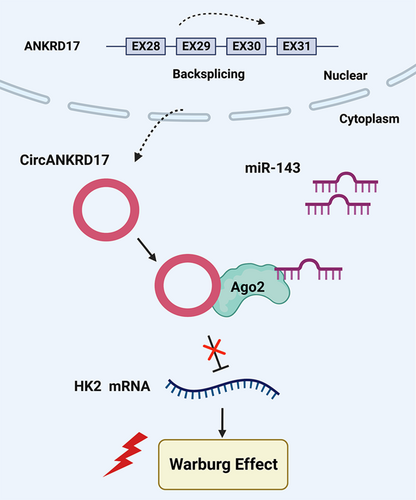
This study demonstrated that circANKRD17 promotes glycolysis by inhibiting miR-143 and may represent a promising therapeutic target to interrupt cancerous glycolysis in BC.
CircRNAs were initially identified as functional sponges for miRNAs (Hansen et al., 2013). This concept has also been extensively studied in BC; for example, circRNA_0025202 regulates tamoxifen sensitivity by controlling the miR-182-5p/FOXO3a signaling (Sang et al., 2019); circIRAK3 facilitates the metastasis of BC by interacting with miR-3607 (Wu et al., 2018), and circEPSTI1-miR-4753/6809-BCL11A axis regulates the progression of triple-negative BC (Chen et al., 2018). However, whether and how circANKRD17 regulates the glycolytic switch in BC remains largely unknown. The role of miR-143 in inhibiting glucose metabolism in tumors has been previously investigated (Jiang et al., 2012; Miao et al., 2016; Zhang et al., 2017). Instead of performing high-throughput microarray sequencing in tissues or cells, we aim to identify the functional circRNAs interacted with miR-143, the well-documented glycolytic repressor. We identified circANKRD17 acted as a sponge of miR-143. Our RIP assay and MS2-based circRNA pulldown assay also showed that Ago2 protein could bind to circANKRD17, further confirming the role of circANKRD17 as a miR-143 sponge.
Cancer cells increase glucose consumption by switching metabolism to glycolysis, which provides a good source of carbon for cancer cell growth. Tumor cell metabolism is implicated in cancer progression and affected by circRNAs. For instance, circARHGAP29 facilitates glycolysis in docetaxel-resistant prostate cancer (Jiang et al., 2022), and circRPN2 inhibits glycolysis in hepatocellular carcinoma (Li et al., 2022). The present study found that circANKRD17 inhibition significantly reduced 18F-FDG uptake and intracellular residual 18F-FDG levels, and accelerated the 18F-FDG efflux rate in BC cells, indicating that downregulation of circANKRD17 inhibited BC glycolysis in vitro. Our results demonstrated that SUVmax and SUVmean acquired from 18F-FDG PET/CT were reduced in circANKRD17-downregulated BC xenografts, indicating that downregulation of circANKRD17 inhibited glycolysis in vivo. We have previously indicated that miR-143 functions as a regulator by suppressing HK2 expression in BC cells (Jiang et al., 2012; Miao et al., 2016). HK2 acted as an integrator for metabolic cues and cell growth (Zhang et al., 2015). Our rescue experiments demonstrated that circANKRD17 promoted glycolysis via sponging miR-143. Collectively, the current study highlights the critical regulatory ability of circANKRD17 in controlling glycolysis in BC.
In summary, these data demonstrated the circANKRD17-miR-143 cascade as a novel mechanism in controlling glucose metabolic reprogramming in BC cells and circANKRD17 is a crucial regulator of aerobic glycolysis (Figure 9). Our results suggest that circANKRD17 may be a promising therapeutic target to interrupt cancerous glycolysis in BC.
AUTHOR CONTRIBUTIONS
Hong Chen conceived the idea. Hong Chen and Ling-Fei Zhang designed the studies. Hong Chen performed the experiments. Hong Chen and Ling-Fei Zhang drafted the manuscript. Ling-Fei Zhang, Lu Zhang, Ying Miao, and Yun Xi assisted with data analysis. Mo-Fang Liu, Min Zhang, and Biao Li conducted the project management. All of the authors approved the final manuscript.
ACKNOWLEDGMENTS
This work was supported in part by the National Natural Science Foundation of China (81671715, 81871378, 82171971, 81501499), Shanghai Jiaotong University Translational Medicine Open Project (TMSK-2020-002), Shanghai Municipal Key Clinical Specialty, China (shslczdzk03403).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Clinical human samples were included with the informed consent of the patient. This study was approved by the ethics committee of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine (Ethical code: 2022-201).