Sustained Inhibition of Proliferative Response After Transient FGF Stimulation Is Mediated by Interleukin 1 Signaling
Abstract
Transient FGF stimulation of various cell types results in FGF memory—a sustained blockage of efficient proliferative response to FGF and other growth factors. FGF memory establishment requires HDAC activity, indicating its epigenetic character. FGF treatment stimulates proinflammatory NFκB signaling, which is also critical for FGF memory formation. The search for FGF-induced mediators of FGF memory revealed that FGF stimulates HDAC-dependent expression of the inflammatory cytokine IL1α. Similarly to FGF, transient cell treatment with recombinant IL1α inhibits the proliferative response to further FGF and EGF stimulation, but does not prevent FGF receptor-mediated signaling. Interestingly, like cells pretreated with FGF1, cells pretreated with IL1α exhibit enhanced restructuring of actin cytoskeleton and increased migration in response to FGF stimulation. IRAP, a specific inhibitor of IL 1 receptor, and a neutralizing anti-IL1α antibody prevent the formation of FGF memory and rescue an efficient proliferative response to FGF restimulation. A similar effect results following treatment with the anti-inflammatory agents aspirin and dexamethasone. Thus, FGF memory is mediated by proinflammatory IL1 signaling. It may play a role in the limitation of proliferative response to tissue damage and prevention of wound-induced hyperplasia. J. Cell. Physiol. 231: 650–658, 2016. © 2015 Wiley Periodicals, Inc.
We recently determined that transient FGF stimulation results in a stable inhibition of proliferative response to repeated FGF treatment (Poole et al., 2014). This phenomenon termed “FGF memory” has been detected in fibroblasts, endothelial cells, mesenchymal stem cells, and adipose-derived stem cells. Cell stress occurring in damaged tissues can results in the nonclassical release of FGF1 (Prudovsky et al., 2008). We hypothesized that FGF memory may serve to moderate the proliferative response after tissue damage, and thus prevent hyperplasia and decrease scar formation. Establishment of FGF memory depends on NFκB signaling and requires histone deacetylase (HDAC) activity (Poole et al., 2014). Based on these results, a study was undertaken to understand the molecular mechanisms underlying FGF memory. We found that FGF enhances the expression of interleukin (IL) 1α, and this effect depends on HDAC activity. Similarly to FGF, transient treatment with IL1α drastically inhibits the proliferative response to subsequent stimulation with FGF but does not interfere with FGF-promoted signaling and cell migration. Moreover, cell pretreatment with both FGF1 and IL1α blocks the proliferative response to an unrelated growth factor, EGF. FGF memory is abolished by IRAP, the competitive inhibitor of IL1 receptor type I, and a specific neutralizing antibody against IL1α. In addition, anti-inflammatory agents aspirin and dexamethasone eliminate it. Collectively, these results show that FGF memory is mediated by IL1α production resulting in enhanced inflammatory signaling.
Materials and Methods
Cell cultures
Swiss 3T3 (ATCC, Manassas, VA) cells and mouse lung endothelial cells Le II (Friesel and Maciag, 1988) were maintained in DMEM (HyClone, Logan, UT) supplemented with 10% bovine calf serum (HyClone) and 1% antibiotic-antimycotic mixture (GIBCO, Grand Island, NY). Quiescence was induced by culturing cells in DMEM containing 0.2% bovine calf serum and 5 units/mL heparin (Sigma, St. Louis, MO).
Cell treatment
Prior to stimulation, cells were transferred for 48 h to quiescence (Q) medium: DMEM with 5 units/mL heparin and 0.2% bovine calf serum. This basic medium was used in all types of stimulation. Upon induction of quiescence as well as withdrawal of growth factors, the cells were washed twice with DMEM medium containing 5 units/mL heparin.
Recombinant human FGF1 was prepared as described (Forough et al., 1991) and applied at 10 ng/mL. Human recombinant EGF (Lonza, Portsmouth, NH) and IL1α (Roche, Nutley, NJ) were used at 10 ng/mL. Human recombinant IRAP (Roche) and neutralizing antibodies against mouse IL1 α (R&D, Minneapolis, MN) were used at 600 or 300 ng/mL.
The following chemical compounds were used: aspirin (acetylsalicylic acid) (Sigma), dexamethasone (Sigma), trichostatin A (TSA) (Sigma) and panabinostat (Selleck, Houston, TX).
Dna synthesis study
Throughout the final 36 h of each stimulation condition, the cells were exposed to 10 μg/mL bromodeoxyuridine (BrdU) (Sigma). Once stimulation schedules were completed, the cells were fixed for a minimum of 10 min in 100% ethanol, washed with PBS, and DNA was denatured by incubation in 1 N HCl at 55°C for 30 min. The residual acid was then washed with PBS. Non-specific binding of antibodies was prevented by a 30 min pre-exposure to blocking buffer (5% bovine albumin, 0.1% Triton X-100, 0.1% sodium azide in PBS), followed by an 1 h incubation in a 1:500 dilution of monoclonal mouse anti-BrdU antibody (Dako, Carpinteria, CA) in blocking buffer. The cells were then washed with PBS and incubated for 30 min in 1:500 dilution of Alexa 546-conjugated anti-mouse IgG antibodies (Invitrogen). Counting of BrdU-positive nuclei in cell populations was performed using an Olympus IX70 microscope with a combination of fluorescence and phase contrast. Two coverslips were studied per each experimental point. For each coverslip, 500 nuclei were counted; the number of fluorescent nuclei was recorded to acquire BrdU incorporation percentages.
Cell migration assay
Linear scratches in cell monolayers were made using a 1 mL pipette tip. Photographs of scratches were taken at 0 and 24 h after monolayer wounding. Per each experimental condition, three independent wells were studied, and in each of them fifteen microscopic fields were photographed using the 10 X objective. The mean distances covered by the migrating fronts of monolayers and corresponding SEM were calculated.
Confocal fluorescence microscopy
The effects of FGF stimulation on the actin skeleton of naïve and IL1α-pretreated Swiss 3T3 cells were studied using confocal fluorescence microscopy. The cells were fixed with 4% neutral formalin, pre-incubated in blocking permeabilizing buffer (PBS with 5% BSA and 0.1% Triton X100), and then stained with Oregon green-conjugated phalloidin and TOPRO3 (both from Invitrogen, Carlsbad, CA). Cell images were taken using Leica SP8 confocal microscope at the MMCRI confocal microscopy facility.
Immunoblot analysis
Lysates were prepared from cell monolayers after various schedules involving IL1α and FGF1 stimulation. The cells were washed and scraped in ice-chilled PBS and centrifuged for collection at 2,500 rpm for 10 min. NPB buffer (20 mM Tris-HCl (pH 7.4), 250 mM sucrose, 60 mM KCl, 20 mM EDTA, 1.5 M NaCl, 1% TritonX-100, 0.1% deoxycholic acid, and a 1:50 dilution of protease inhibitor cocktail from Sigma) was used to lyse the cells. Relative protein concentrations were determined with Coomassie Plus Protein Assay Kit (Pierce, Rockford, IL) using a DU 640 spectrophotometer (Beckman, Fullerton, CA) at an excitation wavelength of 595 nm. The lysate was then mixed with an equal volume of SDS–PAGE sample buffer and incubated at 95°C for 10 min. Equivalent sample amounts were resolved by 12% PAGE and transferred to membranes Hybond-P (GE Healthcare, Little Chalfont, UK). The membranes were blocked in 5% fat-free dry milk diluted in TBS-Tween buffer at 42°C for 2 h, and then blotted with the appropriate primary mouse or rabbit antibody overnight at 4°C.
Membranes blotted with mouse anti-β-actin antibodies (Sigma) served as loading controls. Antibodies against the following proteins were used: rabbit antibodies against phosphorylated Erk1/2 (Sigma) and against cyclin D1 (Santa Cruz Biotechnologies, Santa Cruz, CA), and mouse monoclonal antibodies recognizing cyclin A (Millipore, Temecula, CA). The bound primary antibodies were visualized using horseradish peroxidase-conjugated goat antibodies against rabbit or mouse IgG (BioRad, Hercules, CA) and the ECL detection system (Amersham, Piscataway, NJ).
RT–PCR
RNA was prepared from Swiss 3T3 cells using the RNeasy kit (Qiagen, Hilden, Germany). Expression of IL1β and IL1α was assessed by RT-PCR using the SuperScript kit (Invitrogen). The following pairs of primers were utilized:
IL1α: as. -5′-GTC TCA TGA AGT GAG CCA TAG C-3′, s. -5′-CAA GAT GGC CAA AGT TCG TGA C-3′.
IL1β: as. -5′-CAG GAC AGG TAT AGA TTC TTT CTT TT-3′, s. -5′-ATG GCA ACT GTT CCT GAA CTC AAC T-3′.
β-actin: as. -5′-GTC TCA AAC ATG ATC TGG G-3′, s. 5′-AGA AAA TCT GGC ACC ACA CC-3′.
For qRT-PCR, we used the same IL1α primers and the following cyclophilin primers:
as. -5′-CAG TGC TCA GAG CTC GAA AG-3′, s. -5′-CCA CCG TGT TCT TCG ACA T-3′.
Elisa
IL1α concentration in cell lysates was determined using an ELISA kit from eBioscience (San Diego, CA) according to the instructions of the manufacturer.
Statistical analysis
Each experiment was repeated at least three times with consistent results. In the studies analyzing DNA synthesis, percentage of BrdU-labeled cells with 95% confidence interval was calculated for each condition. In qRT-PCR and ELISA studies of IL1α expression and in the studies of cell growth and migration, Student's t test was used to assess the significance of observed effects.
Results
FGF induces the expression of IL1α
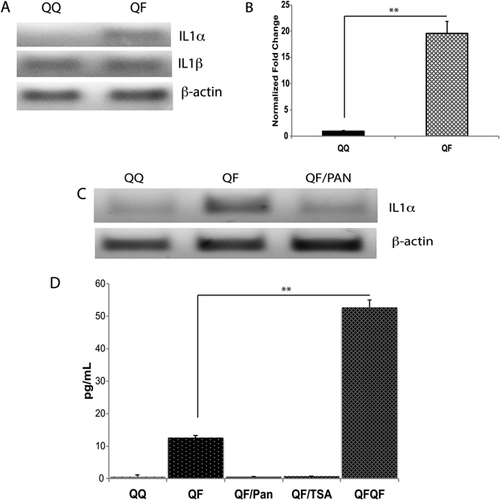
The observations that FGF stimulation activates NFκB signaling, and establishment of FGF memory depends on NFκB pathway (Poole et al., 2014) indicated that FGF may induce the expression of a proinflammatory cytokine. Interestingly, IL1α, a potent inducer of inflammation and activator of NFκB signaling, represses cell proliferation (Ohmori et al., 1988; Ikeda et al., 1991) and plays a key role in some cases of cell senescence (Maier et al., 1990; McCarthy et al., 2013). We used RT-PCR to assess the effect of FGF1 stimulation on the expression of proinflammatory cytokines and inducers of NFκB signaling, IL1α, and IL1β, in Swiss 3T3 cells (Fig. 1A). While IL1β expression remained unchanged, FGF1 stimulated the expression of IL1α. qRT-PCR study has shown a 20-fold induction of IL1α by FGF (Fig. 1B). Interestingly, the induction of IL1α expression by FGF1 was abolished by the HDAC inhibitor panabinostat (Fig. 1C). The RT-PCR results were confirmed by determining the IL1α content in the lysates of Swiss 3T3 cells stimulated with FGF1 in presence or absence of HDAC inhibitors (Fig. 1D). It is noteworthy that the concentration of IL1α in the cell lysates after repeated FGF1 stimulation was approximately five times higher than after the primary stimulation. Thus, FGF induces the expression of IL1α in an HDAC-dependent manner.
Transient pretreatment with IL1α prevents proliferative response to FGF and EGF
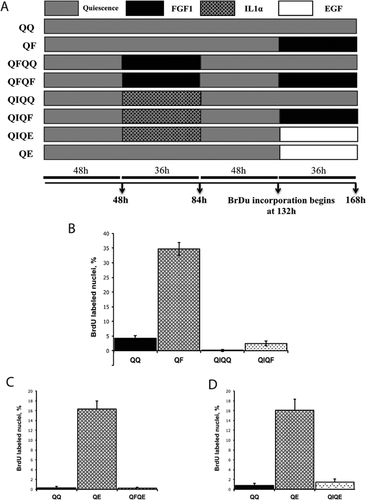
Because FGF1 induces the expression of IL1α, we assessed whether similar to transient FGF1 stimulation, transient application of IL1α to Swiss 3T3 cells prevents proliferative response to further FGF stimulation. The following schedules of cell treatment were applied: QQ – 168 h of quiescence; QF – 132 h quiescence, 36 h FGF1; QIQQ – 48 h quiescence, 36 h IL1α, 84 h quiescence; QIQF – 48 h quiescence, 36 h IL1α, 48 h quiescence, 36 h FGF1. BrdU incorporation during the last 36 h of each schedule was determined (Fig. 2A). IL1α pretreatment (QIQF) resulted in a 10-fold decrease of DNA synthesis in comparison to FGF1 stimulation of naive quiescent cells (QF) (Fig. 2B). In a series of similar experiments, we found that transient FGF1 and IL1α stimulations inhibited the proliferative response to epidermal growth factor (EGF) (Fig. 2C,D).
Transient pretreatment with IL1α does not interfere with the activation of Erk1/2 and induction of cyclin D in response to FGF stimulation but prevents the induction of cyclin A
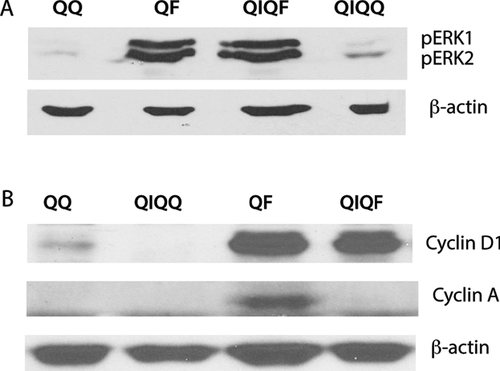
To understand the mechanisms through which the pretreatment of FGF1 inhibits growth factor-induced proliferation, we used immunoblotting to assess the signaling events and expression of key cell cycle proteins in response to FGF1 stimulation of IL1α-pretreated (QIQF) and naïve (QF) Swiss 3T3 cells. Thirty minutes after final FGF1 stimulation, the phosphorylation of Erk1/2 reflecting the early signaling downstream of FGFR was identical in both QIQF and QF cells (Fig. 3A). The same was true for the expression of cyclin D1 24 h after stimulation, a period corresponding to late G1/early S phase after the exit from quiescence (Fig. 3B). In contrast, unlike naive cultures, cells with IL1α history failed to express cyclin A, the key regulator of the S-phase initiation and transition, in response to 24 h FGF stimulation (Fig. 3B).
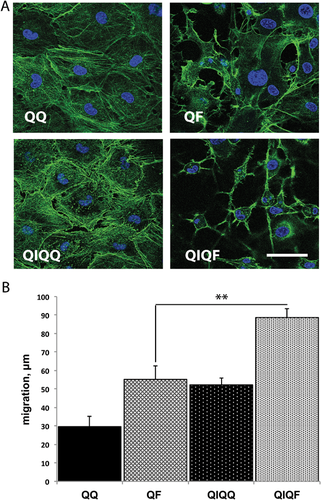
IL1α pretreatment enhances the restructuring of actin cytoskeleton and cell migration in response to FGF stimulation
Cells transiently treated with FGF respond to FGF restimulation by enhanced actin cytoskeleton reorganization and migration (Poole et al., 2014). We used phalloidin staining to compare the status of the actin cytoskeleton after FGF treatment of naïve (QF) and IL1α-prestimulated Swiss 3T3 (QIQF) cells. Confocal fluorescence study demonstrated a stronger formation of actin fibers in cells with the history of IL1α treatment (Fig. 4A). In agreement with these results, the study of wounded monolayers showed that QIQF cells exhibit a faster migration than cells in QF populations (Fig. 4B).
FGF memory depends on IL1 receptor signaling
The induction of IL1α expression by FGF1 and the similarity between the effects of FGF1 and IL1α pretreatment on proliferative and migratory response to FGF stimulation indicate that FGF memory could be dependent on IL1 signaling. To assess this hypothesis, we determined the effect of IRAP, a specific competitive antagonist of IL1 receptor type I, on FGF memory. When IRAP was applied throughout the QFQF schedule of a typical FGF memory experiment, it completely rescued the proliferative response of Swiss 3T3 cells after repeated FGF1 stimulation (Fig. 5A).
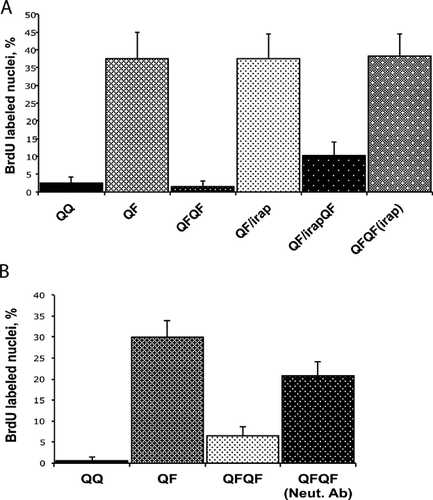
Neutralization of IL1α rescues the proliferative response to the secondary FGF stimulation
To assess the role of IL1α in FGF memory, we evaluated the effect of a specific neutralizing anti-IL1α antibody upon the proliferative response of Swiss 3T3 cells to secondary FGF stimulation. We found that DNA synthesis after repeated FGF treatment was rescued when anti-IL1α antibodies were present throughout the QFQF experimental schedule (Fig. 5B).
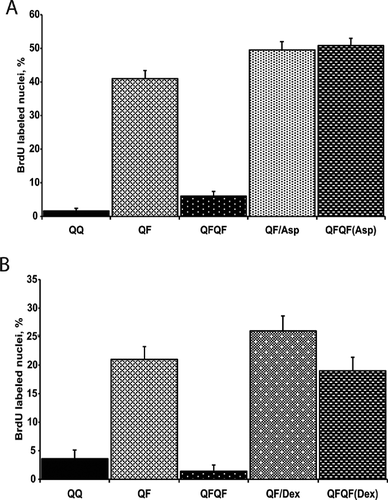
Anti-inflammatory drugs rescue the proliferative response to the secondary FGF stimulation
IL1α is a potent proinflammatory cytokine. To elucidate whether inflammatory signaling is required for FGF memory, we used two anti-inflammatory drugs: aspirin and dexamethasone. Both of them restored the proliferative response of Swiss 3T3 cells to the secondary FGF stimulation (Fig. 6A,B). Decreasing the concentrations of aspirin and IL1α resulted in weaker rescuing effects (Supplementary Figure 1).
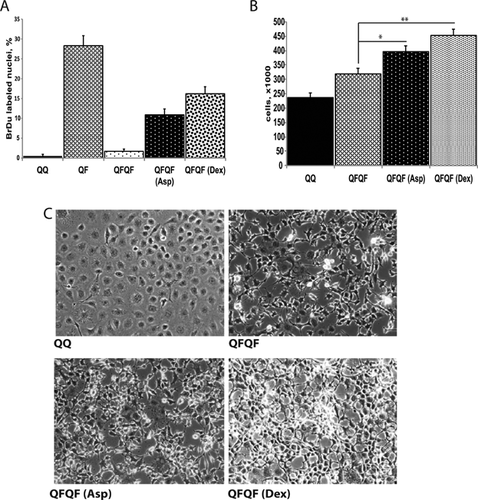
The rescuing effects of aspirin and dexamethasone on DNA synthesis were confirmed in FGF restimulation experiments using Le II mouse lung endothelial cells (Fig. 7A). Moreover, in special experiments where the time of secondary FGF stimulation was extended to 2 days, we detected a significant increase of LeII cell numbers upon the treatment with aspirin and dexamethasone (Fig. 7B,C).
Discussion
The earlier observation that transient stimulation with FGF results in a stable and potent inhibition of proliferative response to repeated growth factor stimulation (Poole et al., 2014) encouraged us to elucidate the key molecular determinant(s) of this phenomenon that had been termed FGF memory. The present study shows that IL1α plays a critical role in FGF memory. The following results underlie this conclusion: (i) FGF treatment results in a HDAC-dependent IL1α expression; (ii) IL1α stimulation generates a cell phenotype similar to that observed in FGF-pretreated cells, i.e., strongly inhibited proliferative and enhanced migratory response to growth factor stimulation; (iii) IRAP and specific neutralizing anti-IL1α antibodies inhibit FGF memory.
It has long been known that IL1α exhibits anti-proliferative effects in non-transformed cells (Ohmori et al., 1988; Ikeda et al., 1991). In particular, it was found that senescence of human endothelial cells can be significantly delayed by the inhibition of IL1α expression (Maier et al., 1990). In recent years, IL1α was identified as a key determinant of the senescence-associated secretory cell phenotype, which is characterized by the inhibition of proliferation and production of several proinflammatory cytokines in addition to IL1α (McCarthy et al., 2013).
We have earlier reported that transient FGF stimulation results in a sustained activation of NFκB signaling, which is critical for FGF memory establishment (Poole et al., 2014). IL1α (Niu et al., 2004; Melisi et al., 2009) and FGF (Muddasani et al., 2007; Salazar et al., 2014) have been demonstrated to activate the proinflammatory NFκB pathway. Moreover, IL1α expression and NFκB signaling constitute an autoregulatory feedback loop that maintains IL1α production (Niu et al., 2004). Proinflammatory signaling can result in the inhibition of DNA synthesis (Vlahos and Stewart, 1999). Concurrently, we have shown that the anti-inflammatory drugs, aspirin and dexamethasone, abolish IL1α-mediated FGF memory. Thus, FGF-induced IL1α expression and proinflammatory signaling limit the proliferative response to continuous FGF stimulation. The experiments involving transient cell treatment with IL1α demonstrated a sustained inhibition of proliferative response to FGF and EGF stimulation. Similar to earlier reported experiments with FGF restimulation (Poole et al., 2014), cells pretreated with IL1α respond to FGF by Erk1/2 phosphorylation and cyclin D1 expression. Moreover, their cytoskeletal and migratory responses to FGF are significantly stronger than in naïve cells. These results indicate that FGFR-mediated signaling, progression from quiescence to G1 phase as well as morphological and migratory changes are resistant to IL1α or increased by it. Indeed, IL1 signaling enhances cell migration in the course of inflammation (Mitchell et al., 2007). On the other hand, FGF-induced IL1α prevents DNA replication. The mechanisms of the sustained inhibition of proliferative response in cells transiently treated with IL1α and FGF1 require further studies. The inability of such cells to express cyclin A in response to FGF indicates the blockage of cell cycle events immediately preceding the onset of the S phase.
FGF memory and induction of IL1α expression require not only NFκB signaling but also HDAC activity. Deacetylation of NFκB p65 is required for its nuclear translocation and retention and thus for efficient NFκB signaling (Grabiec et al., 2012; Liu and McCall, 2013; Ziesche et al., 2013; Zhang et al., 2015). FGF memory could be dependent on HDAC-mediated histone deacetylation or on HDAC-dependent activation of NFκB signaling or on both. As we have reported (Poole et al., 2014), FGF does not change the expression of major HDACs. Thus, the search for a specific HDAC involved in FGF1 memory followed by the identification of its mechanism of action requires a comprehensive siRNA knockdown screen study involving the assessment of FGF memory establishment, NFκB signaling activity and IL1α expression.
The significance of FGF memory for in vivo processes remains to be elucidated. This phenomenon could play a role in the regulation of the proliferative response during repair of tissue damage. It is noteworthy that liver regeneration after partial hepatectomy is accompanied by the increase of IL1 expression, which limits cell proliferation and prevents hyperplasia (Boulton et al., 1997; Sgroi et al., 2011). It is also interesting that endothelial-specific overexpression of FGF1 in transgenic mice paradoxically results not in the improvement of post-ischemic kidney repair, but in the inhibition of reparative processes and increased inflammation (Kirov et al., 2012). We hypothesize that FGF1 memory mediated by IL1α production may regulate the repair of damaged tissues through the limitation of cell proliferation and enhancement of cell migration, which decrease scarring and help restore the normal tissue structure.
Acknowledgments
The study has been supported by a Maine Cancer Foundation grant to IP, NIH grant HL35627 to IP and MMCRI institutional support to IP. In this work, we used the facilities of the Protein, Nucleic Acid and Cell Imaging Core supported by NIH grant P30 GM103392 to Robert Friesel. We are grateful to Robert Friesel and Lucy Liaw for critical reading of the manuscript, to Norma Albrecht for editorial assistance and to Roche for kindly providing us with IRAP. A.P. and E.C. were the University of Southern Maine Independent Study Interns at MMCRI. The authors have no conflict of interest to declare.