Thrombin induces slug-mediated E-cadherin transcriptional repression and the parallel Up-regulation of N-cadherin by a transcription-independent mechanism in RPE cells†
Conflicts of interest: none to declare.
Abstract
The proliferation, directional migration to the vitreous and epithelial–mesenchymal transition (EMT) of quiescent, differentiated retinal pigment epithelium (RPE) cells is a major feature in the development of proliferative vitreoretinopathy (PVR) following exposure of the immuno-privileged eye niche to serum components, thrombin among them. We have previously documented thrombin induction of RPE cell proliferation and migration. We here analyzed the effect of thrombin on the E/N cadherin switch, a hallmark of EMT. Results show that thrombin induces the specific repression of epithelial E-cadherin gene transcription, alongside with the up-regulation of mesenchymal N-cadherin protein in RPE cells. We demonstrate, for the first time, that thrombin induces E-cadherin repression by stimulating snail-2 (SLUG) transcription factor expression, and the concomitant up-regulation of N-cadherin through the transcription-independent increase in protein translation promoted by PI3K/PKC-ζ/mTOR signaling. Our present findings suggest that the activation of protease-activated receptor-1 (PAR-1) by thrombin induces EMT of RPE cells, further supporting a central role for thrombin in PVR pathogenesis. J. Cell. Physiol. 228: 581–589, 2013. © 2012 Wiley Periodicals, Inc.
The retinal pigment epithelium (RPE), a monolayer of differentiated, quiescent cells located between the neural retina and the choroid, plays a central role in the maintenance of the functional and structural integrity of the neural retina (Mund and Rodrigues, 1979). In addition to its function as the main component of the blood–retina barrier (BRB), the RPE is involved in the trans-epithelial transport of nutriments, the storage and metabolism of vitamin A derivatives, and the renewal of photoreceptor outer segments (Mund and Rodrigues, 1979).
RPE cells remain quiescent through adult life; however, under distinct pathological conditions, RPE cell proliferation results in the development of proliferative eye diseases leading to blindness, such as PVR (Campochiaro, 1997). A hallmark feature in PVR pathogenesis is the development of epiretinal membranes on both retinal surfaces as a consequence of (1) RPE proliferation, (2) RPE cell migration into the vitreous, and (3) the trans-differentiation of RPE cells into mesenchymal/fibroblastic-like cells expressing high content of actin stress fibers, a well-characterized physiological process known as epithelial–mesenchymal transition (EMT; Campochiaro, 1997).
EMT plays a relevant physiological role in gastrulation during embryonic development of vertebrates, whereas in differentiated tissue EMT is related to the onset of diseases involving the hyperproliferation of epithelial cells, including fibrosis and cancer development (Acloque et al., 2009).
At the biochemical level, signaling pathways that control EMT converge on the regulation of E-cadherin, the prototypic epithelial adhesion molecule in adherens junctions (AJs). EMT involves the loss of expression of epithelial membrane proteins/markers such as E-cadherin (Cadherin-1; CDH1), and the up-regulation of N-cadherin (Cadherin-2; CDH2), a mesenchymal membrane-associated protein, together with α-smooth muscle actin (α-SMA) expression and cytoskeleton remodeling (Kalluri and Weinberg, 2009; Zeisberg and Neilson, 2009).
Classic cadherins are single pass transmembrane proteins, which mediate Ca2+-dependent adhesive binding through five cadherin-type repeats within the extra-cellular domain. Cadherin cytoplasmic region mediates interaction with the actin cytoskeleton through the binding to p120-catenin and β-catenin. The classic cadherin subfamily includes E-, N-, P-, and R-cadherins, which are essential components of intercellular AJs (Oda and Takeichi, 2011). Cadherin-based junctions in cells are involved in a diversity of biological processes including the regulation of epithelial and endothelial cell junctions which control the passage of water and solutes (Venkiteswaran et al., 2002) and the maintenance of stable tissue organization, while allowing the turnover of rapidly growing tissues like epidermis (Tinkle et al., 2008). Additionally, cadherins are also involved in synapse formation and play an important role in the control of synaptic dynamics (Takeichi and Abe, 2005).
Several studies have demonstrated the participation of transcriptional repressors of the SNAIL family in EMT and plasticity in embryonic development (Nieto, 2002). First described in Drosophila melanogaster, the SNAIL homologues in vertebrates (Snail-1:snail; Snail-2:slug and Snail-3:smuc) encode transcription factors of the zinc-finger type, and act as transcriptional repressors by binding to E-box motifs, a core of six bases (CANNTG) on target gene promoters (Inukai et al., 1999; Nieto, 2002). Direct targets of the snail family repressors are proteins involved in the maintenance of the epithelial phenotype including E-cadherin, claudin-1, occludin, desmoplakin, and the cytokeratin genes (Cano et al., 2000; Bolos et al., 2003; Tripathi et al., 2005; Martinez-Estrada et al., 2006).
Snail-driven EMT results in the transformation of epithelial cells into mesenchymal cells, which display migratory properties contributing to organogenesis in the embryo; however, in the adult stage, snail is known to promote the acquisition of invasive properties in epithelial tumors (Peinado et al., 2007; Nieto, 2011). Snail and Slug over-expression has been described in leukemias (Mancini et al., 2010), esophageal carcinomas (Jethwa et al., 2008) and in breast cancer (Storci et al., 2008). In contrast, no involvement in cancer development has been attributed so far to the snail homolog smuc.
We have demonstrated previously that in vitro treatment of RPE cells with α-thrombin induces cell proliferation through the joint activation of the protein kinase C (PKC)- and the mitogen-activated protein kinase (MAPK) pathways (Palma-Nicolas et al., 2008), as well as the acquisition of cell motility, through the activation of nuclear factor kappa B (NF-κB) and the consequent expression of MCP-1 and CINC-1/GRO chemokines (Palma-Nicolas et al., 2010). More recently, we have also shown that thrombin induces actin stress fiber assembly in RPE cells (Ruiz-Loredo et al., 2011), thus suggesting the involvement of thrombin in RPE epithelial–mesenchymal transformation.
Thrombin actions are mediated by a small family of G protein-coupled receptors (GPCR) named protease-activated receptors (PARs), activated by the proteolytic cleavage of the N-terminal receptor sequence which unmasks a new amino acid sequence that functions as a tethered ligand (Macfarlane et al., 2001; Gandhi et al., 2011).
Four members of the PAR family have been described: PAR-1, PAR-3, and PAR-4, activated by thrombin, and PAR-2 activated by trypsin, tryptase and other trypsin-like proteases. PAR-1, the prototype of this family, triggers intracellular signaling through Gq, Gi/s, and G12/13 (Macfarlane et al., 2001; Soh et al., 2010). Particularly within the retina, PAR-1 activation by thrombin stimulates glial cell proliferation as well as actin cytoskeletal changes and the proliferation in RPE cells (Ruiz-Loredo et al., 2011).
Since E/N cadherin switch is known as the hallmark of epithelial cells ongoing EMT, in the present study we investigated the effect of α-thrombin on the expression of snail-related genes and their possible involvement in cadherin gene modulation in RPE cells. Our results show that α-thrombin induces the transcriptional repression of the epithelial marker E-cadherin through the promotion of snail-2 (SLUG) gene/protein expression, and the transcription-independent up-regulation of the mesenchymal marker N-cadherin by a PI3K/PKC-ζ/mTOR-dependent signaling pathway. These data provide additional support to a role for thrombin in EMT linked to the pathogenesis of proliferative eye diseases such as PVR.
Materials and Methods
Reagents
All reagents used were cell culture grade. The PAR-1 peptide agonist (Ser-Phe-Leu-Leu-Arg-Asn-Pro-Asn-Asp-Lys-Tyr-Glu-Pro-Phe), thrombin, hirudin, and the inhibitory PKC-ζ pseudosubstrate (Myr-Ser-Ile-Tyr-Arg-Arg-Gly-Ala-Arg-Arg-Trp-Arg-Lys-Leu) were obtained from Calbiochem (San Diego, CA). The PI3K inhibitor Wortmannin was from Sigma (St. Louis, MO). MEK inhibitor U0126, PLC-β inhibitor U73122, and the PAR-1 non-peptide antagonist SCH 79797 were purchased from Tocris (Ellisville, MO). The thrombin inhibitor PPACK (D-Phenylalanyl-L-prolyl-L-arginine chloromethyl ketone) was obtained from Enzo Life Sciences (Farmingdale, NY). PAR-3 (Ser-Phe-Asn-Gly-Gly-Pro) and PAR-4 (Gly-Tyr-Pro-Gly-Lys-Phe) peptide agonists were obtained from Bachem (Torrance, CA). Lipofectamine RNAiMAX and Opti-MEM were from Invitrogen (Carlsbad, CA). Serum-free Opti-MEM was used as the standard medium for all assays.
Long-Evans rat RPE cell culture
RPE cells were isolated as previously described (Pacheco-Dominguez et al., 2008). Briefly, 8- to 10-day-old Long-Evans rats were sacrificed following the animal care and use guidelines established by our institution. The eyes were enucleated, rinsed in Dulbecco's modified Eagle's medium (Gibco BRL, Grand Island, NY) containing penicillin (100 U/ml) and streptomycin (100 µg/ml), and incubated for 30 min at 37°C in the presence of dispase (2% v/v). After removal of the sclera and the choroid, the RPE was detached from the neural retina in calcium- and magnesium-free Hanks' balanced salt solution (HBSS), and incubated in the presence of trypsin (0.1%) for 5 min at 37°C. Trypsin digestion was stopped by 1:1 dilution with Opti-MEM containing 4% fetal bovine serum (FBS). The dissociated cells were suspended in Opti-MEM containing 4% FBS, and seeded at a density of 50,000 cells/cm2 in six-well format culture plates. Cell viability (>90%) was assessed by Trypan-blue exclusion (Suppl. Fig. 1).
RT-PCR analysis of Snail/Cadherin gene expression
RPE cells were grown at confluence in six-well plates (Costar, Corning, Inc., Washington D.C) in Opti-MEM containing 4% FBS. After serum deprivation for 24 h, cells were stimulated with 2 U/ml thrombin in serum-free Opti-MEM. Negative control (basal) wells were kept in serum-free Opti-MEM. After stimulation with thrombin for 24–96 h, or with PAR agonists (25 µM) for 72 h, cells were harvested and total RNA extraction was carried out using the TRIzol reagent (Sigma). cDNA was synthesized from 3 µg of total RNA using Molony murine leukemia virus reverse transcriptase (MMLV-RT) and oligo dT12-18 primer, following the recommendations of the supplier (Invitrogen). Three microliter of the reverse transcriptase reaction were amplified in a final volume of 20 µl including 1.5 mM MgCl2, 300 µM dNTPs, 0.5 µM of each primer, 1.25 U of Taq DNA polymerase, and the corresponding buffer. The sequences for the primers used are listed in Table 1. The thermocycler (Master Cycler Personal; Eppendorf, Hamburg, Germany) program consisted of an initial step at 94°C for 5 min, 28 cycles (each consisting of a denaturation step at 94°C for 30 sec, an annealing step at 54°C for 30 sec, an extension step at 72°C for 1 min 45 sec), and a final extension step at 72°C for 5 min. PCR products were resolved by agarose gel electrophoresis (0.8%), and snail or cadherin gene expression relative to HPRT housekeeping gene (hypoxanthine-guanine phosphoribosyltransferase) was quantified by optical densitometry (Fluor-S, MultiImager System, and Quantity One Software; Biorad, Hercules, CA).
| Gene | Primer sequence | Product (pb) |
|---|---|---|
| Snail-1 (snail) | 5′-ATGCCGCGCTCCTTCCT-3′ | 765 |
| 5′-TTGGTGTTTGTGGAGCAA-3′ | ||
| Snail-2 (slug) | 5′-ATGCCGCGCTCCTTCCT-3′ | 788 |
| 5′-CCAGACTCCTCATGTTTAT-3′ | ||
| Snail-3 (smuc) | 5′-ATGCCGCGCTCCTTCCT-3′ | 847 |
| 5′-AGCCGGCATCCTCGTGCCTCAC-3′ | ||
| E-cadherin | 5′-CAAGCAGCAGTACATTCTGCA-3′ | 1291 |
| 5′-ATACATGTCAGCCAGCTTCTTGAA-3′ | ||
| N-cadherin | 5′-TGAAAATCATTCGCCAAGAGG-3′ | 1202 |
| 5′-ATACATGTCAGCCAGCTTCTTGAA-3′ | ||
| P-cadherin | 5′-GGGAACTGGTCTGTGTCTATA-3′ | 1106 |
| 5′-ATACATGTCAGCCAGCTTCTTGAA-3′ | ||
| R-cadherin | 5′-AGACAATGCATATGAGGCCAC-3′ | 1000 |
| 5′-ATACATGTCAGCCAGCTTCTTGAA-3′ | ||
| HPRT | 5′-AGCGTCGTGATTAGTGATGATG-3′ | 580 |
| 5′-TGAAGTACTCATTATAGTCAAG-3′ |
Western blot assays
RPE cells from confluent six-well plates were serum-deprived for 24 h and washed three times with RPE-Krebs Ringer bicarbonate buffer. Cultures were then incubated in thrombin-supplemented serum-free Opti-MEM (2 U/ml). At indicated times, cells were washed twice with Krebs Ringer bicarbonate buffer, and disrupted in lysis buffer containing 50 mM Tris–HCl pH 7.4, 150 mM NaCl, 10 mM EDTA, 0.1% SDS, 1%, Triton X-100, 1% CHAPS, 0.5% NP40, 0.1% BSA, 40 mM β-glycerophosphate, 10 mM sodium pyrophosphate, and a protease inhibitor cocktail (10%; Sigma). Proteins in total cell lysates (30 µg) were resolved by 10% SDS–PAGE and electro-transferred to PVDF membranes. When indicated, cells were stimulated with PAR-1 and PAR-3 agonists (25 µM) for 72h. The specific thrombin inhibitors hirudin (4 U/ml) and PPACK (10 µM) were pre-incubated with thrombin for 15 min prior to cell stimulation. Pseudosubstrate (PS) PKC-ζ inhibitor (2.5–10 µM), U0126 (20 µM), U73122 (5 µM), or Wortmannin (2 µM) were used to identify signaling pathways by pre-incubating RPE cells for 45 min at 37°C; then, the inhibitors were washed-out and the cells were stimulated with thrombin (2 U/ml in serum-free Opti-MEM) for 72 h. Cicloheximide (CHX, 3.6 or 7.2 µM), actinomycin D (ActD, 0.5 or 1 µM), and rapamycin (100–300 nM) were included through the last 18 h in standard 72 h thrombin-stimulated assays.
After blocking for 60 min at room temperature (25°C) with 4% non-fat milk, the PVDF membranes were probed overnight at 4°C with the following primary antibodies: 1:200 rabbit anti-SLUG (0.2 mg/ml), 1:2,000 rabbit anti N-cadherin (1 mg/ml), Abcam (Cambridge, MA); 1:2,000 mouse anti E-cadherin (0.2 mg/ml), Santa Cruz Biotechnology (Santa Cruz, CA); and 1:10,000 mouse anti-actin (clone C4), Chemicon (Temecula, CA). Secondary HRP-conjugated antibodies, raised in the corresponding species, were used at 1:2,000 dilutions, except for actin (1:10,000) and developed using the Immobilon Western AP Chemiluminescent Substrate (Millipore, Billerica, MA). Kodak® film images were digitized using an Alpha Digi-Doc system (Alpha-Innotech, San Leandro, CA), and densitometric analysis was performed using the Quantity One Software v4.6 from Biorad.
SiRNA silencing
Predesigned MISSION siRNAs against SLUG (SASI_Rn01_00108597) and E-cadherin (SASI_Rn01_00046548) were acquired from Sigma. Primary RPE cells were seeded in six-well culture plates at 3 × 105 cells/well density, in 4%-FBS supplemented OptiMEM. Twenty-four hours after plating, RPE cells were transfected with small-interfering RNA duplexes (siRNAs) for slug (150 pmol), and E-cadherin (100 pmol) in antibiotic-free 4%-FBS Opti-MEM. Lipofectamine–siRNA complexes were maintained through 48 h and RPE monolayers were rinsed in serum-free Opti-MEM during 24 h prior to thrombin stimulation. Sixty-five to 75% silencing of the targets by the corresponding siRNA was obtained in our conditions.
Statistical analysis
Raw data for analysis were obtained from pooled RPE cells of 10–12 Long-Evans rats in three independent experiments. One-way ANOVA and Bonferroni post hoc test were applied for statistical analysis, using the Prism v4.0 program from Graph Pad (La Joya, CA). Statistical significance was depicted in graphs as follows: *P < 0.05, **P < 0.01, ***P < 0.001.
Results
Thrombin induces the expression of Snail-2 (SLUG) through PAR-1 activation
To determine if thrombin stimulates the expression of snail-related genes in Rat RPE cells, the time course for snail1 (snail), snail2 (slug), and snail3 (smuc) mRNA expression was quantified by using the primers listed in Table 1. Primer design was based on the GenBank reported sequences for Rat Snail-1 (NM_053805.1), Snail-2 (NM_013035.1), and Snail-3 (NM_001107439.1). mRNA levels were quantified following 24- to 96-h exposure to 2 U/ml thrombin. Figure 1a shows that RPE cells do not express snail or smuc, even after 96 h of thrombin stimulation. In contrast, we detected slug expression, which was up-regulated in a time-dependent manner by thrombin. No changes in snail (1–3) gene expression were observed at thrombin stimulation at early time points (2, 4, and 8 h; data not shown). Consistent with this result, the level of the SLUG protein, as assessed by Western blot, was also increased by thrombin stimulation (Fig. 1b). Maximal SLUG expression was observed at 72-h post-stimulation.
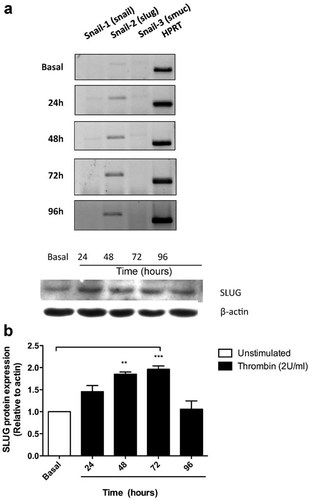
Selective expression of snail-2 (SLUG) induced by thrombin in RPE Cells. Confluent monolayers of RPE cells were serum-starved for 24h, prior to stimulation with thrombin (2 U/ml). a: Cells were harvested in Trizol for RNA isolation at the indicated time points and the expression of mRNA for snail-1 (snail), snail-2 (slug) and snail-3 (smuc) was quantified by RT-PCR, as described in Materials and Methods Section. b: Cell lysates were obtained in Laemmli buffer and total protein was resolved by 10% SDS–PAGE. After blotting, PVDF membranes were probed with: 1:200 rabbit anti-SLUG (0.2 mg/ml) and 1:10,000 mouse anti-actin. Gels show a representative experiment from three independent determinations. HPRT or β-actin housekeeping gene content was used for normalizing. Basal (white bars) corresponds to unstimulated cultures maintained in serum-free Opti-MEM. Results are expressed as the mean ± SEM of three independent experiments; one-way ANOVA plus Bonferroni post hoc test was depicted as: *P < 0.05, **P < 0.01, ***P < 0.001.
Thrombin signaling in Rat RPE cells is mediated by the cleavage/activation of PAR-1 and PAR-3 PARs (Parrales et al., 2010). In order to identify the PAR involved in SLUG expression, RPE cells were stimulated with 25 µM of thrombin-receptor agonist peptides (TRAPs) for PAR-1 (SFLLRNPNDKYEPF) and PAR-3 (SFNGGP) for 72 h. Whereas PAR-3 agonist had no effect, the PAR-1 agonist was able to induce the expression of SLUG repressor at comparable levels as thrombin, indicating that although thrombin activates PAR-1 and PAR-3, PAR-1 cleavage is sufficient for the induction of SLUG expression (Fig. 2).
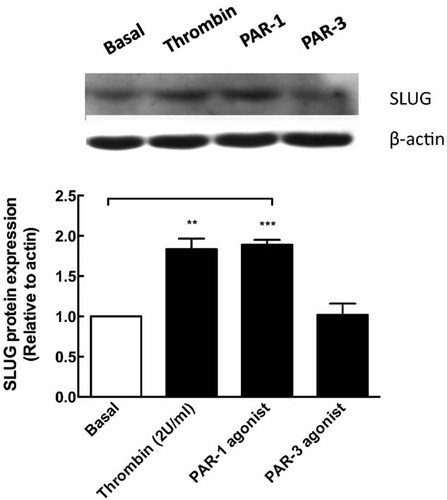
Thrombin induces SLUG through PAR-1 activation in RPE Cells. Confluent RPE cultures were serum-deprived for 24 h prior to stimulation with thrombin (2 U/ml) or with thrombin receptor agonists (TRAPs) for PAR-1 and PAR-3 at 25 µM, during 72 h. Total protein from cell lysates was resolved by 10% SDS–PAGE, blotted onto PVDF membranes and probed with: 1:200 rabbit anti-SLUG (0.2 mg/ml) and 1:10,000 mouse anti-actin. Gel shows a representative experiment from three independent determinations. β-actin housekeeping gene content was used for normalizing. Basal (white bars) corresponds to unstimulated cultures maintained in serum-free Opti-MEM. Results are expressed as the mean ± SEM of three independent experiments; one-way ANOVA plus Bonferroni post hoc test was depicted as: *P < 0.05, **P < 0.01, ***P < 0.001.
Thrombin induces E-Cadherin down-regulation in RPE cells
Primers in Table 1 were designed for quantifying the expression level of classic cadherin genes Cdh-1 (Cadherin E), Cdh-2 (Cadherin N), Cdh-3 (Cadherin P), and Cdh-4 (Cadherin R). Design was based on the GenBank reported sequences for Rat cadherins E- (NM_031334.1), N- (NM_031333.1), P- (NM_053938.1), and R- (D86742.1/XM_001061943.2). We first assessed the expression of E-, N-, P-, and R- cadherins in control, non-stimulated confluent RPE monolayers. We found that while mRNA for E-, N-, and P-cadherins is clearly expressed, R-cadherin mRNA expression was barely detectable (Fig. 3a); hence, further analysis of R-cadherin was discarded.
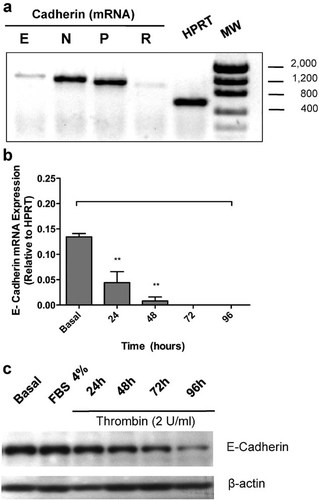
Thrombin induces the transcriptional repression of E-cadherin. a: Confluent RPE cell cultures were harvested in Trizol for RNA isolation and the expression of mRNA for E, N, P, and R-Cadherin was quantified by RT-PCR, as described in Materials and Methods Section. Gel shows representative data for cadherin gene expression in control Rat RPE cells. b: Confluent RPE cultures were serum-deprived for 24 h prior to thrombin (2 U/ml) stimulation for 24–96 h in serum-free Opti-MEM. Cells were harvested in Trizol for mRNA extraction and E-cadherin gene expression was quantified by RT-PCR at the indicated time-points. Data were normalized relative to HPRT mRNA expression. c: Laemmli cell lysates were obtained at indicated time-points of thrombin stimulation (2 U/ml), and total protein was resolved by 10% SDS–PAGE. After blotting, PVDF membranes were probed with: 1:2,000 mouse anti E-cadherin (0.2 mg/ml), and 1:10,000 mouse anti-actin. Basal corresponds to unstimulated cultures maintained in serum-free Opti-MEM. Gel shows a representative experiment from three independent determinations. β-actin housekeeping gene expression was used for data normalization. Results are expressed as the mean ± SEM of three independent experiments; one-way ANOVA plus Bonferroni post hoc test was depicted as: *P < 0.05, **P < 0.01, ***P < 0.001.
Since Snail family of transcriptional repressors has been linked to the down-regulation of epithelial cell markers such as E-cadherin, claudin-1, occludin, desmoplakin, etc. (Cano et al., 2000; Bolos et al., 2003; Ohkubo and Ozawa, 2004; Tripathi et al., 2005; Martinez-Estrada et al., 2006), we investigated the involvement of the thrombin-induced repressor SLUG on epithelial E-cadherin gene expression. We analyzed E-cadherin mRNA level in thrombin-stimulated (2 U/ml) RPE cell monolayers at 24- to 96-h post-stimulation, using RT-PCR. Results in Figure 3b show that thrombin induces the transcriptional shutdown of E-cadherin gene in a time-dependent manner, with maximal repression observed at 72-h post-stimulation, consistent with the alongside up-regulation of the SLUG repressor (Suppl. Fig. 2). Expression of the E-cadherin protein was decreased in parallel to the cognate gene repression, and was undetectable after 96 h of thrombin treatment, as assessed by Western blot (Fig. 3c). FBS (4%) was included as a control for culture medium-contained growth factors.
E-cadherin repression is specific and requires the activation of PAR1
E-cadherin down-regulation by thrombin in RPE cells was shown to be specific, since thrombin-mediated repression was prevented by the inclusion of the thrombin inhibitors hirudin (4 U/ml) and PPACK (10 µM; Fig. 4a).
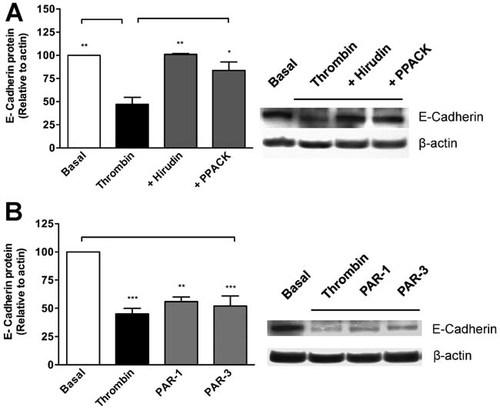
E-cadherin repression requires the activation of PAR-1. Confluent RPE cultures were serum-deprived for 24 h prior to thrombin (2 U/ml) stimulation for 72 h. In (a) thrombin inhibitors hirudin (4 U/ml) or PPACK (10 µM) were included jointly with thrombin. In (b) PAR-1 and PAR-3 agonists (25 µM) were included in serum-free Opti-MEM. Cell lysates were obtained in Laemmli buffer, and total protein was resolved by 10% SDS–PAGE, blotted onto PVDF membranes and probed with: 1:2,000 mouse anti E-cadherin (0.2 mg/ml), and 1:10,000 mouse anti-actin. Gels show a representative experiment from three independent determinations. β-actin gene expression was used for normalization. Basal (white bars) corresponds to unstimulated cultures maintained in serum-free Opti-MEM. Results are expressed as the mean ± SEM of three independent experiments; one-way ANOVA plus Bonferroni post hoc test was depicted as: *P < 0.05, **P < 0.01, ***P < 0.001.
Since thrombin mediates the proteolytic activation of PAR-1 and PAR-3, both expressed by RPE cells, we used specific peptide agonists in order to identify the receptor subtype involved in E-cadherin repression. Surprisingly, peptide agonists for PAR-1 and PAR-3 (25 µM), both induced E-cadherin repression at the same level as thrombin (Fig. 4b), however, the simultaneous inclusion of both agonists was found non-additive (not shown). This result adds to existence of controversy regarding the effect of synthetic peptides modeled on the thrombin-revealed sequence of PAR3 (see Hollenberg and Compton, 2002). Considering that E-cadherin repression is due to thrombin induction of SLUG, and only the PAR-1 agonist increased the expression of the repressor SLUG (Fig. 2), we suggest that, in RPE cells PAR-3 could be involved in maintenance functions but dispensable for thrombin-mediated effect in EMT. Future studies would be necessary to address this question.
E-cadherin down-regulation is due to thrombin-induced SLUG expression
In order to support the involvement of SLUG in thrombin-mediated E-cadherin repression, RPE cells were transfected with siRNA SLUG (75% silencing). Figure 5a shows that siRNA SLUG transfection up-regulates the basal level of E-cadherin in control (non-stimulated) RPE cells, suggesting that the low levels of E-cadherin consistently found despite the epithelial origin of RPE cells, is maintained by the basal expression of the SLUG repressor. In contrast, the basal level of the mesenchymal N-cadherin adhesion molecule was not altered by SLUG knockdown, as expected (Hao et al., 2012). Importantly, slug siRNA transfection of RPE cells prevents thrombin-induced E-cadherin repression, demonstrating that SLUG mediates the repression of E-cadherin induced by thrombin activation of PAR-1 in RPE cells (Fig. 5b).
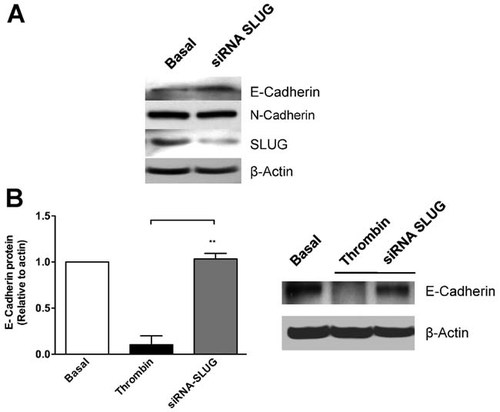
E-cadherin down-regulation is due to thrombin-induced SLUG expression. RPE cells were transfected with small-interfering RNA duplexes (siRNAs) for Slug (150 pmol) in antibiotic-free Opti-MEM during 48 h (75% silencing), and rinsed in serum-free Opti-MEM for additional 24 h. a: Laemmli cell lysates from unstimuated siRNA SLUG transfected RPE cultures were obtained, and total protein was resolved by 10% SDS–PAGE, blotted onto PVDF membranes and probed with: 1:200 rabbit anti-SLUG (0.2 mg/ml), 1:2,000 rabbit anti N-cadherin (1 mg/ml), 1:2,000 mouse anti E-cadherin (0.2 mg/ml), and 1:10,000 mouse anti-actin. b: siRNA SLUG transfected RPE cultures were stimulated with thrombin (2 U/ml) for 72 h and then assayed for SLUG immunoblot. Gels show a representative experiment from three independent determinations. Values were normalized relative to β-actin gene expression. Basal corresponds to unstimulated cultures maintained in serum-free Opti-MEM. Results are expressed as the mean ± SEM of three independent experiments; one-way ANOVA plus Bonferroni post hoc test was depicted as: *P < 0.05, **P < 0.01, ***P < 0.001.
Thrombin induces N-Cadherin up-regulation in RPE cells
Alongside E-cadherin repression, a hallmark of EMT is the up-regulation of the mesenchymal marker N-cadherin. We found that stimulation of RPE cells with thrombin (2 U/ml) induced a sustained increase in N-cadherin protein from 24 to 96 h, as assessed by Western blot (Fig. 6a). FBS (4%) was included as a control for culture medium-contained growth factors. Thrombin-induced N-cadherin protein up-regulation was shown to be specific, since this effect was prevented by hirudin (4 U/ml) or 10 µM PPACK (Fig. 6b).
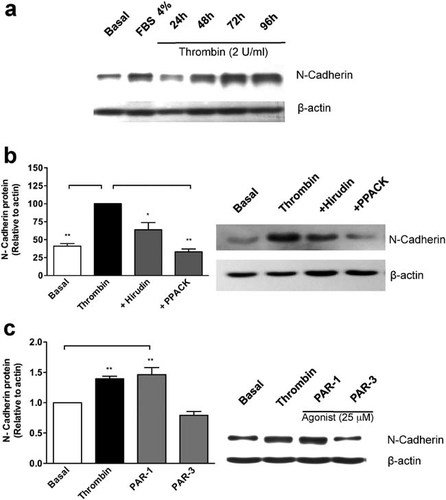
Thrombin induces N-cadherin up-regulation in RPE cells. a: Confluent RPE cultures were serum-deprived for 24 h prior to thrombin (2 U/ml) stimulation for 24–96 h in serum-free Opti-MEM. Laemmli cell lysates were obtained at indicated time-points, and total protein was resolved by 10% SDS–PAGE, blotted onto PVDF membranes and probed with: 1:2,000 rabbit anti N-cadherin (1 mg/ml), and 1:10,000 mouse anti-actin. b: Hirudin (4 U/ml) or PPACK (10 µM) were included together with thrombin (2 U/ml) for 72 h prior to N-cadherin immunoblotting. c: RPE cultures were stimulated with 25 µM PAR-1 and PAR-3 agonists (TRAPs) for 72 h prior to N-cadherin immunoblotting. Basal corresponds to unstimulated cultures maintained in serum-free Opti-MEM. Gel shows a representative experiment from three independent determinations and β-actin gene expression was used for immunoblot normalization. Results are expressed as the mean ± SEM of three independent experiments; one-way ANOVA plus Bonferroni post hoc test was depicted as: *P < 0.05, **P < 0.01, ***P < 0.001.
In order to identify the thrombin receptor involved in the up-regulation of N-cadherin, RPE cells were stimulated with the peptide agonists for PAR-1 and PAR-3. After 72 h of stimulation with 25 µM of each TRAP we found that PAR-1 peptide agonist but not PAR-3 was able to up-regulate N-cadherin protein at the same level as thrombin (2 U/ml; Fig. 6c). PAR-4 peptide agonist had no effect on N-cadherin protein (data not shown), as predicted by the absence of PAR-4 receptor in rat RPE cells (Parrales et al., 2010).
N-cadherin up-regulation by thrombin is transcription-independent
Transcriptional up-regulation of N-cadherin under the control of the transcription factor Twist has been demonstrated both, in normal human trophoblastic cells (Ng et al., 2012), and in colorectal- (Chang et al., 2011) and prostate-carcinoma cells (Alexander et al., 2006). Unexpectedly, thrombin failed to increase the expression of N-cadherin mRNA at the same time points at which the increase in N-cadherin protein was observed (Fig. 7a, filled bars), suggesting that N-cadherin up-regulation by thrombin in RPE cells is unrelated to gene transcription.
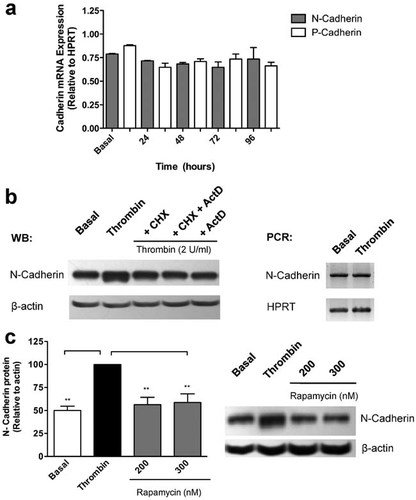
N-cadherin up-regulation by thrombin is transcription-independent. a: Confluent RPE cultures were serum-deprived for 24 h prior to thrombin (2 U/ml) stimulation for 24–96 h in serum-free Opti-MEM. Cells were harvested in Trizol for mRNA extraction and N-cadherin gene expression was quantified by RT-PCR at the indicated time-points. Data were normalized relative to HPRT mRNA expression. b: Twenty-four hours serum-deprived RPE cultures were stimulated with thrombin (2 U/ml) for 72 h. When indicated, CHX (3.6 µM) or ActD (0.5 µM) were included for the last 18 h. WB: Laemmli cell lysates were obtained, and total protein was resolved by 10% SDS–PAGE, blotted onto PVDF membranes and probed with: 1:2,000 rabbit anti N-cadherin (1 mg/ml), and 1:10,000 mouse anti-actin. PCR: Cells were harvested in Trizol for mRNA extraction and N-Cadherin gene expression was quantified by RT-PCR. HPRT expression was used for normalizing. c: mTOR inhibitor rapamycin was included for the last 18h of thrombin-stimulation period (72 h), prior to N-cadherin immunoblotting. Basal corresponds to unstimulated cultures maintained in serum-free Opti-MEM. Gel shows a representative experiment from three independent determinations. Data were normalized relative to β-actin gene expression. Results are expressed as the mean ± SEM of three independent experiments; one-way ANOVA plus Bonferroni post hoc test was depicted as: *P < 0.05, **P < 0.01, ***P < 0.001.
Despite successfully being used in human ARPE-19 RPE cell line (Vogt et al., 2006), the inclusion of the protein translation inhibitor Cycloheximide (CHX: 3.6 µM) or the transcription inhibitor ActD (0.5 µM) for the 72-h period of treatment required in our assays, induced high mortality in rat primary RPE cells (viability <50%; not shown). Due to this finding, the inhibitors were included only for the last 18 h of the 72 h thrombin-stimulation period (viability >90%; not shown). Figure 7b shows that inhibition of protein translation with CHX (3.6 µM) reverses thrombin-induced N-cadherin up-regulation to baseline level; however, the simultaneous inhibition of mRNA transcription with Act D (0.5 µM) did not display additive effect on the inhibition of protein translation, indicating that gene transcription is not required for N-cadherin up-regulation. The inclusion of Act D alone (0.5 µM) also reversed N-cadherin level to basal value, thus discarding any lack of drug effectiveness. As shown for N-cadherin, thrombin had no effect on P-cadherin mRNA gene expression (Fig. 7a, open bars). In contrast to N-cadherin, thrombin did not alter P-cadherin protein level in RPE cells (data not shown). Hence, further analysis of P-cadherin was discarded.
mTORC1 promotes N-Cadherin translation
In order to determine if transcription-independent N-cadherin up-regulation is achieved by an increase in the rate of protein translation, we analyzed a possible role for the mammalian target of rapamycin (mTOR), a major regulator of protein translation, in N-cadherin expression. mTOR forms the catalytic core of two distinct complexes: whereas mTOR association with RAPTOR (regulatory-associated protein of mTOR) and mLST8 gives rise to rapamycin-sensitive mTORC1, association with RICTOR (rapamycin insensitive companion of mTOR), Sin 1 and Protor forms mTORC2. mTORC1 has been involved in the control of protein translation, cell division and cell growth, and mTORC2 activity likely relates to the regulation of cell cytoskeleton (Wang and Proud, 2011). mTORC1 regulates translation at the stage of initiation (the rate-limiting step in protein synthesis), by phosphorylation of 4E-BP1 (eIF4E-binding protein-1) with the consequent release/activation of eIF4E (eukaryotic initiation factor 4E).
Since the transcription-independent up-regulation of N-cadherin requires de novo protein synthesis, we tested the possible involvement of mTORC1 in the translational rate of N-cadherin by including the mTORC1 inhibitor rapamycin (200 and 300 nM) for the last 18 of 72 h thrombin-stimulation period. As depicted in Figure 7c, rapamycin inclusion reverts the thrombin-induced N-cadherin up-regulation to basal levels, suggesting that thrombin increases the translational rate of N-cadherin by a transcription-independent mechanism mediated by mTORC1 activation.
N-cadherin up-regulation is mediated by PI3K/PKC-ζ signaling
Transcription-independent mechanisms driven by PAR-1-induced mTORC1 activation have been identified in thrombin-stimulated platelets (Pabla et al., 1999), and has been shown to require PI3K activation (Weyrich et al., 1998). In this line, our previous studies have determined that RPE cell proliferation induced by thrombin action on PAR-1, requires the activation of the atypical protein-kinase C-zeta (PKC-ζ), induced by PI3K (Palma-Nicolas et al., 2008). In an attempt to provide insight on the mechanism mediating thrombin-induced N-cadherin up-regulation, we analyzed key signaling pathways known to be activated by thrombin stimulation of PARs in rat RPE cells (Palma-Nicolas et al., 2008, 2010). Among these pathways, ERK 1/2 signaling cascade has been shown to promote protein synthesis through the regulation of mTORC1 (Carriere et al., 2011). The lack of effect of the MEK inhibitor U-0126 (20 µM) and of the PLC-β inhibitor U-73122 (5 µM) on thrombin-induced N-cadherin up-regulation, rules-out the involvement of ERK1/2 MAPK and PLC-β downstream signaling (data not shown). In contrast, we found a dose-dependent inhibition by the inclusion of the PKC-ζ PS (Fig. 8a), and Wortmannin (Fig. 8b), the inhibitor of the upstream PKC-ζ activator PI3K, demonstrating the requirement of atypical PI3K/PKC-ζ signaling in the transcription-independent up-regulation of the mesenchymal marker N-cadherin in RPE cells by thrombin.
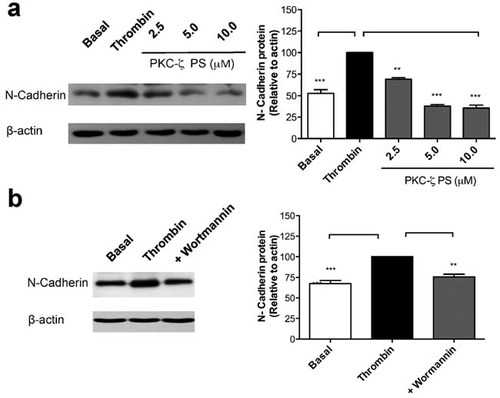
N-cadherin up-regulation is mediated by PI3K/PKC-ζ signaling. Confluent RPE monolayers were pre-treated for 45 min with (a) PKC-ζ pseudosubstrate (PS) inhibitor peptide (2.5–10 µM) or (b) the PI3K inhibitor, wortmannin (2 µM). Inhibitors were washed-out and RPE cultures were stimulated with thrombin (2 U/ml) for 72 h. Total protein was resolved by 10% SDS–PAGE as described blotted onto PVDF membranes and probed with: 1:2,000 rabbit anti N-cadherin (1 mg/ml), and 1:10,000 mouse anti-actin. Basal corresponds to unstimulated cultures maintained in serum-free Opti-MEM. Gel shows a representative experiment from three independent determinations. Data were normalized relative to β-actin gene expression. Results are expressed as the mean ± SEM of three independent experiments; one-way ANOVA plus Bonferroni post hoc test was depicted as: *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
EMT of RPE cells is a well-documented feature in the pathophysiology of PVR, an ocular disease leading to blindness after ocular traumatic injury or as a consequence of chirurgic intervention for primary retinal detachment or corrective laser ablation (Grisanti and Guidry, 1995; Campochiaro, 1997; Casaroli-Marano et al., 1999). Such ocular trauma involving the disruption of the BRB integrity frequently exposes the eye immunoprivileged environment to serum components, thrombin among them (Campochiaro, 1997). Our previous work showed that in vitro treatment of RPE cells with thrombin induces RPE cell proliferation and the acquisition of cell motility through a common, PKCζ-dependent signaling pathway triggered by PAR-1 activation (Palma-Nicolas et al., 2008, 2010). In order to further support a role for thrombin in PVR pathogenesis, in the present study, we analyzed the effect of thrombin on EMT and the involvement of the SNAIL family of transcriptional repressors in EMT. We demonstrated that exposure of RPE cells to thrombin induces the expression of Snail-2 (SLUG) through PAR-1 activation. Moreover, the induction of the transcription factor SLUG occurred alongside with E-cadherin down-regulation, consistent with the well-documented role of SLUG as a transcriptional repressor of epithelial markers during developmental or cancer-associated EMT (Nieto, 2002).
Characteristic morphologic/biochemical changes associated with EMT have been shown to occur in RPE cells upon exposure to serum-contained factors. Whereas thrombin activation of PAR-1 induces actin stress fiber assembly in rat RPE cells (Ruiz-Loredo et al., 2011), TGF-β treatment represses E-cadherin and Zonula occludens-1 (ZO-1) expression in ARPE-19 human RPE cell line (Li et al., 2011). Furthermore, increased snail-1 transcription factor expression in RPE cells ongoing EMT has been detected in patients suffering from choroidal neovascularization (CNV) secondary to age-related macular degeneration (AMD; Hirasawa et al., 2011).
E-cadherin expression in RPE is controversial. In contrast to the ubiquitous expression of the mesenchymal marker N-cadherin in cultured adult human RPE, E-cadherin expression was found restricted to patches of cells in the confluent cultures (Burke et al., 1999). Also on this line, epithelial P-cadherin has been reported to substitute for E-cadherin in adherent junctions (AJs) of porcine RPE cells; moreover, the expression of P-cadherin was found to be down-regulated upon treatment with TGF-β, a well-documented EMT-inducer (Tamiya et al., 2010). Similar repression of E-cadherin was found in ARPE-19 RPE human cell line following TGF-β treatment (Li et al., 2011). These data, together with recent work showing the differential expression of E-cadherin, ZO-1 and other epithelial markers in cultured RPE cells obtained from RPE central and peripheral regions suggest the in vivo existence of RPE subpopulations in different maturation/senescence stages (Kokkinopoulos et al., 2011). In rat primary RPE cells, we observed low but consistent expression of E-cadherin throughout the assays, and showed that thrombin induces E-cadherin repression in a specific manner, prevented by hirudin and PPACK.
E-cadherin expression has been suggested to be controlled by the transcriptional repressors SNAIL and SLUG (Cano et al., 2000; Bolos et al., 2003). We demonstrated the involvement of SLUG in the repression of E-cadherin gene by siRNA silencing of the transcriptional repressor. After SLUG knockdown, the basal level of E-cadherin was up-regulated in resting cells, suggesting that the low levels of the epithelial E-cadherin consistently observed in this and other studies could be maintained by SLUG expression (Fig. 5a). Additional studies regarding the silencing/overexpression of SLUG in RPE cells could be informative about how these cells maintain an epithelial morphology despite the low levels of E-cadherin, the major adhesion molecule involved in intercellular AJ assembly in epithelial cells (Oda and Takeichi, 2011).
As previously described in porcine RPE (Tamiya et al., 2010), high P-cadherin expression was found in rat RPE cells; however, in contrast to P-cadherin down-regulation in porcine RPE following TGFβ-induced EMT, thrombin treatment did not down-regulate P-cadherin expression in rat RPE as could be expected if the epithelial P-cadherin were involved in functional AJ formation. The possible involvement of P-cadherin in AJ assembly in rat RPE cells thus, remains an open question. Another epithelial cadherin involved in AJ formation is R-cadherin, but it was early discarded from this study because of the faint level of expression consistently found in rat RPE. In agreement with this finding, R-cadherin developmental expression in chick RPE cells is strongest at E12-13 and decreases thereafter to undetectable levels in post-hatching RPE (Liu et al., 1997). To the best of our knowledge, here we show for the first time that thrombin activation of PAR-1 induces E-cadherin repression by up-regulating the snail-2 (SLUG) transcription factor in RPE cells.
N-cadherin up-regulation is a well-documented feature of cells ongoing EMT, both in embryonic development and in metastatic-related EMT (Alexander et al., 2006; Acloque et al., 2009; Hao et al., 2012; Ng et al., 2012). The expression of N-cadherin in distinct normal and cancer cells is regulated at the transcriptional level, controlled by the transcription factor Twist (Alexander et al., 2006; Chang et al., 2011; Ng et al., 2012). In our study, although thrombin stimulation was found to up-regulate N-cadherin protein in RPE cells, it failed to increase N-cadherin gene expression at transcriptional level (Fig. 7). In contrast, N-cadherin up-regulation was prevented by the translation inhibitor cycloheximide (CHX). Since the simultaneous addition of CHX and the transcriptional inhibitor ActD did not further decrease N-cadherin content, our results suggest that gene transcription is not essential for N-cadherin up-regulation in RPE cells (Fig. 7b).
Recent data have shown that N-cadherin levels in melanoma cells are down-regulated by E-cadherin expression induced by transfection of either full-length E-cadherin or the E-cadherin cytoplasmic domain, suggesting that N-cadherin is directly regulated by E-cadherin content (Kuphal and Bosserhoff, 2006). Our results show that slug siRNA transfection (which restores E-cadherin expression) did not down-regulate N-cadherin content (Fig. 5a), nor did E-cadherin knockdown by siRNA up-regulate N-cadherin protein (data not shown).
The down-regulation of N-cadherin by transcription-independent mechanisms has been shown to impair thrombin-induced migration of vascular smooth muscle cells (VSMCs; Jagadeesha et al., 2012). In contrast, although information regarding N-cadherin up-regulation by transcription-independent mechanisms is scarce, a recent study has shown that alphaIIb/beta3 integrin engagement upon thrombin-induced platelet aggregation up-regulates the synthesis of Bcl-3 by a translation-dependent pathway regulated by mTOR, demonstrating that integrins can directly control expression at translational checkpoints (Pabla et al., 1999). Since this specialized translation pathway triggered by PAR-1 activation in platelets was shown to depend on PI3K and mTOR activation (Weyrich et al., 1998), we analyzed the effect of the respective inhibitors wortmannin and rapamycin on thrombin-induced N-cadherin up-regulation. Results demonstrated that both kinases are required for thrombin-mediated effect. Moreover, in agreement with previous data regarding the involvement of atypical PKC-ζ in PI3K-mediated effects following PAR-1 activation in RPE cells, we found a strong inhibition of N-cadherin up-regulation upon the inhibition of PKC-ζ by the specific inhibitor PKC-ζ PS, thus demonstrating that the transcription-independent up-regulation of N-cadherin requires the activation of PI3K/PKC-ζ/mTOR translation pathway. Since the expression of AlphaIIb/beta3 integrin (CD41) has been demonstrated in human RPE cells and moreover, CD41 is down-modulated in RPE cells by vitreous humour treatment in vitro (Ganti et al., 2007), future studies could provide insight into a possible role of AlphaIIb/beta3 integrin (CD41) in PAR-1 mediated thrombin effect on N-cadherin expression in RPE cells.
Several G-protein triggered signals are known to activate mTOR; among these, Ras-regulated MAPK pathway leads to RSK-mediated raptor phosphorylation, which promotes mTORC1 kinase activity (Foster and Fingar, 2010). Also on this matter, the members of the AGC family kinases, PKC included, are direct mTOR targets (Su and Jacinto, 2011) which could mediate an indirect effect on cadherin expression. The participation of these pathways in our results was ruled-out by the lack of effect of the specific MEK inhibitor U0126 and the PLC-β inhibitor U-73122.
The involvement of thrombin in PVR has been proposed, based on data which demonstrate that thrombin induces the proliferation, actin cytoskeleton rearrangement, and migration of RPE cells (Palma-Nicolas et al., 2008, 2010).
Results from this study demonstrate that thrombin activation of PAR-1 in RPE cells induces E/N cadherin switch, considered as a hallmark of EMT, which further supports a role for thrombin in the induction of proliferative eye diseases.
Acknowledgements
This study was partially supported by grants IN-200209 from Proyectos de Investigación e Innovación Tecnológica (PAPIIT/UNAM) and CB-80398 from Consejo Nacional de Ciencia y Tecnología (CONACyT) to A.M.L.C. Authors acknowledge the expert technical assistance of Q.F.B. Edith López.




