The role of cancer stem cells and miRNAs in defining the complexities of brain metastasis†
All authors declare no conflict of interest.
Abstract
Researchers and clinicians have been challenged with the development of therapies for the treatment of cancer patients whose tumors metastasized to the brain. Among the most lethal weapons known today, current management of brain metastases involves multiple therapeutic modalities that provide little, if any, for improving the quality of life and overall survival. Recently the role of cancer stem cells (CSCs) in the development of cancer has been studied extensively, and thus its role in the prognosis, diagnosis, and treatment is now being investigated even in the realm of brain metastasis (BM). Recognizing the molecular make-up of CSCs as well as understanding the role of these cells in resistance to treatment modalities is expected to benefit cancer patients. Additionally, past decade has witnessed an increase in awareness and understanding of the role of microRNAs (miRNAs) in various cancer types, and the deregulation miRNAs are critically important for the regulation of genes during the development and progression of human malignancies. The role miRNAs in BM is being investigated, and has also shown tremendous promise for future research. In this review, we discuss the problem and lethality of brain metastases and the current state of management, and further provide insight into novel avenues that are worth considering including the biological complexities of CSCs and miRNAs for designing novel therapies. J. Cell. Physiol. 228: 36–42, 2013. © 2012 Wiley Periodicals, Inc.
Cancer is among the most lethal diseases plaguing our society today. Its perplexing microbiology and pathophysiology has been a challenge for physician, scientists and clinicians for many years. Of greatest concern is the progression of localized cancer to a systemic metastatic site, and amongst the most lethal is brain metastasis (BM). It is well established that BM can occur in up to 30% of patients with various cancers (Kyritsis et al., 2012) with other reports of over 40% developing metastasis to the brain (Fidler, 2011) and an estimated 8–10% of adults develop symptomatic BM (Eichler et al., 2011) resulting from the primary tumors originating in the lung (40–50%), breast (15–25%), melanoma (5–20%), renal, and colorectal tissues (Eichler et al., 2011; Fidler, 2011). Furthermore, metastatic brain tumors are the most common intracranial neoplasm in adults, exceeding gliomas in prevalence by nearly tenfold (Chamberlain, 2010; Steeg et al., 2011) with an incidence of 200,000 cases in the U.S (Eichler et al., 2011). This is in part attributed to the large amounts of blood flow to the brain; 15–20% of the entire body's blood circulation covers the brain, increasing the chances of circulating tumor cells to reach and seed in the brain (Seol et al., 2011). Metastatic brain cancer has also now become an area of increased attention due newer development of therapeutic modalities, resulting in relatively longer patient survival compared to previous management strategies that did not improve the mortality from late diagnosis of BM (Fidler, 2011; Steeg et al., 2011). However, much research to be done for optimal treatment strategies for brain metastatic disease.
Emerging evidence suggests that only a small but distinct subset of tumor cells, also known as cancer stem cells (CSCs), has the capacity to self-renew, differentiate, and initiate tumor growth in vitro and in vivo with as few as 5,000 cells in immunodeficient mice (Bao et al., 2011a). A recent study has reported that as low as 200 stem cell markers CD44+ and CD24− breast cancer cells when implanted in NOD-SCID mice formed tumors as opposed to 20,000 cells that lacked these surface protein expression (Al-Hajj et al., 2003). Moreover, these CSCs have the ability to detach from the primary site and invade the surrounding tissue by acquiring epithelial-to-mesenchymal transition (EMT) phenotype, and are believed to be the main cause of death in cancer patients. These cells are also believed to be highly resistant to both chemo- and radiotherapy, and also escape targeted treatment. Hence, complete cure cannot be achieved unless and until all CSCs are totally ablated (Li et al., 2012). The discovery of CSCs has the potential to develop targeted therapies by blocking the essential signaling pathways such as Notch, Wnt, and hedgehog that are involved in self-renewal, differentiation, resistance to chemotherapy within the tumor microenvironment (Grotenhuis et al., 2012). Recently, microRNAs (miRNAs), small nucleotides that control the expression of many target genes at the posttranscriptional level, have been reported to be frequently deregulated in many types of human cancers. They can function either as tumor suppressors or as oncogenes and initiate tumor growth, invasion, metastases, the process of EMT, as well as regulate the overall “stemness” of cancer cells (Ali et al., 2011, 2012; Kong et al., 2011; Zhao et al., 2011). They have also been proposed to be novel diagnostic plasma biomarkers in differentiating normal and patients (Ali et al., 2010). Hence, targeting these miRNAs both in cancer research and clinical management of cancer by novel strategies appears to be very promising.
The Problem and Incidence of Brain Metastases
Prognosis of patients with cancer drastically worsens once a patient develops BM, with the median survival of untreated patients of 1–2 months, and those treated could have a median survival of 6 months (Fidler, 2011). These months however is complicated with morbid conditions such as neurological complications, headache, loss of motor and sensory function, seizures, and cognitive impairment (Eichler et al., 2011; Steeg et al., 2011) leaving patients with a complicated course and poor quality of life during the final few months of their lives. This disappointing outcome calls for drastic measures, which can only come from innovative research.
Among several solid tumors, lung cancer is of particular concern when discussing brain metastases. Twenty-five percent of patients with lung cancer will develop brain metastases; there is a 15–30% incidence of BM in non-small cell lung cancer patients where 33% metastases occur in the brain (Chamberlain, 2010; Steeg et al., 2011). Similar, if not worse, results are seen in patients with small cell lung cancer. Patients with breast cancer, the second leading cause of BM, will develop BM in 6–16% of cases. While the cause remains unclear, recent data has suggested a possible chemokine-mediated chemotaxis in breast cancer with a particular tropism for the brain (Chamberlain, 2010; Steeg et al., 2011). Melanoma is the third most common source of BM, and clinically recognized in 30% metastatic melanomas; 20–50% of all melanoma deaths are believed to be due to BM, and similar to breast cancer, BM are presented with an average of 2.5 years after initial diagnosis (Chamberlain, 2010; Steeg et al., 2011). Cancers that rarely metastasize to the brain include hepatocellular carcinoma, bladder, prostate cancer, and ovarian cancer (Kyritsis et al., 2012).
What Makes Brain Metastases so Lethal
In order to best understand what makes brain metastases so lethal, one must first appreciate the complexities involved in the development and progression of brain metastases. Colonization of the brain by metastatic cancer cells is initiated by extravasation of the primary tumor cells into the brain, eventually leading to a clinically detectable metastatic lesion (Steeg et al., 2011). When these primary tumor cells traverse the vascular system, they use the outside of the vessels to situate and migrate while interacting the brain's microglial and astrocytic cells, and subsequently utilize the inflamed brain's microenvironment to settle (Steeg et al., 2011). This microenvironment contains damaged axons, edema, and vascular changes, such as disruption of the blood brain barrier (BBB), and associated with neuronal damage (Steeg et al., 2011). Furthermore, metastasis to the brain is unique in comparison to other sites. The location of brain metastases appears to correlate with the rich blood supply as well as tissue volume (80% cerebral hemispheres, 15% cerebellum, and 5% brainstem; Eichler et al., 2011).
Reaching sites of BM for therapeutic purposes is quite complicated, adding to the lethality of brain metastases. The brain parenchyma contains a rich microvasculature. This is lined by continuous, nonfenestrated endothelium with tight junctions, resulting in limited transport of macromolecules through blood vessel, restricting the entry of pathogens and microorganisms (Fidler, 2011). This construction, further strengthened with pericytes and astrocytic perivascular endfeet, forms a selective barrier between systemic circulation and cerebrospinal fluid, otherwise known as the BBB (Eichler et al., 2011). Unfortunately many chemo-therapeutic drugs and molecular-targeted drugs are made up of large hydrophilic molecules, which are restricted to cross the BBB, and thus limiting treatment options for metastatic brain tumors (Eichler et al., 2011). While it seems true that the BBB is compromised to some degree where brain metastases has occurred, chemotherapy and cytotoxic agents are still unable to adequately penetrate. Pharmacokinetic studies have recently revealed that despite the increased permeability when compared to a normal brain, only 10% showed a cytotoxic response to chemotherapy (Steeg et al., 2011). Also contributing to this lack of brain permeability are drug efflux pumps (Steeg et al., 2011) and contributing to treatment failure.
In addition, because of the macro- and microanatomy of the brain, surgical resection of brain tumors is not always practical, further complicating the lethality of brain metastases. While it is widely accepted that brain metastases have distinct, sharp borders that can be surgically removed, in comparison to the infiltrative nature of primary brain tumors, there is a 50% recurrence rate when radiation is not employed; furthermore, although lesions of several millimeters in size are radio-graphically detectable (Steeg et al., 2011) there is a possibility of tiny metastatic deposits that need treatment and are not readily visualized by MRI (Eichler et al., 2011). In addition, 1/3rd or more of patients found to have BM contain four or more tumors (Chamberlain, 2010; Steeg et al., 2011) making resection risky and often impossible. In approximately 33% of patients, after initial workup, the site of origin of BM is not apparent (Kyritsis et al., 2012).
Current State of Management of Brain Metastases
Management of BM has certainly evolved over the last several years. The treatments may be divided into two types: symptomatic and definitive. Symptomatic treatment includes the administration of steroids, anticonvulsants, etc. (Chamberlain, 2010; Steeg et al., 2011). Due to the presence of vasogenic edema secondary to BM, patients are at increased risk for intracranial mass effect. Dexamethasone is the most common treatment for such condition, and has long been utilized though primarily reserved for patients with a symptomatic presentation (Chamberlain, 2010; Steeg et al., 2011). Anticonvulsants are used in patients who present with seizures secondary to BM and are not recommended prophylactically. Furthermore, when these become necessary, nonenzyme-inducing anti-epileptic drugs are preferred (Chamberlain, 2010; Steeg et al., 2011) so as to limit interaction with concurrent chemotherapy regimens.
Definitive therapy varies widely, and includes surgery, stereotactic radiosurgery (SRS), whole-brain radiation therapy (WBRT), and chemotherapy (Eichler et al., 2011) and is dependent on factors such as number, size, and location of brain tumors as well as extent of systemic disease. Presently, surgical resection is indicated for treatment of large symptomatic solitary BM in a manageable location, whereas stereotactic radiotherapy is utilized for patients with solitary, small tumors that are not easily surgically resectable (Chamberlain, 2010; Steeg et al., 2011). Multiple studies have demonstrated that solitary BM from non-small cell lung cancer (NSCLC) and breast cancer were better treated with surgical resection followed by WBRT, rather than WBRT alone (Kyritsis et al., 2012). Surgery for solitary metastasis also has proven to improve local control when combined with WBRT (Kyritsis et al., 2012).
Radiotherapy is the most definitive treatment in most patients. WBRT is the most common form used, primarily because brain metastases tends to be multifocal and local therapies are not useful for distant disease and causes treatment failure (Chamberlain, 2010; Steeg et al., 2011). WBRT increases survival in patients to 4–6 months, and occasionally 12–24 months (Eichler et al., 2011). For patients with a single intracranial metastatic tumor, clinical trials have shown that surgery or SRS in combination with WBRT improves overall survival in comparison with WBRT alone (Eichler et al., 2011). However, WBRT has its reservations and limitations. These include radiation-induced neurotoxicity and failure to treat radio-resistant tumors such as malignant renal cell carcinoma, sarcoma, and melanoma (Chamberlain, 2010; Steeg et al., 2011; Kyritsis et al., 2012). In addition, radiation therapy may cause side effects including impaired cognitive function and radiation necrosis (Seol et al., 2011).
WBRT also plays a role in prevention of BM, through a process known as prophylactic cranial irradiation (PCI). A 73% reduction of brain metastases in patients with small-cell lung cancer was noted, with an increased survival from 5.4 to 6.7 months, though a review of randomized trials suggested no overall survival benefit (Steeg et al., 2011). PCI has recently been shown to reduce the incidence of BM and improve disease-free survival, 1-year survival, and overall survival in patients with small-cell lung carcinoma (Kienast and Winkler, 2010). Nevertheless, the potential adverse effects must be compared when making clinical decisions.
Presently available clinical data indicate that chemotherapeutic drugs may be most effective in the prevention of metastatic lesions to the brain rather than treatment (Steeg et al., 2011). Excluding SCLC, chemotherapy is rarely a first-line treatment for BM (Kyritsis et al., 2012). However, its role has been limited due to several factors. Firstly, as mentioned earlier, the BBB is difficult to penetrate with currently available chemotherapeutic drugs. In addition, many patients have had multiple rounds of chemotherapy prior to development of BM, with possible induction of drug resistance (Seol et al., 2011). Lastly, patients with BM, historically speaking, have been excluded from clinical trials testing new chemotherapeutic agents (Eichler et al., 2011). Other intervention includes intracavitary chemotherapy in patients with surgically resected BM (Chamberlain, 2010; Steeg et al., 2011).
Kyritsis et al. (2012) present an excellent summary for the current management recommendations for patients with BM. In patients with SCLC, induction therapy should be the first line treatment with or followed by WBRT. For all other cancer sources, BM should be treated with either surgery if tumors are few in number (1–4) and surgically resectable, or simply radio-surgery followed by WBRT in nonsurgical cases; WBRT may be unnecessary in some cases of radio-resistant tumors. In all other cases, for example, four or more tumors, WBRT should be administered. Following therapy, serial MRI scans every 3 months for the first year are recommended, and if in remission, extended steadily to 4–6 months (Kyritsis et al., 2012).
How Evolving Research Can Help Tackle the Complexity of Brain Metastases
Research has shown that brain metastases are characterized by changes in a variety of cellular pathways, implicated and suggested in clinical and pre-clinical models of BM. Chen and Davies (2012) have outlined these pathways in excellent detail. Included are descriptions of vascular endothelial growth factor (VEGF) signaling, human epithelial growth factor receptor family receptors (HER), wingless-type (WNT) pathway, Janus Kinase (JAK) signal transducer and activator of transcription (STAT) pathway, PI3K-AKT pathway, hexokinase (HK), and ECM proteins. It is evident that multiple signaling pathways are involved, which allows several ways of targeting brain metastases. One method is identifying signaling nodes that are similar across pathways, and in focusing on these salient targets, affecting multiple pathways at once. Additionally, multiple pathways can be targeted concurrently. Nevertheless, focused pre-clinical and clinical trials on patients with BM are needed. Although many pathways are similar between primary tumors and extracranial metastases, several are selective to BM, such as ST6GALNAC5, GSTA5, and TWIST1 (Chen and Davies, 2012). Molecular targeted therapy has been on the rise although the majority of which are tyrosine kinase inhibitors and monoclonal antibodies targeting EGF or angiogenesis pathway (Kienast and Winkler, 2010) suggesting that newer agents must be developed that will specifically target multiple pathways at the same time. Such developments would certainly improve therapeutic outcome in the future.
Metastasis in general is a complicated process that requires multiple steps, which has posed difficulty in the laboratory setting. Individual aspects of cancer biology and biochemistry, such as cell cycle control and apoptosis, have been studied and modeled in cell cultures and test tubes in vitro; however, the study of metastasis requires a measure of the interactions between tumor cells and neighboring host environment, which is not optimally feasible in vitro. This resulted heavily on the reliance of transplantable animal models of metastasis, including both syngeneic and xenograft models (Kang, 2009). With the development of improved preclinical models that recapitulate the clinical scenario (Eichler et al., 2011) there will be much needed assistance to the study of brain metastases. Presently one of the most commonly utilized models involves injection of metastatic tumor cells into the cardiac ventricle or the internal carotid artery of the mice. Although this model is useful, it does not take into account the several steps often found during the development of metastasis, including primary tumor involvement and tumor cell migration (Chen and Davies, 2012). Novel methods and approaches to the management and treatment of brain metastases will be proven useful in the near future once the complexities on brain metastases are molecularly understood.
Cancer Stem Cells as Determinants of Metastasis and Chemo-Resistance
Cancer stem cells
Cancer stem cells are rare sub-populations of tumor cells that are capable of tumor propagation in vitro and in immunodeficient mice and are resistant to chemotherapy, radiation therapy, resulting in the development of metastatic disease (Kang, 2009; Bao et al., 2011a; Chu and Allan, 2012; Grotenhuis et al., 2012). These cells have the ability to self-renew and differentiate. Increasingly, researchers are beginning to investigate strategies for targeting CSCs for the treatment of cancer. Under normal conditions, stem or progenitor cells function in self-renewal, dormancy, and pluripotency, although when genetic damage is sustained, cells will acquire tumorigenic characteristics that will contain CSCs with excessive generative and proliferative ability (Pommier et al., 2010). The hierarchy theory suggests that there is a small, phenotypically identifiable subpopulation of cancer cells which possess stem cell-like characteristics (Chu and Allan, 2012) and only this fraction of tumor cells are even capable of extensive renewal capacity (Guo et al., 2011).
The CSC model is being established as a foundation for understanding the mechanisms of tumor initiation and progression. The development of a tumor is compared with the development of an abnormal or deficient organ. Under normal organogenesis, the self-renewal and differentiation of stem cells produces a meticulous organ of heterogeneous cellular make-up. However, in tumorigenesis, self-renewal and differentiation are dysregulated, producing hyper-proliferative and abnormally differentiated tissue (Sullivan et al., 2010). The CSC hypothesis, as described by Sullivan et al., consists of a few components. Firstly, that cancer arises from stem cells that have acquired adequate oncogenic mutations for transformation; in other words, a tumor cell of origin, otherwise known as the tumor-initiating cell, is a stem cell that is already capable of self-renewal and differentiation. Another component of the hypothesis is that tumor progression is driven by a subpopulation of self-renewing tumor cells (Sullivan et al., 2010). According to the CSC model, cancer should not be thought of as simple monoclonal expansions of tumor cells of equal function; rather only a subset of these cells have the ability to maintain malignancy (Artells et al., 2010). Since its discovery, solid tumor CSCs have been identified from human brain, breast, prostate, colon, lung, and pancreatic cancer (Sullivan et al., 2010).
The discovery of rare tumor cells with features of stem cells, such as the ability to self-renew and differentiate, was first in observed leukemia, and later in solid tumors. These CSCs are the primary cellular component within a tumor that impel disease progression and metastasis (Sullivan et al., 2010). Recent studies over the last 4–5 years have provided evidence in support of the existence of CSCs capable of proliferation, self-renewal, and differentiation into various cell types (Artells et al., 2010; Bao et al., 2011a; Kong et al., 2011). Furthermore, these CSCs are also enriched in cells that are resistant to conventional radiation therapy and chemotherapy (Sullivan et al., 2010; Bao et al., 2011a, 2012; Yu et al., 2012).
Role of CSCs in metastasis of primary cancers
The CSC model as discussed earlier, postulates that only a subpopulation of cancer cells are able to proliferate independently, and in xenograft transplantation studies, are the only cells thought to have the ability to produce secondary and tertiary tumors that reproduce the heterogeneity of primary disease (Sullivan et al., 2010; Yu et al., 2012). It is widely being accepted that stem cells play a significant role in the metastasis of primary cancers. Pommier et al. (2010) presented work on breast cancer elucidating the discrete subpopulations of cancer stem/progenitor cells, all of which displayed invasive potential and ability after acquiring damage that leads to malignant function. Guo et al. (2011) demonstrated in the GI-101A human breast cancer cell line that cells remaining after treatment had a significantly higher rate of experimental BM as compared with untreated cells, suggesting a role of metastasis-initiating cells.
CSCs' role in determining resistance of tumors to chemotherapy as well as radiation therapy
Cancer has historically been resistant to conventional chemotherapy. Part of the high relapse rates can be attributed to the presence of CSCs. BMI1, for instance, has recently been linked with stem cell-like 11 gene expression microarray signature predictive of short interval to disease recurrence following therapy, increased likelihood of metastasis, and poor response to therapy in cancers such as medulloblastoma, glioma, prostate, lung, ovarian, acute myeloid leukemia, and breast cancer (Hoenerhoff et al., 2009). The roles of CSCs in causing resistance to conventional therapeutics are an intense area of research, which is likely contribute to the development of next generation of therapeutics that will help to overcome resistance, and thus will result in the improvement of therapeutic outcome.
Markers of cancer stem cell-like phenotype such as Notch 1, CD133, BMI1 that can potentially be targeted for reducing brain metastases
Targeted therapeutic gene delivery via stem cells is an area of growing research. Although only one syngeneic study of BM has been published for targeting metastatic brain tumor (Seol et al., 2011) using genetically engineered stem cells have advantages that are useful for gene therapy in the treatment of brain tumors (Seol et al., 2011). More recently, with the finding of inherent tumor-tropic properties of neural stem cells (NSCs), the challenge in developing chemotherapeutic treatments must be revisited (Seol et al., 2011). NSCs carrying therapeutic suicide genes such as carboxyl esterase, for example, may be useful. Recent studies have suggested that CE-producing NSCs, responsible for converting prodrug CPT-11 to SN-38 migrate to tumor cells, and have a therapeutic effect on the tumors with a 71% reduction in tumor volume and increased survival outcome in xenograft mouse model (Seol et al., 2011).
Researchers have been investigating several markers of CSC-like phenotype. McGowan et al. (2011) presented data indicating Notch signaling plays a significant role in the formation of brain metastases from breast cancer, partially due to its role in maintaining CSC (CD44hi/CD24lo) putative cancer stem-like cells. Notch proteins consist of a family of four transmembrane receptors, and the signaling is found to be dysregulated in many human cancers (Sethi et al., 2010; Wang et al., 2010a, c, 2011a, b; Ji et al., 2011, 2012; Bao et al., 2011b). A reduction in the proportion of CSC (CD44hi/CD24lo) significantly reduces formation of brain metastases from breast cancer, coinciding with a previous study using breast cancer cells with the same markers and phenotype which enhanced cell growth, colony formation, invasion in vitro, and enhanced metastases formation in multiple organs in NOD/SCID-IL2Rγ null mice (McGowan et al., 2011).
Another membrane protein that has been studied is human prominin-1 (CD133), which is a pentaspan membrane protein that historically has been a marker for primitive haematopoeitic and NSCs. CD133 has been identified as a stem cell marker capable of identifying tumor-initiating subpopulations in brain, colon, melanoma, and other solid tumors (Yu et al., 2009; Artells et al., 2010). Artells et al. (2010) in their study of colorectal cancer have demonstrated that CSC expression levels in a tumor may be associated with increased aggressiveness of clinicopathological features and worse outcome.
Hoenerhoff and associates elaborated on the role of BMI1, a transcription repressor that operates in stem cell maintenance and oncogenesis through inhibition of INK4A/ARF tumor suppressor locus. Over-expression of BMI1 has been linked with increased incidence of metastasis in human gastric and breast cancer as well as melanoma and other cancer types. In this study, they were able to show that BMI1 in conjunction with H-RAS in human mammary epithelial cells (HMECs) results in an aggressive phenotype that includes spontaneous metastasis to liver and spleen as well as novel metastasis to brain (Hoenerhoff et al., 2009). In addition to above studies, intense research is on-going which clearly show many additional markers of CSCs such as miRNAs as discussed below.
MicroRNAs (miRNAs) in Brain Metastases
MicroRNAs (miRNAs)
MicroRNAs are small non-coding RNA molecules that ranges in size from 19 to 30 nucleotides (on average 20 nucleotide) and are thought to regulate 60% of genes, acting on differentiation, and cell cycle control (Ali et al., 2010, 2011, 2012; Zhao et al., 2011; Lu et al., 2012). They are aberrantly expressed in many types of cancer, and play a major role in regulating a variety of targets and pathways, which are beginning to be appreciated, which making them a useful tool for early detection of disease, management, and prognosis. One miRNA regulates several hundred genes, and thus its profiling could be superior compared to just gene expression profiling (Ali et al., 2010; Chen et al., 2011) provided novel tools are used to identify specific but handful of target genes of a given miRNA, which may also be context dependent.
Role of miRNAs in cancer metastasis
Recently, research has shown that miRNAs could play a significant role in tumor invasion and metastasis of various cancers, and alter many signaling pathways (Wang et al., 2010b; Ahmad et al., 2011; Sreekumar et al., 2011; Zhao et al., 2011). In addition to the role of CSCs in human cancer as discussed above, emerging evidence has shown that one of the first steps in tumor progression may be mediated through the acquisition of EMT phenotype of cancer cells, which is a process by which epithelial cells lose their cell to cell contacts and subsequently attain characteristics of mesenchymal phenotype. These cells detach from the primary tumor site and enter into circulation which is believed to be responsible for tumor cell metastasis (Korpal and Kang, 2008; Iliopoulos et al., 2009; Ahmad et al., 2011; Kahlert et al., 2011; de Krijger et al., 2011; Zhang et al., 2011b). Evidence has shown that the acquisition of EMT phenotype could be regulated by deregulation in the expression of miRNAs in the context of metastasis. To that end, studies have shown that detection of circulating miRNAs can be associated with clinical parameters such as relapse with metastatic disease (Ali et al., 2010; Wu et al., 2012).
MicroRNA expression profiling has proven to be useful in human pancreatic, colorectal, ovarian, and breast cancer as well as glioblastoma and other cancer types (Pfeffer et al., 2009; Ali et al., 2010; Chang et al., 2011; Niyazi et al., 2011; Shahab et al., 2011; Wu et al., 2012; Zhang et al., 2012). According to Niyazi et al. (2011) a distinct miRNA expression pattern was possible with miRNA expression profiling showing prognostic subgroups associated with early death versus long-term survival. Similarly, in colorectal cancer miRNA expression profiling on stage II tumor and normal tissues identified few miRNAs predictive of tumor status in stage II disease Chang et al. (2011) and others have identified the miRNAs that are associated with colorectal cancer metastasis (Zhang et al., 2012).
Role of miRNAs in brain metastases
Emerging evidence indicates critical roles of miRNAs in brain metastases from different primary tumors (Table 1). Over 50% of BM are associated with NSCLC (Lu et al., 2012) and ∼25% of NSCLC patients will develop BM during their lifetime (Arora et al., 2011). In a study by a group of collaborators, 10 miRNAs were identified to be associated with BM. The miR-145 is one of these, which has been shown to suppress cell invasion and metastasis by targeting metastasis gene mucin 1 (MUC1; Lu et al., 2012). Other studies have looked into biomarkers that predict BM in breast cancer patients using gene expression analysis (Arora et al., 2011). In the following sections, we will summarize the role of several specific miRNAs that are associated with brain metastases.
The role of miR-1258 in brain metastases of breast cancer through targeting of heparanase
As mentioned above, breast cancer is among the leading sources of metastases to the brain. Novel methods are necessary to help us better understand and manage such a devastating complication. Zhang et al. published a study in which they showed that miR-1258 suppresses breast cancer BM by targeting heparanase (HPSE), a potent pro-tumorigenic and pro-metastatic enzyme that is over-expressed in brain metastatic breast cancer. Mechanistic studies have shown that miR-1258 inhibited the expression and activity of heparanase, and adjusting heparanase blocked phenotypic effects of the miRNA. Functional experiments demonstrated that stable expression of miR-1258 in brain metastatic breast cancer hampered heparanase, cell invasion, and experimental BM (Zhang et al., 2011a). These findings suggest the potential of heparanase-based therapies for breast cancer patients with BM.
The role of miR-328 in brain metastases of lung cancer
MicroRNA-328 has also been studied extensively (Eiring et al., 2010; Li et al., 2010, 2011; Arora et al., 2011; Lu et al., 2012; Turrini et al., 2012). It is inversely correlated with ABCG2 expression in glioblastoma CSCs (Li et al., 2010) and breast cancer cells (Pan et al., 2009) and is also associated with chemo-resistance. Modulation of either miR-328 or ABCG2 protein expression increased the efficacy of chemotherapeutic agents (Pan et al., 2009; Li et al., 2010). Arora and colleagues conducted miRNA microarray profiling on samples from seven NSCLC patients with BM and five without BM. Differential expression of miR-328 properly classified patients with BM and without. It was determined to be over-expressed in patients with BM, and appears to play a role in establishing migratory potential of NSCLC cells, in particular through the deregulation of PRKCA gene. Elevated expression of miR-328 in both primary lung cancer specimens as well as BM samples points toward its possible “brain-seeking” metastatic potential (Arora et al., 2011). Although sample size was a limitation in this study, it showed tremendous potential for further investigation.
The role of miR-378 in brain metastasis of non-small cell lung cancer (NSCLC)
In a study involving miR-378, Chen and colleagues were able to determine that this miRNA was significantly and differentially expressed in NSCLC patients with BM versus without BM. The miR-378 is associated with promotion of cell migration, invasion, and tumor angiogenesis. When used as a potential biomarker, it could assist clinicians in classifying patients that are high-risk for BM and may benefit from additional treatment opportunities, such as clinical trials and PCI (Chen et al., 2011). In addition to the above specific examples, accumulating evidence are emerging and many such reports cannot be fully summarized in this short review article, and thus would become the subject of further review articles in the future; however, this article would likely stimulate further research in elucidating the role of specific miRNA in brain metastases, which would be useful in the development of targeted novel therapeutic strategies.
Conclusions and Perspectives
Among the most devastating complications from illness today is the development of brain metastases of patients diagnosed with cancers. Aside from the shortened life expectancy and generalized symptoms commonly associated with systemic illness, these patients suffer from numerous neurological sequelae that further complicate their final few months of life. Current management of patients with BM is limited, with therapies such as surgical resection, radiotherapy, and chemotherapy that only provide for a few extra weeks or months of survival. Therefore, novel approaches are urgently needed in order to tackle the disease process more efficiently and successfully. Two areas of growing research with tremendous potential are emerging such as the area of CSCs and miRNAs (miRNAs), and emerging evidence suggests a connection between up-regulated CSC markers with deregulated expression of miRNAs consistent with brain-seeking behavior of cancer cells (Fig. 1). The science of brining stem cell therapy to the clinic is not yet ready, as many molecular avenues still needs to be refined along with many other issues such as safety, route of administration, required number of cells, and immune-rejection (Seol et al., 2011). However, we are beginning to smell the tremendous potential of stem cells, CSCs, and miRNAs in the molecular mechanism of tumorigenesis, and their roles in diagnosis, prognosis, and treatment although we have to wait to appreciate their taste. Newer techniques such as induction of pluripotent stem cells from adult fibroblasts and the production of NSC-like cells from human bone marrow are showing some promise (Seol et al., 2011). In addition, over the last decade, the field of cancer biology became excited regarding the tremendous potential of miRNAs as targets of designing novel therapies not only for primary tumors but also for the treatment of brain metastases. Translating the concept of miRNA targeted therapy into the clinical setting would likely be useful to realize the dream of personalized medicine in the next decade.
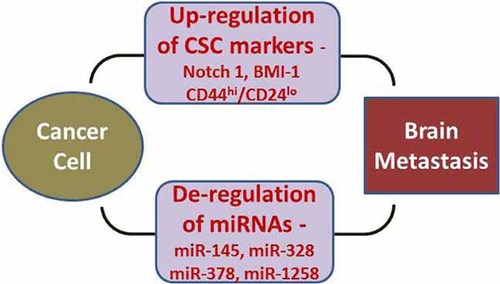
Early evidence has suggested a role of CSC phenotype and novel miRNAs in influencing the brain-seeking behavior of cancer cells leading to preferential homing of metastatic cells in brain. It is expected that selective targeting of these putative targets can help reduce the clinical incidence of brain metastatic disease.
Acknowledgements
This work was funded by grants from the National Cancer Institute, NIH 5R01CA131151, 3R01CA131151-02S109, 1R01CA132794, and 1R01CA154321 awarded to FHS. We also thank Puschelberg and Guido foundations for their generous contribution.