Osteogenic Potential of Human Oral-Periosteal Cells (PCs) Isolated From Different Oral Origin: An In Vitro Study
Abstract
The periosteum is a specialized connective tissue containing multipotent stem cells capable of bone formation. In this study, we aimed at demonstrating that human oral periosteal cells derived from three different oral sites (upper vestibule, lower vestibule, and hard palate) represent an innovative cell source for maxillo-facial tissue engineering applications in terms of accessibility and self-commitment towards osteogenic lineage. Periosteal cells (PCs) were isolated from patients with different ages (20–30 yy, 40–50 yy, 50–60 yy); we then analyzed the in vitro proliferation capacity and the bone self-commitment of cell clones culturing them without any osteogenic supplement to support their differentiation. We found that oral PCs, independently of their origin and age of patients, are mesenchymal stem cells with stem cell characteristics (clonogenical and proliferative activity) and that, even in absence of any osteogenic induction, they undertake the osteoblast lineage after 45 days of culture. These results suggest that oral periosteal cells could replace mesenchymal cells from bone marrow in oral tissue-engineering applications. J. Cell. Physiol. 231: 607–612, 2016. © 2015 Wiley Periodicals, Inc.
Abbreviations
-
- PCs
-
- periosteal cells
-
- UV
-
- upper vestibule
-
- LV
-
- lower vestibule
-
- P
-
- palate
-
- BM-MSCs
-
- bone-marrow mesenchymal stem cells
-
- CFU
-
- colony forming unit
-
- PDT
-
- population doubling time
-
- ALP
-
- alkaline phosphatase
-
- PER
-
- periostin
-
- OPG
-
- osteoprotegerin
The periosteum is a complex structure composed of two tissue layers: an outer fibrous layer that provides structural integrity as well as attachment to soft tissue and a cambial region that possesses osteogenic potential containing undifferentiated mesenchymal cells, osteogenic progenitor cells that support bone formation (Colnot et al., 2012; Saimbi et al., 2014). During growth and development, the periosteum contributes to bone elongation and modeling, and when the bone is injured, participates in its recovery (Ringe et al., 2008). Clinical and in vivo experimental data demonstrate that periosteum plays a pivotal role in bone autograft healing and remodeling (Zhang et al., 2008).
Periosteum contains multipotent mesenchymal stem cells that are capable of differentiating into bone and cartilage (Ringe et al., 2008; Colnot et al., 2012), giving the periosteum a great potential in maxillo-facial tissue engineering applications. The advantages of using an autologous periosteal membrane are that it requires only one surgical procedure, minimizes any adverse tissue responses during healing and stimulates new bone formation (Hassan et al., 2008). In this context, cell-based bone reconstruction therapies with stem cells derived from periosteum offer new therapeutic opportunities for the repair of bone damaged by disease or injury, not only in cranio-facial defects. For this reason, it is necessary to gain further insight into the characteristics of periosteal-derived mesenchymal precursor cells by analyzing their mesenchymal features. In this study, we tried to demonstrate that human oral-periosteum represent an innovative cell source for bone tissue engineering applications in terms of accessibility and self-commitment towards osteogenic lineage. We collected periosteum from 30 patients in three different sites of buccal region: from upper vestibule (UV), from lower vestibule (LV), and from hard palate (P). From these samples, we established, by enzymatic digestion, clones of cells that were subsequently confirmed as mesenchymal by FACS analysis (Dominici et al., 2006). In order to demonstrate their innate capacity to differentiate into cells of osteogenic/odontogenic phenotype, cells were left in culture without any osteogenic medium to support their differentiation. The osteogenic potential was evaluated by qRT-PCR on genes involved in osteogenic differentiation (OPG, BMP-2, ALP, RUNX-2, and Periostin) and measuring the activity of Alkaline Phosphatase, as marker of osteogenic differentiation (Owen and Friedenstein, 1988; Ogasawara et al., 2004; Burns et al., 2010; Komori, 2010; Gurban and Mederle, 2011; Yang et al., 2011; Gerbaix et al., 2014; Heo et al., 2015; Park et al., 2015). Taken together these results demonstrate the osteogenic potential of the oral periosteum isolated from different oral origin and from patients with different ages. Nevertheless, in contrast to bone marrow-mesenchymal stem cells (BM-MSCs), the oral periosteal cells are easier to achieve from the patient, possess a greater expansion capacity and stemness. These features make them an exciting new tool for approaching tissue engineering.
Subjects and Methods
Patients selection
Thirty patients of both sexes (15 Female, 15 Males), healthy for systemic pathologies, were selected from the periodontology department of the Dental School of the University of Turin (Italy) and divided into three age groups (20–30 yy, 40–50 yy, 50–60 yy). Briefly, after signing an informed consent, the oral cavity was cleaned using chlorexidine 0, 12% rinse for 5 min (Dentosan, Milan, Italy) and during the dental extraction, three samples of periosteum were taken from three different oral sites using sterile micro-surgical forceps (Hu-Friedy, Milan, Italy) and a micro-surgical blade (BD, Milan, Italy). The samples were divided: 10 from the palate (“P”), 10 from upper vestibule (“UV”), and 10 from lower vestibule (“LV”). The samples were immersed in sterile saline solution and kept refrigerated at 4°C before being processed within 24 h. Data reported are representative of patients of different ages and samples of periosteum origin. Samples collected from these different patients are listed in the Table 1.
| Patients | Surgical site | Cell clone |
|---|---|---|
| Patient 1 (22 years old) | Upper vestibule | 31 |
| Patient 1 (22 years old) | Lower vestibule | 32 |
| Patient 1 (22 years old) | Palate | 33 |
| Patient 2 (46 years old) | Upper vestibule | 37 |
| Patient 2 (46 years old) | Lower vestibule | 39 |
| Patient 2 (46 years old) | Palate | 38 |
| Patient 3 (55 years old) | Upper vestibule | 35 |
| Patient 3 (55 years old) | Lower vestibule | 34 |
| Patient 3 (55 years old) | Palate | 36 |
Cells isolation and tissue processing
Periosteum samples were digested in a collagenase I (3 mg/ml) and dispase (4 mg/ml) solution for 1 h at 37°C. Cells “released” from the digestion were filtered through a 70 μm strainer and then plated in α-MEM medium supplemented with 20% FBS, 100 μM 2 p ascorbic acid, 2 mM l-glutamine, 100 U/ml penicillin, and 1,000 mg/ml streptomycin. Flasks were incubated at 37°C at 5% of CO2 and the medium changed twice a week. The clones (derived from each sample and numbered) were expanded to have a sufficient number of cells (2 million approximately) to be cryopreserved. Of each clone, at least two vials of early passages (two, three) were frozen and cells expanded for further experiments.
FACS analysis
After a minimum of five passages, cells were challenged for the following mesenchymal surface antigens: CD34, CD117, CD45, CD90, CD14, CD73, HLA-DR, HLA-ABC (all from BD Bioscience, Buccinasco, Italy) and CD105, CD29 (AbD Serotec). Briefly, for each clone cells were detached with trypsin and re-suspended in PBS with 0.5% BSA, incubated with antibody diluted 1:10 for 30 min in the dark. After two washes with PBS 0.5% BSA, the cells were analyzed with FACS-calibur instrument (Becton Dickinson; BD, Heidelberg, Germany).
Colony forming unit (CFU) assay and population doublings time analysis
For CFU assay, periosteal cells from different origin (P, UP, and LV) were diluted and seeded in a 96-well culture plate to obtain approximately one cell per well. The cells were observed daily for 1–2 weeks to examine colony formation, without any medium changing. At the end of the culture period, the cells were stained with Wright's staining and CFUs were quantified by counting the colonies. In addition, population doubling time during the culture period (96 h) was evaluated for all clones of periosteal cells isolated from P, UV, and LV.
Osteogenic potential of periosteal cells without osteogenic medium
Each sample derived from palate, lower and upper vestibule was maintained in α-MEM medium supplemented with 20% FBS, 100 μM 2 p ascorbic acid, 2 mM l-glutamine, 100 U/ml penicillin, and 1,000 mg/ml streptomycin for 45 and 60 days, changing medium twice a week. We did not add any osteogenic supplement to verify the self-commitment and the osteoblast genotype of human periosteum-derived cells (PCs) from different patients and from different buccal origin.
Extraction of RNA and qRT-PCR analysis
At the end of the culture period (45 days), total RNA was extracted from cells using NucleoSpin RNA kit from Macherey-Nagel (Germany). Quantitative Real-Time PCR (Mini-Opticon® Real-Time PCR System, BioRad, Hercules, CA) was performed to evaluate the gene expression of alkaline phosphatase (ALP), Runt-related transcription factor 2 (Runx-2), Bone-morphogenetic protein 2 (BMP-2), Periostin (PER), and Osteoprotegerin (OPG). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as housekeeping gene. The genes analyzed in our study are extensively used to evaluate stem cell bone differentiation. Primers were designed according to published gene sequences and they are listed in Table 2.
| Genes | FW | RW |
|---|---|---|
| ALP | 5′-CTATCCTGGCTCCGTGTCC-3′ | 5′-AGCCCAGAGATGCAATCG-3′ |
| OPG | 5′-ATCTCCATTAAGTGGACCACCCA-3′ | 5′-TTGCACACGGGGCTGCAGTAT-3′ |
| RUNX2 | 5′-ACAGTAGATGGACCTCGGGA-3′ | 5′-ATACTGGGATGAGGAATGCG-3′ |
| BMP-2 | 5′-CCTCCGTGGGGATAGAACTT-3′ | 5′-CACTGTGCGCAGCTTCC-3′ |
| PER | 5′-GAGGTCACCAAGGTCACCAAA-3′ | 5′-GGGTGTGTCTCCCTGAAGC-3′ |
Alkaline phosphatase (AP) staining
In order to detect AP activity normally present in all osteoblastic cells, the BM Purple AP substrate (Roche®) was used. At each time point (45 and 60 days), a suspension of 105 cells was incubated for 5 h at 37°C in BM Purple solution. The reaction product has a dark purple color, which is insoluble in water; the intensity of the color is proportional to the activity of the membrane-bound enzyme. Spectrophotometric quantification was performed with Nanodrop at wavelength of 615 nm.
Statistical analysis
Each experiment was repeated three times. Results are expressed as the mean ± standard deviation. In order to evaluate statistical differences between each group of clones, a one-way analysis of variance (ANOVA) was performed. In addition, Tukey's post-hoc test was accomplished to determine which samples show statistically significant differences. Statistical significance was established at P ≤ 0.05, for which P is the null hypothesis (*P < 0.05; **0.01 < P < 0.05; ***P < 0.01).
Results
Mesenchymal markers expression of cells from periosteum derived from different oral sites
FACS analysis revealed that all cell clones isolated from UV, LV, and P express surface markers of mesenchymal stem cells. In fact, cells showed simultaneous expression of mesenchymal surface markers as CD73, CD90, CD105, and CD29 with a positivity >98% for all antigens tested and the concomitant absence of hematopoietic markers CD45 and CD34. In addition, FACS analysis confirmed the presence of a subpopulation that expressed at low percentage (2.2%) CD117 (c-Kit), a stem antigen. CD117 (tyrosine-kinase receptor) is an important cell surface marker expressed especially in mesenchymal stem cells that are yet committed to bone phenotype (Suphanantachat et al., 2014). Results are listed in Table 3. FACS results demonstrate that collected clones display the same mesenchymal surface markers pattern regardless of specific site of origin.
| Antigens | UV | LV | P |
|---|---|---|---|
| HLA-DR | − | − | − |
| HLA-ABC | + | + | + |
| CD34 | − | − | − |
| CD45 | − | − | − |
| CD117 | +2.2% | +2.3% | +1.5% |
| CD14 | − | − | − |
| CD90 | +100% | +100% | +100% |
| CD105 | +97% | +98% | +99% |
| CD73 | +100% | +100% | +99% |
| CD29 | +100% | +100% | +100% |
Periosteal cells from different oral locations show a remarkable clonogenical activity and a similar doubling time period
Results of population doubling and CFU assays are shown in Table 4. The clonogenical activity of oral-periosteal cells from palate, lower vestibule and upper vestibule is included in a range between 25 and 30 percent (± 5%). Moreover, these cells, during “in vitro” expansion from passage 2 to passage 11 revealed a population doubling time between 61 and 65 h, independently of their origin and age of patients. These cells maintain a mesenchymal-like appearance with a spindle morphology even after 11 passages (Fig. 1). ANOVA analysis with Tukey's post-hoc test do not reveal any statistical differences between samples.
| Samples | CFU (colony forming unit)-%- | PDT (population doubling time)-h- |
|---|---|---|
| UV (31,35,37) | 25% ± 4.5 | 63 h ± 7.1 |
| LV (32,34,39) | 30.6% ± 5.4 | 61.1 h± 7.2 |
| P (33,36,38) | 29% ± 5.6 | 65.4 h± 4.9 |

Gene expression results
Gene expression results of, RUNX-2, ALP, BMP-2, PER, and OPG, after 45 days of culture in basal conditions, are presented in Figure 2. In addition, expressions of the same genes in T0 samples are reported in Supplementary Figure S2. One-way ANOVA analysis with Tukey's post-hoc test was performed between samples collected from same surgical sites. Statistical analysis revealed that, in all genes analyzed, samples 34 (LV) and 35 (UV) deviated from the others in a significant manner.
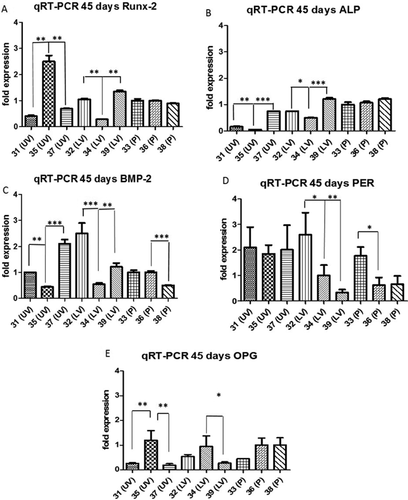
The expression of Runx-2, the master gene of bone differentiation, was in sample 35 (UV) twofold higher with respect to all others clones (Fig. 2A P < 0.05), that anyway express Runx-2 in a similar manner (1–1.5-fold expression). On the other hand, sample 34 (LV) showed the expression of Runx-2 at the lower level with respect to all other samples (0.05 < P < 0.01). In panel B clones collected from palate showed a slightly higher expression of ALP with respect to clones from LV and especially from UV. The expression of BMP-2 (Fig. 2C) showed differences between each group, sample 37 (UV) and sample 32 (LV) showed a moderate higher expression with respect to all other clones (2 and 2.5-fold expression respectively, P < 0.01). The expression of Periostin gene was very similar between samples, exception for sample 39 (lower expression with respect to other clones, 0.05 < P < 0.01) (Fig. 2D). OPG mRNA expression is considerable present in all the clones with small differences between samples (Fig. 2E). The presence of these two last genes, which are associated with homeostatic mechanism of normal bone remodeling, underlies the capability of oral-PC cells to acquire osteoblast-like genotype independently from surgical sites and patient age. These data are consistent with the expressions of the same genes achieved by PCs in osteogenic medium (Supplementary Fig. S1).
While the expressions of osteogenic genes are very similar between samples collected from palate, samples of surgical origin LV and UV present statistical differences. Some of these differences can be ascribed to biological variability; samples 35 and 34 that exhibit the major differences, derive from the same patient (Table 1). Moreover, although the end point of our culture was 45 days, we cannot exclude that single clones acquire different level of osteogenic induction during culturing time.
In summary, apart from the differences outlined by ANOVA test, all clones expressed osteogenic genes at a higher level with respect to the average of expressions of the same cells at time 0 of culture (Supplementary Fig. S2).
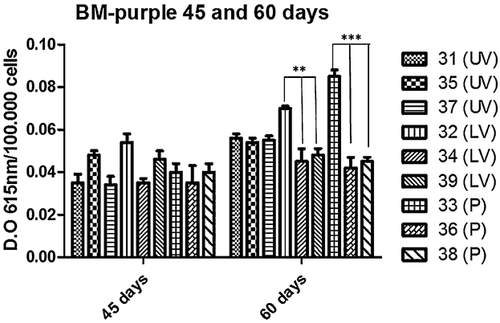
Alkaline phosphatase activity assays
BM-purple Roche® was used to measure ALP activity at 45 and 60 days of culture in proliferative medium. All cell clones tested exhibited similar ALP positivity, with no significant differences at 45 days. ALP positivity is maintained even at 60 days of culture with some minimal statistical differences (samples 32 LV, 0.05 < P < 0.01 and 33 P, P < 0.01) (Fig. 3). These results are consistent with the data of gene expression and display that oral-PC cells are self-committed towards the osteogenic lineage when cultured in basal conditions.
Discussion
Stem cells have become very prominent candidates for tissue engineering cell therapy approaches because of their pronounced expansion capacity, their remarkable plasticity, and therefore, their potential to regenerate complex tissue defects. Nevertheless, adult stem cells and in particular mesenchymal stem cells present some limitations, like loss of proliferative potency and the difficulty of sample harvesting. For example, in vitro, BM-MSC cells within 7–12 passages, demonstrated morphological abnormalities, enlargement, attenuated expression of specific surface markers, and ultimately proliferation arrest and loss of plasticity to differentiate in mesodermal lineage (Wagner et al., 2008). Periosteum plays a major role in bone growth, bone development, bone fracture healing, and in cortical blood supply (Decker et al., 1996; Colnot et al., 2012). It has been demonstrated by several studies that periosteum-derived progenitor cells (PCs) are real “mesenchymal stem cells” for their characteristics to differentiate into osteoblasts and chondroblasts (Ferretti and Mattioli-Belmonte, 2014; Tanabe, 2014). In this study, we focused on periosteal cells derived from the oral region. In particular, our aim was to demonstrate that oral-periosteum derived cells, regardless of age of patient and origin site, are self-committed to differentiate in vitro towards osteoblast lineage without any osteogenic medium to support their differentiation. We isolated periosteal cells from three different oral sites: the palate (P), the upper vestibule (UV), and lower vestibule (LV). We have selected patients of different ages, ranging from 22 to 66 years old and from all of them we have obtained stable clones of PCs. The enrollment of the patient is listed in Table 1.
We used FACS analysis to investigate whether PCs express hematopoietic and mesenchymal stem cell markers (Table 3). All the clones obtained from different patients showed a marked expression of CD73, CD90, CD105, and CD29 with a concomitant absence of hematopoietic markers CD45 and CD34 (Dominici et al., 2006). Interestingly, oral-PCs demonstrated a minimum presence of CD117 surface marker (2.2% average positivity), indicating that these cells have a probable origin from Schneider membrane in maxillary sinus cavity (Aimetti et al., 2010; Graziano et al., 2012). Since we demonstrated their mesenchymal features, we evaluated the “stemness” and the proliferative potency of all cell clones collected. CFU analysis, calculation of PDT and the maintenance of morphological characteristics during culturing time allow us to consider these cells as mesenchymal-like stem cells, although we are aware that these clones are composed of a mixed population likely containing progenitor cells and differentiated mesodermal cells (Table 4, Fig. 1).
To verify their self-bone commitment, we cultured oral-periosteal cells for 45 days in proliferative medium. We chose to reach this long culturing time because of the absence of osteogenic supplement. Results of qReal-time PCR assay for genes characterizing bone differentiation of PCs are shown in Figure 2. These genes are normally used to evaluate bone differentiation. In particular, RUNX-2 is required for determination of the osteoblast lineage. The protein level of Runx2 in osteoblasts is reduced during bone development while osteoblasts acquire mature phenotype (Smith et al., 2011). Bone morphogenetic protein 2 (BMP-2) represent an important signaling ligand for osteoblast differentiation and bone formation (Owen and Friedenstein, 1988; Yamaguchi et al., 2000; Heo et al., 2015). Periostin is a non-collagenous matrix protein expressed in osteoblasts and osteocytes, it seems to be involved in bone response to mechanical stress (Gerbaix et al., 2014; Heo et al., 2015). OPG is a soluble member of the TNFR family produced also by osteoblasts, and it is involved in the osteoblast/osteoclast balance (Gurban and Mederle, 2011; Yang et al., 2011). Nevertheless the presence in our periosteal cells of ALP, BMP-2, RUNX-2, together with Periostin and Osteoprotegerin genes indicates that the overall trend of oral-PC cells is to acquire an osteoblast gene expression pattern in long term culture (Fig. 2). The average expression level of investigated genes in proliferative conditions (45 days) results anyway higher than the same gene expression at time 0 and consistent with the level of expression achieved by PCs in osteogenic medium (Supplementary Fig. 1 and 2). Anyway some samples exhibited differences in gene expression. These differences can be related to intra-patients variability but also to the innate capacity of different clones in reaching “bone differentiation” during culture. The presence of ALP activity, evaluated by BM-purple staining, confirms their osteoblast commitment in a time dependent manner showing a trend of further maturation of PC cells during culturing time (Fig. 3).
Taken together, these results confirm the bone potential of oral-PC cells collected from palate, UV and LV. PCs could replace mesenchymal cells from bone marrow in oral tissue-engineering applications not only for the ease of collection, but also for the rapid in situ engraftment. Our aim was to study the capacity of these cells to undertake the osteogenic lineage in order to use periosteal cells as a source of stem cells in creating bio-complexes with a 3-D structure. It has been demonstrated that, especially for bone, 3-D culture is the pivotal point of proper deposition of matrix (Guerrero et al., 2015). Further studies should be focused on the potentiality of oral PCs in functionalizing 3D porous scaffolds for the creation of bio-complexes supporting bone neo-tissue formation for the engraftment in cranio-facial defects. In this prospect, our findings of the expressions of OPG and Periostin genes will be useful to evaluate the balance between osteo-production and reabsorption in experiments on scaffolds in basal conditions. In fact, the absence of osteo-induction can be overcome by culture conditions as formation of bio-complexes between oral PC cells, 3-D scaffolds, and bioreactors.
Acknowledgments
We are grateful to M.A Avanzini (San Matteo Hospital, Pavia, Italy) for the assistance in FACS analyses and to L. Trovato for the contribution to the study.