Photoactivation of bone marrow mesenchymal stromal cells with diode laser: Effects and mechanisms of action†
Marco Giannelli and Flaminia Chellini contributed equally to the study.
Abstract
Mesenchymal stromal cells (MSCs) are a promising cell candidate in tissue engineering and regenerative medicine. Their proliferative potential can be increased by low-level laser irradiation (LLLI), but the mechanisms involved remain to be clarified. With the aim of expanding the therapeutic application of LLLI to MSC therapy, in the present study we investigated the effects of 635 nm diode laser on mouse MSC proliferation and investigated the underlying cellular and molecular mechanisms, focusing the attention on the effects of laser irradiation on Notch-1 signal activation and membrane ion channel modulation. It was found that MSC proliferation was significantly enhanced after laser irradiation, as judged by time lapse videomicroscopy and EdU incorporation. This phenomenon was associated with the up-regulation and activation of Notch-1 pathway, and with increased membrane conductance through voltage-gated K+, BK and Kir, channels and T- and L-type Ca2+ channels. We also showed that MSC proliferation was mainly dependent on Kir channel activity, on the basis that the cell growth and Notch-1 up-regulation were severely decreased by the pre-treatment with the channel inhibitor Ba2+ (0.5 mM). Interestingly, the channel inhibition was also able to attenuate the stimulatory effects of diode laser on MSCs, thus providing novel evidence to expand our knowledge on the mechanisms of biostimulation after LLLI. In conclusions, our findings suggest that diode laser may be a valid approach for the preconditioning of MSCs in vitro prior cell transplantation. J. Cell. Physiol. 228: 172–181, 2013. © 2012 Wiley Periodicals, Inc.
Mesenchymal stromal cells (MSCs) have been shown to be a promising source of adult stem cells for cell transplantation and tissue engineering (Quattrocelli et al., 2010; Wang et al., 2011). These cells, in fact, can be easily isolated from different sources of the adult body, typically the bone-marrow, and are capable of providing significant functional benefits after implantation in the damaged tissue (Doorn et al., 2012). They have the potential to acquire lineage of any-mesenchymal-derived tissue in vitro. However, there is a general consensus that their beneficial effects on tissue repair/regeneration do not require differentiation of MSCs at target sites and mostly depend on their ability to secrete a broad panel of growth factors and cytokines, which instruct the neighboring cells and provide cues for stimulating neo-angiogenesis and extracellular matrix remodeling and assisting the endogenous regenerative response (Caplan, 2007; Sassoli et al., 2012). Moreover, the immunosuppressive properties of MSCs allow them to be used in autologous and allogenic contexts (Bernardo et al., 2012). The use of these cells in cell therapy requires their expansion in vitro in order to obtain an adequate amount of cells to be implanted in the injured tissue. Therefore, improvements of their proliferative potential during in vitro culture can significantly shorten cell preparation time and avoid contamination, thus contributing to the further development of cell-based tissue regeneration. In this context, we have previously shown that the treatment with platelet-derived rich plasma (PRP) may represent a promising approach to stimulate cell proliferation and influence stemness gene expression in cultured human MSCs (Formigli et al., 2012). Of interest, there is recent evidence suggesting that proliferation of various cultured stem cells, including MSCs, cardiac and pulpal dental stem cells, could also be enhanced by low-level laser irradiation (LLLI; Tuby et al., 2007; Eduardo Fde et al., 2008; de Villiers et al., 2011; AlGhamdi et al., 2012). This approach is widely applied in different branches of regenerative medicine and dentistry, where it is used to improve the tissue healing and repair processes. Indeed, LLLI has been found to stimulate cutaneous wound repair (Mendez et al., 2004; Maiya et al., 2005), cornea and nerve regeneration (Ratkay-Traub et al., 2001; Barbosa et al., 2010), to enhance bone remodeling and formation (Fávaro-Pípi et al., 2011) and promote cartilage tissue regeneration (Kamali et al., 2007). In particular, we have recently demonstrated that diode laser irradiation is able to improve periodontal healing in patients affected by chronic periodontitis (Giannelli et al., 2012). Despite these data, the molecular mechanisms underlying the effects of laser bio-stimulation remain to be fully clarified. Most of the studies in this field, in fact, have been focused on the classical mechanisms related to the conversion of the laser photons into metabolic energy which, in the form of ATP, stimulates a variety of biochemical pathways leading to normalization of the cell function and promotion of cell proliferation (Basso et al., 2012).
On these bases, and with the aim of expanding the therapeutic applications of LLLI in regenerative medicine, in the present study we evaluated the effects of diode laser (4 × 4 Dental Laser, General Project, Montespertoli, Italy) at a wavelength of 635 nm, on bone marrow-derived MSC proliferation. We demonstrated that the mitogenic effects in the laser-irradiated cells were dependent on the activation of specific plasma membrane K+ channels (Kir) previously suggested to coordinate upstream and downstream signals regulating cell cycle (Ahidouch et al., 2010; Margheri et al., 2012; Zhang et al., 2012) and involved stimulation of Notch-1 signaling.
Materials and Methods
Mesenchymal stromal cell (MSC) isolation, characterization, and culture
Mouse bone marrow mesenchymal stromal cells (MSCs) were kindly provided by Dr. Benedetta Mazzanti from the Department of Haematology, Placental Blood Bank, Careggi Hospital, University of Florence, Italy. The cells were isolated from femura and tibiae of male C2F1 mice, following the Dobson procedure and characterized as reported previously (Dobson et al., 1999; Sassoli et al., 2011). Briefly, bone marrow pellet was re-suspended in 10 ml of Hank's Balanced Salts Solution (HBSS, w/o calcium and magnesium; Euroclone, Milan, Italy) + 1% Fetal Bovine Serum (FBS, HyClone, South Logan, UT) and centrifuged (300g for 7 min). After the cells were passed through a 22 gauge needle, they were resuspended in growth medium (DMEM-Low Glucose, with L-glutamine, HEPES 25 mM and pyruvate, GIBCOTM, Invitrogen, Milan, Italy, supplemented with 20% FBS), and cultured at 37°C in a humidified atmosphere containing 95% air and 5% CO2. At sub-confluence, the adherent cells were detached and sub-cultured to remove hematopoietic cells. After the 4th- and 5th-passage, the morphologically homogeneous population of MSCs was analysed for the expression of specific cell surface antigen, using flow cytometry. Aliquots of resuspended cells (1.5 × 105 cells/100 µl) were incubated with the following conjugated monoclonal antibodies: CD45-FITC, CD34-FITC (in order to quantify hemopoietic-monocytic contamination); CD90-PE, CD73-PE (BD Pharmingen, San Diego, CA) for 20 min. Non-specific fluorescence and morphologic parameters of the cells were determined by incubation of the same cell aliquot with isotype-matched monoclonal antibodies (Becton Dickinson, San Diego, CA). 7-aminoactinomycin D (7-AAD; BD Pharmingen) was added in order to exclude dead cells from the analysis. Flow cytometric acquisition was performed by collecting 104 events on a FACScalibur (Becton Dickinson) instrument and data were analyzed on DOT-PLOT bi-parametric diagrams using CELL QUESTPRO software (Becton Dickinson) on Macintosh PC. After the exclusion of dead cells, cell population resulted uniformly positive for CD90 and CD73. There was no significant contamination of hematopoietic cells, as flow cytometry was negative for markers of hematopoietic lineage, including CD45 and CD34.
Finally, the isolated cells were tested for their osteogenic and adipogenic potential. To this aim, MSCs (104 cells/cm2) were grown near confluence in 25 cm2 flasks and then incubated in osteogenic medium (DMEM-LG with 10% FBS; 10 nM dexamethasone, 50 µg/ml ascorbic acid and 10 mM β-glycerophosphate, all from Sigma, Saint Louis, MO) or in adipogenic medium (DMEM-LG with 10% FBS; 0.25 mM isobutyl methylxanthine, 10 nM dexamethasone, 5 µg/ml insulin, all from Sigma. The medium was replaced every 3–4 days and the deposition of mineral nodules or the accumulation of lipid-containing vacuoles was revealed after 21 days with Alizarin Red-S or with Oil Red O staining respectively.
For the experiments, MSCs were grown in 96- or 6-well culture plates or on glass coverslips (placed on the bottom of a well of a 6 well-plates), stimulated or not (control) with diode laser (time 0, T0), and cultured in growth medium for 24, 48, and 72 h. During laser irradiation the cells were incubated in Red Phenol free medium (GIBCO™, Invitrogen). In some experiments MSCs were treated with 0.5 mM BaCl2 for 24 h prior laser irradiation in order to inhibit Kir channels (Tennant et al., 2006; Ma et al., 2011).
Low level laser irradiation (LLLI)
Irradiation was carried out with a diode laser (4 × 4 Dental Laser, General Project) operating at a wavelength a 635 nm in continuous irradiation mode. The beam power was set at 0.89 W and a 600 µm diameter optic fiber was used. The detailed irradiation parameters were reported in Table 1. During the treatment, the temperature of the laser-irradiated cells was monitored by a thermo-graphic micro-camera, able to show a thermal map of the treated area. This information allowed to finely tune the laser irradiation and keep the temperature below the cell damage threshold. To avoid overlapping or scattered irradiation, the cells of each cell preparation and experiment were seeded in wells or dishes spaced apart. A black background in the irradiation area was used to minimize light reflection. During the period of laser irradiation, the cover plate was removed and all the procedures were performed under “clean bench” conditions to prevent bacterial contamination. Eye protection of the operator and assistant was assured by wearing safety glasses.
| Modality | Photoinductive | Photoinductive |
|---|---|---|
| Irradiation parameters | ||
| Treated surface diameter (mm) | 18 | 30 |
| Distance from laser output (mm) | 40 | 65 |
| Irradiation time (sec) | 10 | 26 |
| Laser type | Diode 635 | Diode 635 |
|---|---|---|
| Laser beam parameters | ||
| Wavelenght (mm) | 635 | 635 |
| Irradiation mode | Continuous wave | Continuous wave |
| Power output (mW) | 89 | 89 |
| Target power (mW) | 89 | 89 |
| Fiber diameter (µm) | 600 | 600 |
| Laser spot parameters | ||
| Spot diameter/area at target level (mm/mm2) | 18.6/273 | 30/703 |
| Power density (mW/cm2) | 32.6 | 12.6 |
| Treatment mode | Without contact | Without contact |
|---|---|---|
| Surface treatment data | ||
| Treated surface diameter (mm) | 18 | 30 |
| Treated area (mm2) | 273 | 703 |
| Irradiation time (sec) | 10 | 26 |
| Total energy delivered (mJ) | 890 | 2,314 |
| Total energy density (mJ/cm2) | 326 | 329 |
MTS cell viability assay
Cell viability was determined by 3-(4.5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2Htetrazolium (MTS) assay (Promega Corp., Madison, WI) essentially as reported previously (Chellini et al., 2010). To this purpose, the cells were plated in 96-well culture plates, grown until 80% confluence stimulated or not with diode laser. Soon after diode laser exposure, the washed cells were incubated with the provided MTS solution for 4 h and then the optical density (OD) of soluble formazan was measured using a multi-well scanning spectrophotometer (ELISA reader; Amersham, Pharmacia Biotech, Cambridge, UK) at a wavelength of 492 nm.
Time-lapse videomicroscopy
The dynamic features of the MSCs in culture irradiated or not with diode laser were analyzed for 24 h by time-lapse videomicroscopy (1 frame/min, exposure time 0.5 sec) using a Zeiss inverted phase-contrast microscope (Nikon, Tokyo, Japan) equipped with a 10× objective, Panasonic (Osaka, Japan) CCD camera and JVC BR9030 time lapse video recorder.
Cell proliferation analysis
Cell counting
MSCs cultured in 96-well cultures plates, subjected or not to diode laser irradiation were grown for 72 h after diode laser exposure (T0). Cell number was evaluated in 10 random 691.200 µm2 optical square fields (20× ocular) under an inverted phase contrast microscope Nikon Diaphot 300 (Nikon) in each cell preparation at different times (T0, 24, 48, and 72 h) by two different operators.
EdU (5-ethynyl-2´-deoxyuridine) incorporation assay
MSC proliferation was also evaluated by the EdU proliferation assay using the fluorescent Click-iT® EdU Cell Proliferation Assay (Invitrogen) according to manufacturer's instructions essentially as reported previously (Sassoli et al., 2011). Briefly, MSCs grown on glass coverslips subjected or not to diode laser irradiation were incubated in the presence of the provided solution of 10 µM EdU for 24 h. After that, the cells were fixed with 0.5% buffered paraformaldehyde (PFA) for 10 min at room temperature (RT), and incubated with the Alexa Fluor 488 EdU detection solution for 30 min at RT. Nuclei were counterstained with propidium iodide (PI, 1:30; Molecular Probes, Eugene, OR) for 30 sec. After washing, the coverslips containing the labeled cells were mounted with an antifading mounting medium (Biomeda Gel mount, Electron Microscopy Sciences, Foster City, CA) and observed under a confocal Leica TCS SP5 microscope Leica Microsystems, Mannheim, Germany) using a Leica Plan Apo 40×/1.43NA oil immersion objective. The number of the cells with EdU positive nuclei was evaluated on the digitized images and expressed as percentage of the total cell number/nuclei. At least 10 images were analyzed by two different operators for each experimental condition.
Total RNA extraction and reverse transcription RT-PCR
Expression levels of mRNA of Notch-1, Jagged-1, and Hes-1 were assayed by RT-PCR. Total RNA was isolated by extraction with TRIzol Reagent (Invitrogen), according to the manufacturer's instructions. One microgram of total RNA were reverse transcribed and amplified with SuperScript One-Step RT-PCR System (Invitrogen). After cDNA synthesis for 30 min at 55°C, the samples were pre-denatured for 2 min at 94°C and then subjected to 35 cycles of PCR performed at 94°C for 15 sec, alternating with 56°C for Notch-1 and Jagged-1, 57°C for β-actin and 53°C for Hes-1 for 30 sec and 72°C for 1 min; the final extension step was performed at 72°C for 5 min. The following mouse gene-specific primers were used: Notch-1 (NM_008714.3), forward 5′-GTC CCA CCC ATG ACC ACT AC-3′ and reverse 5′-CCT GAA GCA CTG GAA AGG AC-3′ (327 bp); Jagged-1 (NM_013822.4), forward 5′-CAG GAC ACA CAA CTC GGA AG-3′ and reverse 5′-CCA GCC AAC CAC AGA AAC TAC-3′ (383 bp); Hes-1 (NM_008235), forward 5′-AAT TTG CCT TTC TCA TCC CCA-3′ and reverse 5′- CAG TCA CTT AAT ACA GCT CTC-3′ (342 bp); β-actin (NM_007393.3), forward 5′-ACT GGG ACG ACA TGG AGA AG-3′ and reverse 5′- ACC AGA GGC ATA CAG GGA CA-3′(249 bp). β-actin mRNA was used as internal standard. Blank controls, consisting in no template (water) were performed in each run. PCR products were separated by electrophoresis on a 2% agarose gel and the ethidium bromide-stained bands were quantified by densitometric analysis by using the Scion Image Beta 4.0.2 image analysis program (Scion Corp., Frederick, MD). β-actin normalization was performed for each result.
Western blotting
Cells were resuspended in appropriate volume of cold Cell Extraction Buffer (10 mM Tris/HCl, pH 7.4, 100 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM NaF, 20 mM Na4P2O7, 2 mM Na3VO4, 1% Triton X-100, 10% glycerol, 0.1% SDS, 0.5% deoxycholate; Invitrogen) supplemented with 50 µl/ml Protease Inhibitor Cocktail and 1 mM Phenylmethanesulfonyl fluoride, PMSF (Sigma, Milan, Italy). Upon centrifugation at 13,000g for 10 min at 4°C, the supernatants were collected and the total protein content was quantified using Qubit Protein assay Kit (Molecular Probes) following the manufacturer's instructions. Forty micrograms of total proteins were electrophoresed on NuPAGE® 4–12% Bis–Tris Gel (Invitrogen; 200 V, 40 min) and blotted onto polyvinylidene difluoride (PVDF) membranes (Invitrogen; 30 V, 1 h). The membranes were blocked with Blocking Solution included in the Western Breeze®Chromogenic Western Blot Immunodetection Kit (Invitrogen) for 30 h at RT on rotary shaker and incubated overnight at 4°C with rabbit monoclonal anti-Notch-1 antibody (1:2,000; Abcam Cambridge, UK) rabbit polyclonal anti-Hes-1 (1:2,000; Millipore, Milan, Italy) and rabbit polyclonal anti-β-actin antibody (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA), assuming β-actin as control invariant protein. Immunodetection was performed as described in the Western Breeze® Chromogenic Immunodetection protocol (Invitrogen). Densitometric analysis of the bands was performed using ImageJ software (http://rsbweb.nih.gov/ij) and the values normalized to β-actin.
Confocal immunofluorescence
Cells grown on glass coverslips were fixed with 0.5% buffered PFA for 10 min at RT. After permeabilization with cold acetone for 3 min, the fixed cells were blocked with 0.5% bovine serum albumin (BSA; Sigma) and 3% glycerol in PBS for 20 min and then incubated with the following primary antibodies: rabbit monoclonal anti-Notch-1 (1:200), goat polyclonal anti-Jagged-1 antibody (1:100; Santa Cruz Biotechnology) and rabbit polyclonal anti-Hes-1 (1:200, Millipore) over night at 4°C. The immunoreactions were revealed by incubation with specific anti-rabbit Alexa Fluor 488 or anti-goat Alexa Fluor 568-conjugated IgG (1:200; Molecular Probes) for 1 h at RT. Negative controls were carried out by replacing the primary antibodies with non immune serum; cross-reactivity of the secondary antibodies was tested in control experiments in which primary antibodies were omitted. After washing, the coverslips containing the immunolabeled cells were mounted with an antifade mounting medium (Biomeda Gel mount) and observed under a confocal Leica TCS SP5 microscope (Leica Microsystems) equipped with a HeNe/Ar laser source for fluorescence measurements. Observations were performed using a Leica Plan Apo 63×/1.43NA oil immersion objective. Series of optical sections (1,024 × 1,024 pixels each; pixel size 204.3 nm) 0.4 µm in thickness were taken through the depth of the cells at intervals of 0.4 µm. Images were then projected onto a single “extended focus” image. Densitometric analyses of the intensity of Notch-ICD, Jagged-1 and nuclear Hes-1 fluorescent signals were performed on digitized images using ImageJ software (http://rsbweb.nih.gov/ij) in 20 regions of interest (ROI) of 100 µm2 for each confocal stacks (at least 10).
Patch clamp analysis
The electrophysiological properties of voltage-gated K+ currents of cultured MSC were investigated on glass coverslip-adherent single cells by the whole cell patch-clamp technique, using voltage-clamp mode. The electrode junction potential was evaluated before making the patch and was subtracted from the recorded intracellular potential. All experiments were carried in control physiological solution (150 mM NaCl, 5 mM KCl, 2.5 mM CaCl2, 1 mM MgCl2, 10 mM D-glucose, and 10 mM HEPES) with the K+ channel blocker 4-aminopyridine, 4-AP (2 mM) to avoid the occurrence of the transient outward potassium current Ito and with Nifedipine (10 µM) to block L-type Ca2+ current (ICa,L). The standard solution to fill the electropipettes contained 100 mM potassium glutamate, 35 mM KCl, 5 mM MgCl2, 10 mM HEPES, and 5 mM EGTA; pH was adjusted to 7.2 and had tip resistances of 2–3 MΩ. To record IK,DR only, BaCl2 (0.5 mM) was added to the external solution to block IKir and to evaluate the presence of IBK and IKs, we used iberiotoxin, Ibtx (100 nM) and chromanol, Chr (50 µM), respectively. The RMP was evaluated in current-clamp mode. The delayed-rectifier K+ current (IK,DR) was recorded in voltage-clamp mode by applying voltage steps in 10 mV increments from −80 to 50 mV with holding potential (HP) of −30 mV to block Na+ current (INa) and T-type Ca2+ current (ICa,T). Linear leak, voltage independent ionic currents and capacitive currents were withdrawn online using the P/4 procedure. To this end, the pClamp9 program applied eight negative sub-pulses with voltage fourfold lower than the test voltage at a HP of −80 mV in order to elicit only linear leak, voltage independent ionic currents and capacitive currents. The average of the currents generated by the sub-pulses was subtracted on-line from the test currents. The occurrence of the Kir current was assessed in voltage-clamp by recording the currents in response to voltage ramps ranging from −120 to 50 mV over a period of 0.5 sec, which were imposed every 1 min from a holding potential of −60 mV and digitized at a rate of 5 kHz; two runs repeated every 20 sec were averaged. First, we used the control physiological solution without Ba2+ (control) to record any K+ current (IK,DR and IKir) followed by Ba2+ addition to block IKir (Tennant et al., 2006; Ma et al., 2011). Finally, the average of two ramps elicited in the presence of Ba2+ was subtracted from control currents to evaluate IKir. The role of Kir channel on MSC grown and ion channel activity was evaluated by blocking Kir with Ba2+ (0.5 mM for 24 h. The Cm value was considered as an index of the cell surface area assuming that membrane specific capacitance is constant at 1 µF/cm2. To allow comparison of test current recorded from different cells, the resting membrane conductance, Gm, was normalized to Cm, Gm/Cm (in nS/pF). The current amplitudes (I) were normalized to cell linear capacitance (Cm) to allow comparison of test currents recorded from different cells. The ratio I/Cm is then proposed as current density. For RMP evaluation the small (2–5 mV) liquid junction potential was corrected. The patch pipette was connected to a micromanipulator (Narishige International Inc., East Meadow, NY) and an Axopatch 200B amplifier (Axon Instruments, Inc., Burlingame, CA). Voltage-clamp protocol generation and data acquisition were controlled by using an output and an input of the A/D and D/A interfaces (Digidata 1200; Axon Instruments, Inc.) and pClamp9 software (Axon Instruments).
Presentation of data and statistical analysis
For each experiments at least three cell preparations were analyzed and the experiments were performed in triplicate. The data obtained were reported as mean ± SEM statistical significance was determined by one-way ANOVA and Newman–Keuls multiple comparison test or Student's t-test. A P-value ≤0.05 was considered significant. Calculations were performed using GraphPad Prism software (GraphPad, San Diego, CA). Mathematical and statistical analysis of the electrophysiological data was performed by pClamp9 (Axon Instruments), SigmaPlot and SigmaStat (Jandel Scientific, Erkrath, Germany).
RESULTS
Diode laser stimulates MSC proliferation and notch-1 pathway
Morphological features of MSCs grown on cover slips were determined in time lapse by phase contrast video microscopy; they grew as monolayer of flat, spindle-shaped cells resembling fibroblasts in culture (Fig. 1A). The irradiation with a diode laser at a wavelenght of 635 nm, did not affect cell viability as judged by MTS assay (Fig. 1B) nor caused any substantial difference in morphology in the irradiated cells over a period of 24 h (Fig. 1A). However, compared with the non-irradiated MSCs, the treated cells showed increased cell proliferation, which was visible even in the firts 24 h (Fig. 1A,C). Quantitative analyses obtained from microscopic cell counting showed that the number of irradiated cells was increased of about three times, whereas that of non-irradiated controls was increased of two times after 72 h of culture (Fig. 1C). The induction of cell proliferation by the diode laser was further confirmed by increased EdU incorporation, taken as an index of DNA synthesis, in the irradiated MSC cells as compared with control cells (Fig. 1D).
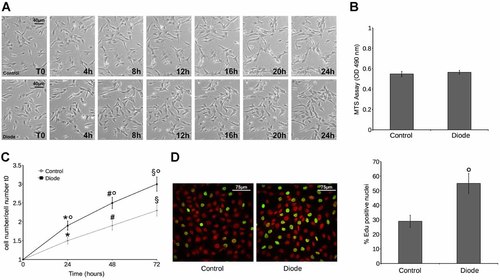
Diode laser irradiation promotes MSC proliferation. A: Time lapse videomicroscopy of control and irradiated cells. Note that irradiated cells show no morphological changes when compared with control cells and proliferate more actively. Proliferating cells were identified and scored on the basis of their peculiar cell rounding and increased luminescence. B: MTS assay using a multi-well scanning spectrophotometer. C: The graph shows the mean increase of cell number during the indicated times. D: Confocal microscopy showing EdU incorporation: the cells were exposed to EdU for 24 h prior fixation and stained with Alexa Fluor 488 EdU detection solution (green) and PI to reveal nuclei (red). The nuclei that had incorporated EdU were counted and plotted as the % of EdU positive over the total nuclei. The images are representative of at least three independent experiments with similar results. *,#,§,° denote P < 0.05 versus T0, 24 h, 48 h and control, respectively.
In order to find the molecular mechanisms underlying the stimulatory effects of the diode laser light on MSC cell viability and growth, we investigated the involvement of Notch-1 pathway, a key determinant of MSC self-renewal (Chen et al., 2012). Notch-1 is a transmembrane receptor which is activated upon interaction with membrane-bound ligands, such as Jagged-1, on the surface of neighbouring cells. Notch-1 intracellular domain (Notch-ICD) is cleaved after the receptor activation and traslocates to the nucleus, where it activates trascription of genes involved in cell proliferation (Chillakuri et al., 2012). By RT-PCR, Western blotting and confocal microscopy analyses, mRNA and protein levels of Notch-1 and Jagged-1 increased progressively over a period of 72 h in control cells, and their expression was significantly up-regulated after laser irradiation (Figs. 2 and 3). In particular, using an antibody capable of recognizing the receptor and activated (Notch-ICD) forms, we were able to reveal that the cytoplasmic and nuclear levels of Notch-ICD were significantly higher 72 h post-irradiation as compared with controls, indicating that increased Notch-1 expression was associated with enhanced Notch-1 signal trasduction in the laser-treated MSCs (Fig. 3). This assumption was further supported by the data showing that the expression of Hes-1, a canonical Notch-1 downstream effector (Borggrefe and Liefke, 2012), was also increased (Figs. 2 and 3) and was particularly evident in the nucleus of the irradiated cells (Fig. 3). Of interest, Hes-1 levels peaked at 24 h and then decreased, reaching the baseline at 72 h, while Notch-1 continued to be highly expressed over the whole period of observation (Figs. 2 and 3). All these findings, taken together, suggested that diode laser irradiation could stimulate MSC proliferation via the activated Notch pathway.
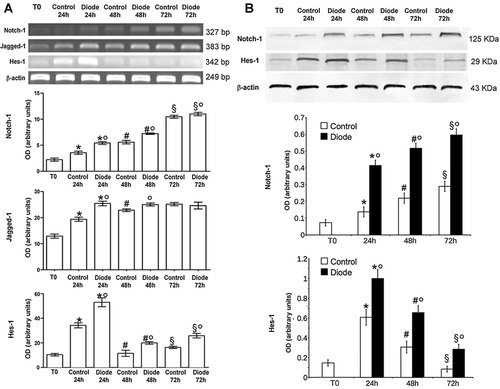
Diode laser irradiation stimulates the expression of Notch-1 and related genes in MSCs. A: RT-PCR and (B) Western blotting analysis of Notch-1, Jagged-1, and Hes-1 expression in control and irradiated cells. The results were normalized with the house-keeping gene β actin. The quantitative analysis of the bands are reported in the bar graphs. The results shown are representative of at least three independent experiments with similar results. *,#,§,° denote P < 0.05 versus T0, 24 h, 48 h and control, respectively.
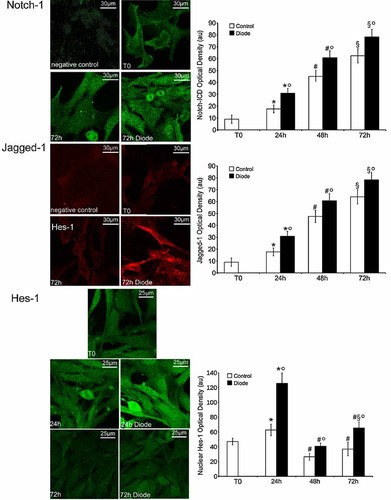
Diode laser irradiation stimulates Notch-1 signaling in MSCs. Confocal immunofluorescence was used to study the cellular localization of Notch-1 receptor and its activated form Notch-ICD (green), Jagged-1 (red), and Hes-1 (green) in control and irradiated cells. The cells were cultured for the indicated times, fixed, incubated with the specific antibodies and immunorevealed with Alexa 488 or 568-conjugated antibodies. Note that diode laser irradiation increases the cytoplasmic and nuclear levels of Notch-ICD, and the membrane expression Jagged-1. Hes-1 nuclear expression also increases in the irradiated cells, particularly after 24 h. Cross-reactivity of the secondary antibodies was tested in control experiments in which the primary antibodies were omitted (negative controls).The graphs show the quantitative analysis of the data. *,#,§,° denote P < 0.05 versus T0, 24 h, 48 h and control, respectively.
Diode laser stimulates MSC proliferation and notch-1 pathway via Kir channel activation
We next performed electrophysiological whole patch-clamp analysis to investigate deeper into the mechanisms underlying stimulation of MSC growth by diode laser. Changes in the membrane properties were recorded soon after laser irradiation. As shown in Figure 4, diode laser caused a significant decrease in cell membrane capacitance (Cm; Fig. 4A), consistent with a reduction of the individual cell size in the treated cells, and concomitantly increased the resting and specific membrane conductance (Gm, Fig. 4B and Gm/Cm, Fig. 4C, respectively). The values of Resting Membrane Potential (RMP, Fig. 4D) did not change significantly, suggesting that diode laser was capable of activation peculiar membrane ion channels (see below) at RMP. In particular, most of MSCs exhibited two distinct types of currents (Figs. 5 and 6): (i) a Ca2+-activated, large conductance K+ current (IBK), which was under-threshold at RMP (−71.2 ± 8.8 mV), and (ii) an inward-rectifier K+ current (IKir), which displayed a reversal potential at −63.1 ± 7.4 mV and was followed by a small outward component at more positive potential. BK current was identified by the relatively rapid activation followed by small inactivation, noisy oscillations and sensitivity to iberiotoxin, whereas IKir was characterized by sensitivity to Ba2+. Of interest, both these currents were greatly potentiated by laser treatment (Figs. 5 and 6), denoting that diode could act as a positive modulator of these channels in MSCs. After irradiation, IKir increased at any voltages and the reversal potential shifted to −72.4 ± 7 (P < 0.05). These findings indicated that current through Kir channels maintained RMP values after irradiation. Moreover, L-type Ca2+ current (ICa,L) was detected in 20 ± 3% and ICa,L and T-type Ca2+ currents (ICa,T) were both identified in 11 ± 2% of the control cells (Figs. 5 and 6). These currents also increased after irradiation; ICa,L and ICa,L+ ICa,T were recorded with significantly higher amplitude in 32 ± 4 and 21 ± 3% of the irradiated cells (Figs. 5 and 6). Since increased IKir have been shown to reflect the proliferative activity of various stem cell types, including MSCs (Tao et al., 2007), we next treated the cells with Ba2+ to block Kir channels and evaluate their role in MSC proliferation. We found that incubation with Ba2+ 5 mM for 24 h suppressed Kir currents (Figs. 5 and 6) and significantly reduced MSC proliferation as well as Notch-1 expression and activation (Fig. 7). The channels' inhibition was also able to attenuate the stimulatory effects induced by laser irradiation on MSCs (Fig. 7), suggesting that activation of Kir channels played a crucial role in controlling MSC proliferation and Notch-1 activation by the diode laser.
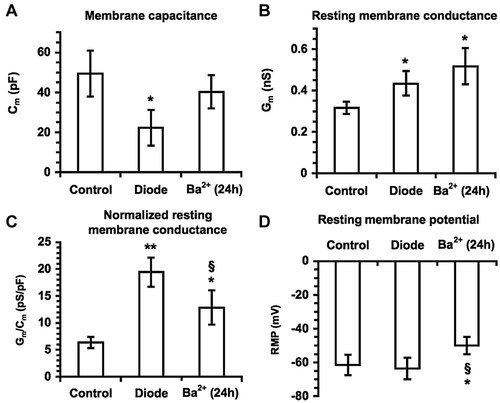
Diode laser affects plasma membrane electrical properties in MSCs. A: Cell membrane capacitance, Cm; B: resting membrane conductance, Gm; C: normalized resting membrane conductance Gm/Cm; D: resting membrane potential, RMP, in the indicated experimental conditions. Ba2+ (24 h), indicates cells pre-treated with BaCl2 (0.5 mM) for 24 h to block IKir. *, ** P < 0.05 and <0.01 versus control; § P < 0.05 versus diode. Experiments were performed in control solution without the presence of channel blockers on 30 (control and diode) and 12 (pre-treated with Ba2+ for 24 h) cells. Data are mean ± SEM.
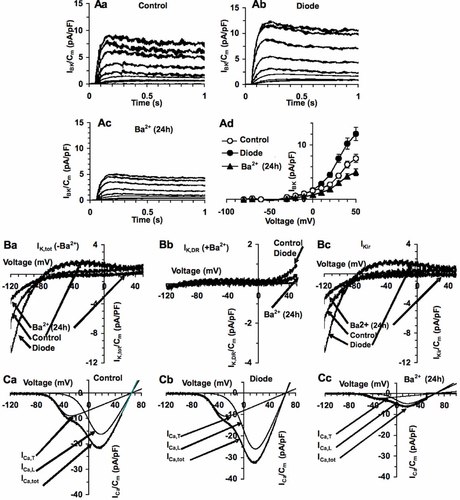
Diode laser irradiation increases normalized K+, BK, Kir, and T-and L-type Ca2+ currents. A: Representative IBK traces induced by pulse protocol from HP of −40 mV to inactivate Na+ and T-type Ca2+ currents in control (a), diode irradiated (b), and Ba2+-treated for 24 h (c) cells. (d) Mean values ± SEM of the IBK-V curves recorded in the indicated experimental conditions (n = 10, control; n = 7, diode; n = 7, Ba2+-treated) at a voltage threshold, −35 ± 3 mV. B: Representative recordings to evaluate IKir with a voltage ramp starting from HP of 0 mV in the indicated experimental conditions. (a) Ik,tot currents recorded in the absence of Ba2+, (b) IK,DR in the presence of Ba2+, (c) IKir obtained by subtracting the trace in (b) from the corresponding traces in (a). Note the different ordinate scale in a,b,c. In c, control and diode traces are superimposed. The experiments were performed in control external solution in the presence of 4-AP (2 mM), Chr (50 µM), Nifedipine (10 µM) and in the presence or absence of BaCl2 (0.5 mM). (C) Representative total Ca2+ currents with a voltage ramp starting from HP at −80 mV in control (a), diode irradiated (b) and Ba2+-treated for 24 h (c). Superimposed are the fit of the sum of two Boltzmann functions; each function is also reported as single fit to identified the voltage dependence of T- and L-type Ca2+ current (ICA,T and ICa,L).
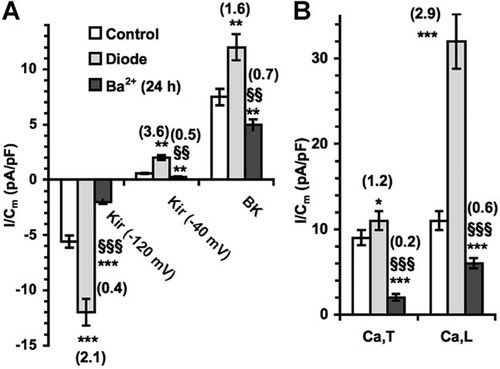
Normalized IKir, IBK, ICa,T, and I Ca,L mean current amplitudes. The currents were evaluated at −120 and −40 mV 40 mV (Kir, n = 12; BK, n = 10, and T- and L-type Ca2+, n = 6 in control, after diode irradiation (n12, 10 and 6, respectively) and after pre-treatment for 24 h with Ba2+ (0.5 mM; n = 6, 7, and 5, respectively). *, **, *** P = 0.05, <0.01 and <0.001 versus the related control; §, §§, §§P < 0.05, 0.01, and 0.001 versus diode. In brackets are the number of fold changes with respect to control.
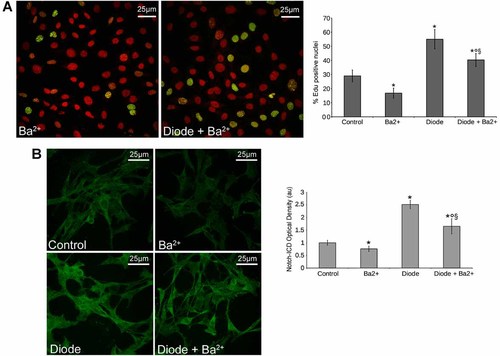
Inhibition of Kir channels affect cell proliferation and Notch-1 expression in control and irradiated cells. The cells were pre-treated with Ba2+ (0.5 mM) for 24 h to inhibit Kir current and irradiated or not with diode laser and cultured for 24 h. A: EdU incorporation assay showing reduced cells proliferation after pre-treatment with Ba2+ both in diode laser unstimulated and stimulated cells. B: Confocal immunofluorescence analysis to reveal the effects of the channel's blocker on Notch-1 expression and activation in unstimulated and irradiated cells. The images are representative of at least three independent experiments with similar results. *,°,§ denote P < 0.05 versus control, versus diode, and versus Ba2+, respectively.
Discussion
The issue of in vitro pre-conditioning of mesenchymal stromal stem cells prior transplantation to improve the current methods for MSC-based regeneration is an intriguing approach in the field of regenerative medicine and dentistry. In such a view, the results of the present study suggest that diode laser (635 nm, 4 × 4) can be employed for stimulating MSC proliferation in vitro and to improve their regenerative potentials, allowing these cells to be transplanted in the right quantity and within a short time interval in the injured tissue. Moreover, we have demonstrated that the enhancement of cell proliferation by diode laser irradiation was associated with the up-regulation and activation of Notch-1 pathway. Notch signaling is emerging as a crucial regulator of widely divergent cellular processes, including cell proliferation, cell-fate specification, survival and apoptosis in many cell types (Borggrefe and Oswald, 2009; Gering and Patient, 2010; Cave, 2011; Chen et al., 2012). Its involvement in MSC cell maintenance and growth has also been recently demonstrated (Chen et al., 2012). We showed that the temporal expression of Notch-1 and its canonical target gene, Hes-1, a transcriptional repressor of differentiation (Cave, 2011; Borggrefe and Liefke, 2012), was quite similar in both the control and irradiated cells, giving strong evidence that diode laser irradiation exerted a controlled (physiological) induction of MSC proliferation. In particular, the levels of Notch-1 continued to progressively increase over 72 h of culture, whereas those of Hes-1 displayed a peak expression at 24 h and decreased thereafter, reaching the baseline at 72 h. It is possible that the decline in Hes-1 expression when Notch pathway continued to be activated (after 24 h) may be dependent on the ability of Notch to drive the expression of individual downstream genes in a spatially and temporally distinct pattern, and on the proposed function of Hes-1 as a negative regulator of its own promoter activity (Cooper et al., 2000; Iso et al., 2003; Cave, 2011). The involvement of Notch-1 in the promotion of MSC proliferation by diode laser is consistent with previous observation obtained by cDNA microarray analysis showing that LLLI regulates in these cells, the expression of several genes involved in the cell cycle progression and apoptosis, including Akt1, a downstream serine/threonine kinase of Notch-mediated pathway (Zhao et al., 2010; Wu et al., 2012).
It is becoming increasingly clear that plasma membrane ion channels are extremely effective in transducing surface events, namely, chemical, electrical and physical signals, into biological responses within the cells (Levin, 2012). They are, in fact, implicated in the control of many essential cellular cell functions, such as cell proliferation/differentiation, migration, and gene expression in normal and neoplastic cells (Arcangeli et al., 2009). The ion channels more commonly associated with control of proliferation are those associated with cell volume decrease, such as Cl− channels (Becchetti, 2011), and those mediating Ca2+ influx (Ahidouch et al., 2010; Margheri et al., 2012). MSCs from bone marrow display multiple functional currents, including: (i) delayed-rectifier K+ currents (IK,DR), showing a considerable noise and a rapid activation/slow inactivation, which corresponds to the Ca2+-activated, large conductance K+ current (IBK or Maxi K+ current); slow activating K+ currents named IKs, and; (iii) inward-rectifier K+ current (IKir) which contribute to membrane hyperpolarization (Heubach et al., 2003; Li et al., 2005; Benvenuti et al., 2006; Tao et al., 2007). Of interest, the contribution of these currents in MSC cycle progression have been previously demonstrated by our group (Benvenuti et al., 2006) and more recently by Zhang et al. (2012). In such a view, the present study, while extending these observations, provide novel evidence that the activity of these channels can be modulated by diode laser irradiation. In particular, the inhibition of the endogenous Kir currents with Ba2+ for 24 h strongly attenuated the cell growth and concomitantly reduced Notch-1 up-regulation in control and irradiated cells. These data, contribute to expand our knowledge on the mechanisms mediating laser-induced proliferation, suggesting that modulation of channel gating by laser light may be a critical step in the up-regulation of Notch-1 signaling in MSCs. Although the detailed mecahnisms are unknown, we may suggest that stimulation may depend, at least in part, on augmented release of growth factors by MSCs by the laser treatment. This on the basis of previous observations showing that ion channels can regulate cell proliferation through the modulation of exocytosis of paracrine and autocrine mediators (Becchetti, 2011) and that LLLI stimulates the release of VEGF and NGF by MSCs (Hou et al., 2008). Along this line, it is worth noting our recent findings underscoring the key role played by MSC-derived VEGF in the regulation of Notch-1 signal in proliferating myoblastic cells (Sassoli et al., 2012).
In conclusion, the results of the present study indicate that diode laser, operating at a wavelength of 635 nm stimulates the proliferative potential of MSCs. The enhancement of cell growth after irradiation is dependent on the activation of physiological processes, including membrane ion channel activity and Notch-1 up-regulation. We suggest that irradiation with diode laser (4 × 4) may provide a novel and safe approach for the in vitro modulation of MSCs before cell therapy and expanding its therapeutic applications for tissue regeneration in medicine and dentistry.