Abstract
Fat accumulation in obese individuals worsens the clinical outcomes of cardiovascular disease (CVD). Paradoxically, increased circulating adipocytokines secreted from visceral fat may confer cardioprotective effects. Visfatin, a novel adipocytokine, has anti-diabetic, anti-tumor, and pro-inflammatory properties. However, its effects on cardiomyocytes and the underlying mechanisms remain unknown. This article demonstrated that visfatin counteracted H2O2-induced apoptotic damage in H9c2 cardiomyocytes in a time-dependent manner. Qualitative immunofluorescence approaches demonstrated that visfatin pretreatment attenuated H2O2-induced DNA fragmentation (TdT-mediated dUTP-biotin nick end-labeling), phosphatidyl serine exposure (Annexin V/PI staining), and mitochondrial membrane potential (ΔΨm) depolarization (JC-1 staining). Biochemical studies on cardiomyoctes showed improved cell viability and reduced caspase-3 activation caused by visfatin pretreatment. Visfatin did not inhibit the death receptor-dependent apoptotic pathways, as characterized by its absence in both Fas and TNFR1 down-regulation. Instead, visfatin specifically suppressed the mitochondria-dependent apoptotic pathways, as characterized by changed levels of p53 and its downstream Bcl-2 family genes. Visfatin also up-regulated the protein levels of phosphorylated AMPK, and the anti-apoptotic action of visfatin was attenuated by the AMPK-specific inhibitor compound C. These results suggested that visfatin plays a critical role in cardioprotection by suppressing myocardial apoptosis via AMPK activation. These findings may be the missing link between obesity and CVD. J. Cell. Physiol. 228: 495–501, 2013. © 2012 Wiley Periodicals, Inc.
The link between obesity and cardiovascular disease (CVD) has gained increasing attention in recent years (Curtis et al., 2005; Habbu et al., 2006; Poirier et al., 2006; Zalesin et al., 2008; Davenport et al., 2009). Fat accumulation in obese people is closely associated with the development of CVD, as demonstrated by worse clinical outcomes of acute myocardial infarction in obese individuals (Schwartz et al., 2005; Zeller et al., 2005). On the other hand, emerging studies have shown that some adipocytokines secreted from visceral white adipocytes is involved in the development of CVD (Matsuzawa, 2006). Obesity may increase the risk factors of CVD (Zalesin et al., 2008). However, increased amounts of circulating adipocytokines secreted from visceral fat may confer cardioprotective effects, which is also known as the so-called “obesity paradox” (Curtis et al., 2005; Habbu et al., 2006). This phenomenon resulted in further studies on the missing link between obesity and CVD.
A complex interplay of many factors, including cardiomyocyte apoptosis, cardiac remodeling, and ventricular hypertrophy, occur during the progression of heart failure in obese individuals (Fujita and Ishikawa, 2011). Myocardial apoptosis plays a central role in the development of CVD. The release of excessive reactive oxygen species (ROS), which frequently occurs during clinical cardiac bypass surgery, is a major cause of myocardial apoptosis (Zhao, 2004). Thus, in the present study, cardiomyocytes were treated with H2O2 to mimic myocardial apoptosis caused by excessive ROS release in vitro. Apoptosis was subdivided into two distinct pathways, namely, the mitochondria-dependent and death receptor-dependent apoptotic pathways (Circu and Aw, 2010). The former begins when an injury occurs within the cell and is initiated by p53. p53 also transactivates a series of Bcl-2 family genes, which causes the corruption of mitochondrial potential and cytochrome c release. The extrinsic apoptotic pathway begins outside the cell and is initiated by death receptors on the cell surface. There were mainly two types of signaling complexes for death receptor signaling. The first group comprises the death-inducing signaling complexes known as DISCs, which are formed at the CD95 (APO-1/Fas) receptor, TRAIL R-1 (DR4), or TRAIL R-2 (DR5). These receptors recruit DISCs with similar composition DISC formation of caspases-8, which has a central function in the transduction of apoptotic signals. The second group comprises TNFR1/DR3/DR6 and EDAR. These receptors recruit a different set of molecules and are transducers of both apoptotic and survival signals (Lavrik et al., 2005). In both intrinsic and extrinsic apoptotic pathways, the activation of caspases, a family of cysteine proteases, leads to a proteolytic cascade to dismantle and remove the dying cell.
Obese individuals have increased circulating levels of visfatin, which is positively correlated with the body mass index (Berndt et al., 2005). Visfatin, a novel adipocytokine, is originally known as pre-B cell colony-enhancing factor (PBEF; Samal et al., 1994), and then later identified as nicotinamide phosphoribosyltransferase (Nampt; Rongvaux et al., 2002). In early, Fukuhara et al. (2005) found a new adipocytokine secreted from visceral fat and named it “visfatin.” Visfatin has multiple functions, such as insulin mimetic effects (Fukuhara et al., 2005) as well as anti-tumor (Khan et al., 2006) and pro-inflammatory properties (Moschen et al., 2007). The mechanistic pathways involved in the effects of some well-known adipocytokines such as adiponectin, leptin, and apelin are commonly correlated with pro-survival signaling pathways, including PI3K/Akt, ERK1/2-dependent mitogen-activated protein kinases (MAPK), and AMP-activated protein kinase (AMPK) pathways (Shibata et al., 2005; Smith et al., 2006; Simpkin et al., 2007). Thus far, the effects of visfatin on CVD and the underlying mechanisms remain unclear.
Whether visfatin exerts direct effects on cardiomyocytes is now of great interest in understanding the pathophysiological properties of obesity and CVD. In the present study, a series of experiments was designed to evaluate the effects of visfatin on cardiomyocytes. We found that visfatin pretreatment effectively attenuated apoptotic damage in cardiomyocytes. Further investigations revealed that increased AMPK activation may partially contribute to the cardioprotective effects of visfatin.
Materials and Methods
Materials
Visfatin was purchased from ADIPO BIOSCIENCE (Santa Clara, CA). All tissue culture materials were obtained from GIBCO (Grand Island, NY). JC-1 was purchased from Molecular Probes (Eugene, OR). All other antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). All other chemicals were purchased from Sigma (St. Louis, MO).
Cell culture and treatment
H9c2 cardiomyocytes were obtained from the cell bank of the Chinese Academy of Sciences (Shanghai, China) and cultured as previously described (Sun et al., 2012; Xiao et al., 2012). H9c2 cardiomyocytes were maintained in Dulbecco's modified Eagle's medium with 4.5 g/L glucose supplemented with 10% (v/v) fetal bovine serum and 1% penicillin/streptomycin (v/v) at 37°C in a humidified atmosphere containing 5% CO2. H9c2 cardiomyocytes were seeded at densities of 5 × 104 cells/ml and were routinely grown to subconfluency (>80% by visual estimate). H9c2 cardiomyocytes were treated with a vehicle (0.1% DMSO) or visfatin (200 ng/ml) in the presence or absence of 150 µM H2O2 for various time periods (1 or 24 h). After pretreatment with or without visfatin (200 ng/ml), the cells were exposed to H2O2 for 4 h. 50 µM LY294002, a selective inhibitor of PI3K (Selleck Chemicals, Houston TX, cat#S1105), 5 µM compound C; a selective inhibitor of AMPK (Calbiochem, San Diego, CA, cat#171260) or 25 µM PD98059; a selective inhibitor of ERK MAPK (Sigma, cat#P215) were added and incubated for 30 min before visfatin treatment to examine the role of PI3K, AMPK, and ERK MAPK pathways in mediating the anti-apoptotic effects of visfatin. The cells were harvested at 4°C for further molecular and biochemical analyses.
Assessment of cell viability and apoptosis
Cell viability was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay as previously reported (Sun et al., 2012). Cell apoptosis was determined by the terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) assay using an in situ cell death detection kit and fluorescein (Roche Applied Science, Quebec, Canada) as previously described (Sun et al., 2012; Xiao et al., 2012). The TUNEL apoptotic index was determined by calculating the ratio of TUNEL-positive cells to total cells.
Assessment of SOD and CAT activities
SOD and CAT activities were measured using a detection kit according to the manufacturer's instructions.
Western blot analysis
Cell lysate preparation and Western blot analysis were performed as previously described (Xiao et al., 2010). The protein concentration was determined using a Bio-Rad (Hercules, CA) DC Protein Determination Kit. Bovine serum albumin (BSA) was used as the standard. The immunoblots were developed using an ECL kit. The primary antibodies used in this study were the anti-phospho AMPK (dilution 1/200), anti-phospho-Bad (dilution 1/200), anti-Bad (dilution 1/200), anti-Bid (dilution 1/500), and anti-truncated Bid (tBid; dilution 1/200). For loading control, the same membranes were probed with anti-GAPDH. The signals were quantified by scanning densitometry and the results from each experimental group were expressed as relative integrated intensity compared with that of controls.
Caspase-3 activity assay
Caspase-3 activities were measured using a Fluorometric Assay Kit (BioVision, MountainView, CA) according to the manufacturer's instructions. The samples were read using a Fluoroskan Ascent FL fluorometer (Thermo Fisher Scientific, Waltham, MA) with 400 nm excitation and 505 nm emission wavelengths. The results were expressed as fold change over the control.
Measurement of mitochondrial membrane potential (ΔΨm) using JC-1
After each treatment, cells grown on coverslips were incubated with 5 mM JC-1 dye (Molecular Probes) for 15 min at 37°C. The cells were washed with PBS and immediately analyzed using a confocal microscope. JC-1 fluorescence was measured from a single excitation wavelength (488 nm) with dual emission (shift from green at 530 nm to red at 590 nm). Quantitative analysis of JC-1 staining was also performed (green to red fluorescence ratio).
Real-time polymerase chain reaction (RT-PCR)
Total RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA). About 2 µg of total RNA was reverse transcribed using a SuperScript First-Strand Synthesis System (Invitrogen). cDNA was synthesized from the isolated RNA, and cycle time (Ct) values were obtained by real-time RT-PCR with the Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA), iQ5 Real-Time PCR Detection System, and analysis software (Bio-Rad) as previously described (Xiao et al., 2010). The primers were designed using the Applied Biosystems Primer Express Software (version 2.0). The mRNA levels were normalized to those of GAPDH. Relative mRNA levels are shown using arbitrary units, and the value of the control group is defined as one.
Statistical analysis
Data are expressed as mean ± SE. The significance of the differences between the means was assessed by Student's t-test, and P-values <0.05 were considered significant. One-way ANOVA with Bonferroni corrections was used to determine the significance for multiple comparisons. Statistical calculations were performed using SPSS (version 11.0).
Results
Pretreatment of visfatin for 1 h but not 24 h reduces H2O2-induced cell death in H9c2 cardiomyocytes
As is shown in Figure 1, exposure of H9c2 cardiomyocytes to H2O2 for 4 h led to decreased cell viability, and this effect was attenuated upon 1 h of pretreatment with 200 ng/ml visfatin but not upon 24 h of pretreatment. The dose was chosen based on our preliminary experiments (data not shown), and was relevant to the circulating levels observed in obesity (Chen et al., 2006; Filippatos et al., 2007).
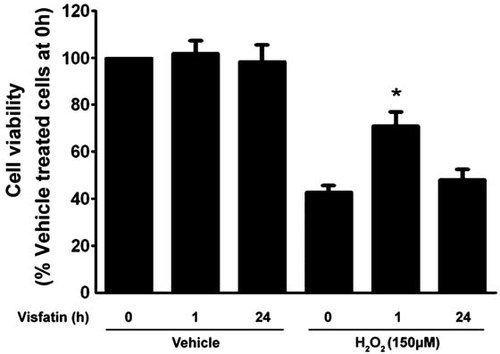
Visfatin pretreatment for 1 h but not 24 h attenuated the effects of H2O2 on cell viability. H9c2 cells were exposed to H2O2 for 4 h. The percentage of cell viability was quantified by the MTT assay. The results are expressed as mean ± SE (n = 8 per group). *P < 0.05 versus 0 h.
Visfatin pretreatment protects cardiomyocytes against DNA fragmentation and phosphatidylserine exposure
DNA fragmentation and phosphatidyl serine (PS) exposure on the extracellular side of the cell membrane are two critical signs of apoptosis (Danial and Korsmeyer, 2004). H9c2 cells were exposed to H2O2 for 4 h. As shown in Figure 2A, the TUNEL assay revealed that visfatin pretreatment attenuated H2O2-induced DNA fragmentation. Annexin V/PI staining results revealed that visfatin pretreatment also inhibited H2O2-induced PS exposure, characterized by the decreased proportion of Annexin-V-stained cells (Fig. 2B). A few PI-counterstained cells were observed, indicating that neither H2O2 nor visfatin resulted in cell necrosis.
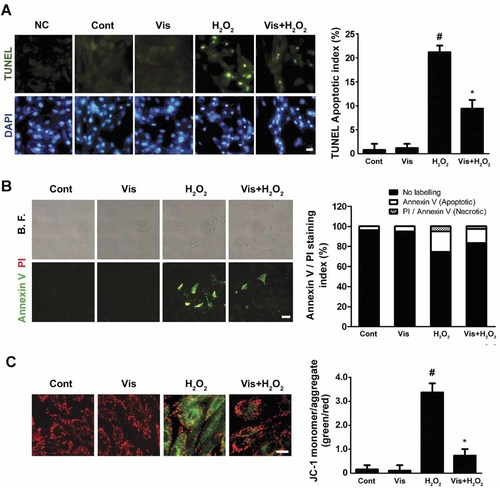
Effects of H2O2 and visfatin on apoptosis in H9c2 cardiomyocytes. A: H9c2 cells were stained and quantified by the TUNEL assay (circles indicate the nuclei of apoptotic cells, scale bar = 10 µm). Negtive control (NC) group: incubated sections with label solution only (without terminal transferase) instead of TUNEL reaction mixture. B: H9c2 cells were labeled and quantified by the Annexin V/PI staining assay (top parts: bright field; bottom parts: Annexin V/PI staining; scale bar = 10 µm). C: H9c2 cells were stained with JC-1 dye and the quantitative analysis of JC-1 staining was evaluated (green to red fluorescence ratio; scale bar = 10 µm). The results are expressed as mean ± SE (n = 8 per group). #P < 0.05 versus control; *P < 0.05 versus H2O2-treated cells.
Visfatin preserves the mitochondrial membrane potential (ΔΨm)
Mitochondrial membrane potential (ΔΨm) depolarization caused corrupted mitochondrial integrity and was closely related to the subsequent regulation of apoptosis (Danial and Korsmeyer, 2004). The cells were stained with the ΔΨm indicator JC-1, which emits red fluorescence with high ΔΨm values and green fluorescence in low ones. As shown in Figure 2C, 4 h H2O2 exposure caused less intense JC-1 red fluorescence in H9c2 cardiomyocytes, whereas pretreatment with visfatin maintained ΔΨm values.
Visfatin inhibited the mitochondria-dependent apoptotic pathway
Apoptosis is generally subdivided into mitochondria-dependent and death receptor-dependent apoptotic pathways (Danial and Korsmeyer, 2004; Circu and Aw, 2010). H9c2 cells were exposed to H2O2 for 4 h. Our results suggested that visfatin did not act via the death receptor-dependent apoptotic pathway, as confirmed by the unchanged mRNA levels of Fas and TNFR1, the two best-characterized death receptors (Fig. 3A). In addition, visfatin exhibited no obvious effect on TRAIL R-1 (also known as DR4) and TRAIL R-2 (also known as DR5) mRNA levels, which may further discard the possible involvement of this death receptor in this mechanism. However, p53, a stressor of the mitochondria-dependent pathway, was downregulated by visfatin pretreatment (Fig. 3A).
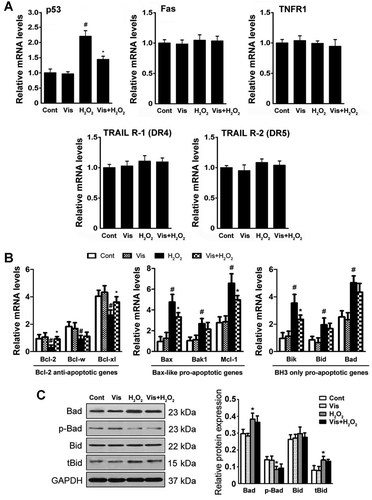
Visfatin specifically acted on the mitochondria-dependent apoptotic pathway. A: The mRNA levels of Fas, TNFR1, p53, TRAIL R-1 (DR4), and TRAIL R-2 (DR5) were determined by real-time RT-PCR. B: The expression profiles of Bcl-2 anti-apoptotic, Bax-like, and BH3-only pro-apoptotic genes were determined by real-time RT-PCR. The values of Bcl-2, Bax, or Bik were set as one. C: The protein levels of Bad, phospho-Bad, Bid, and truncated Bid were evaluated were determined by Western blot analysis. The results are presented as mean ± SE (n = 8 per group). #P < 0.05 versus control; *P < 0.05 versus H2O2-treated cells.
Visfatin maintained the ΔΨm values and counteracted the activation of the mitochondria-dependent apoptotic pathway. Consequently, we next investigated the mechanisms by which ΔΨm was maintained by evaluating the mRNA levels of a series of Bcl-2 family genes upstream of ΔΨm regulation (Danial and Korsmeyer, 2004). As shown in Figure 3B, a series of Bcl-2 family genes upstream of ΔΨm regulation was evaluated. The mRNA levels of Bcl-2 anti-apoptotic genes decreased after H2O2 exposure, whereas the Bax-like and BH3-only pro-apoptotic-related genes increased after H2O2 exposure. Visfatin pretreatment selectively elevated the levels of Bcl-2 anti-apoptotic genes, namely, Bcl-2 and Bcl-xl, whereas pretreatment with visfatin preserved the levels of the Bax-like pro-apoptotic-related genes Bax and Mcl-1, as well as the BH3-only pro-apoptotic-related gene Bik. Subsequently, the protein levels of Bad, p-Bad (phospho-Bad), Bid, tBid (truncated Bid) were evaluated. Our Western blot analysis showed that 4 h H2O2 exposure caused an increase in Bad and tBid (cleaved form of Bid). Bad dephosphorylation is also observed here, at 4 h stimulation with H2O2 (Fig. 3C). However, visfatin pretreatment showed no preservation in all these events. Meanwhile, the protein levels of Bid were not significantly changed upon H2O2 and/or visfatin exposure.
The AMPK signaling pathway was involved in the anti-apoptotic effects of visfatin
Several kinase inhibitors were used to determine the pathway by which visfatin prevented apoptosis. H9c2 cells were exposed to H2O2 for 4 h. As shown in Figure 4A, compound C (an AMPK-specific inhibitor) suppressed the visfatin-induced maintenance of cell viability, whereas LY294002 (a PI3K-specific inhibitor) and PD98059 (an ERK-specific inhibitor) had little or no influence on this event. Visfatin pretreatment also caused elevated protein levels of phosphorylated AMPK in a time-dependent manner (Fig. 4B). AMPK inhibition abrogated the anti-apoptotic effects of visfatin, as characterized by increased caspase-3 activation and DNA fragmentation (Fig. 4C,D). These results demonstrated the intimate involvement of the AMPK signaling pathway in the anti-apoptotic effects of visfatin.
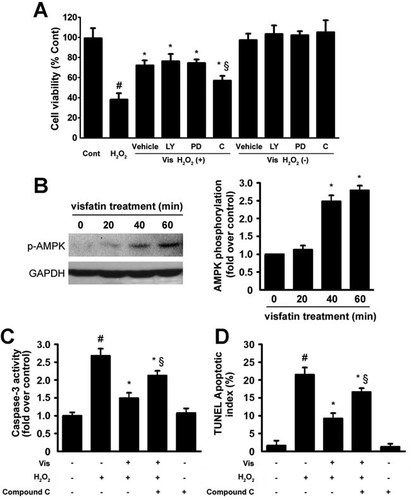
The AMPK signaling pathway was involved in the anti-apoptotic effects of visfatin. A: The effects of the pharmacological inhibitors LY294002 (a PI3K-specific inhibitor), PD98059 (an ERK1/2-specific inhibitor), and compound C (an AMPK-specific inhibitor) on cell viability were determined. #P < 0.05 versus control; *P < 0.05 versus H2O2-treated cells; §P < 0.05 versus H2O2-treated cells in presence with visfatin. B: H9c2 cells were pretreated with visfatin for different durations. The protein levels of phospho-AMPK (p-AMPK) were determined by Western blot analysis. *P < 0.05 versus 0 min visfatin pretreatment. C and D: Effects of compound C on caspase-3 activity (C) and the TUNEL apoptotic index (D). The results are presented as the mean ± SE (n = 8 per group). #P < 0.05 versus control; *P < 0.05 versus H2O2-treated cells; §P < 0.05 versus H2O2-treated cells in presence with visfatin.
Discussion
The link between circulating adipocytokine levels and the development of CVD has gained increased attention in recent years (Curtis et al., 2005; Shibata et al., 2005; Matsuzawa, 2006; Poirier et al., 2006; Smith et al., 2006; Simpkin et al., 2007). Studies have shown that the adipocytokines adiponectin (Shibata et al., 2005), apelin (Simpkin et al., 2007), and leptin (Smith et al., 2006) exert direct cardiprotective effect on cardiomyocytes. Visfatin, a newly discovered adipocytokine, has been shown to possess anti-diabetic (Fukuhara et al., 2005), anti-tumor (Khan et al., 2006), and pro-inflammatory properties (Moschen et al., 2007). However, the pathophysiological role it may share with other adipocytokines is unknown. An excellent recent study has suggested that the administration of visfatin dramatically reduces the myocardial infarct size using an in vivo infarction model in mice (Lim et al., 2008). However, the direct effect of visfatin on cardiomyocyte apoptosis and the intracellular mechanisms involved are not yet fully understood. The pivotal findings of the present study were as follows. (1) We demonstrated for the first time that pretreatment with the novel adipocytokine visfatin, specifically, short-term (1 h) exposure of H9c2 cells to visfatin, dramatically reduced cardiomyocyte apoptosis. (2) Visfatin protected against cardiomyocyte apoptosis via a direct cellular effect, as evidenced by improved cell viability as well as reduced cell death, DNA fragmentation, and caspase-3 activation. (3) Visfatin maintained the mitochondrial membrane potential (ΔΨm) levels and counteracted the activation of the mitochondria-dependent apoptotic pathway. (4) Most importantly, the cardioprotective effect depended on the activation of the pro-survival kinase AMPK.
Cardiomyocyte apoptosis, a well-established component of cardiac remodeling and heart failure, is a common cause of various CVDs (Fujita and Ishikawa, 2011). H9c2 cells combined with the use of H2O2 to induce apoptosis are widely used as an in vitro model to study the regulation of cardiomyocyte apoptosis (von Harsdorf et al., 1999). Using this model, we investigated the effects of short-term (1 h) and long-term (24 h) exposure of H9c2 cells to visfatin on H2O2-induced cardiomyocyte apoptosis. Pretreatment with visfatin for 1 h effectively protected cardiomyocytes from H2O2-induced apoptosis. On the other hand, pretreatment with visfatin for 24 h showed no obvious effect on this event. This finding was not entirely without precedent because a recent study has shown that acute and chronic adipocytokines treatments exert distinct effects (Eguchi et al., 2008). These results suggested that transient intracellular effects stimulated by acute visfatin treatment played an important role in the cardioprotective role of visfatin, which may be closely correlated with the time-dependent effect on AMPK activation. Previous studies have shown that visfatin activates downstream signaling pathways, including PI3K/Akt, ERK1/2, and AMPK of the reperfusion injury salvage kinase pathway, which are commonly linked to cardioprotection (Shibata et al., 2005; Smith et al., 2006; Simpkin et al., 2007). Using specific inhibitors of ERK1/2 MAPK, PI3K, and AMPK, we found that only AMPK inhibition caused a decrease in cell death when pretreated with visfatin in the present model. Based on the data showing the time-dependent effect of AMPK activation upon short-term (1 h) exposure, we proposed that visfatin may play a direct cardioprotective role via the AMPK signaling pathway. This result was in accordance with later data showing that the inhibition of AMPK neutralizes the visfatin-induced reduction in caspase-3 activation and DNA fragmentation (Fig. 4). These results demonstrated the intimate involvement of the AMPK signaling pathway in the anti-apoptotic effects of visfatin. The decline in AMPK activation upon 1 h to 24 h of exposure to visfatin (data not shown) may partially explain why long-term (24 h) visfatin exposure caused no obvious cardioprotective effects. The effects observed after a short period of visfatin exposure may have physiological relevance because circulating visfatin levels fluctuate with the diurnal rhythm and are not consistently high for 24 h (Froy, 2010; Benedict et al., 2012).
The inactivation of several apoptotic markers, including PS exposure at an early stage of apoptosis, DNA fragmentation and caspase-3 activation at a late stage of apoptosis, suggested that visfatin pretreatment effectively antagonized H2O2-induced apoptotic damage. A few PI-positively stained cardiomyocytes indicated that 4 h of treatment with H2O2 did not induce significant necrosis in cardiomyocytes. Our results suggested that visfatin did not act via the death receptor-dependent apoptotic pathway, as confirmed by the unchanged mRNA levels of Fas and TNFR1, the two best-characterized death receptor. We observed that the activation of the mitochondria-dependent or intrinsic apoptotic pathway induced by H2O2 exposure was significantly attenuated by visfatin pretreatment. Notably, the mRNA level of p53 was down-regulated by visfatin exposure, whereas no significant change was observed in the mRNA levels of Fas and TNFR1. Our findings also demonstrated that a series of Bcl-2 family proteins upstream of ΔΨm regulation (Danial and Korsmeyer, 2004) were tightly regulated by visfatin. Further studies indicated that visfatin pretreatment regulated the mRNA levels of some Bcl-2 family genes, including Bcl-2 anti-apoptotic genes, Bax-like pro-apoptotic-related genes, and BH3-only pro-apoptotic-related genes. Based on the data in the present study, the effect of visfatin on the expression of Bcl-2 family genes was selective. Although the underlying molecular mechanisms associated with this effect need to be determined, this visfatin-induced expression profiling alteration in Bcl-2 family genes may have a direct influence on mitochondrial membrane pore formation, thereby leading to the activation of the mitochondria-dependent apoptotic pathway. The regulation of Bcl-2 family genes may be directly transactivated by p53, a sensor of cellular stress that initiates intrinsic apoptosis at the transcriptional level (Danial and Korsmeyer, 2004).
In summary, the present study revealed the protective role of visfatin against H2O2-induced cardiomyocytes apoptosis. This protective role was possibly realized via the inhibition of p53-mediated, mitochondria-dependent intrinsic apoptotic signaling and via involvement in the AMPK signaling pathway. Recent studies have shown that increased circulating adipocytokines secreted from visceral fat may confer cardioprotective effects, that is, the so called “obesity paradox.” Therefore, the findings of the present study elucidated the pathophysiological role of the novel adipokine visfatin in cardioprotection, which may be the missing link between obesity and CVD.




