Autophagy and ionizing radiation in tumors: The “survive or not survive” dilemma†
Conflicts of interest: nothing to declare.
Abstract
Autophagy is a so-called “self-eating” system responsible for degrading long-lived proteins and cytoplasmic organelles, whose products are recycled to maintain cellular homeostasis. This ability makes autophagy a good candidate for a survival mechanism in response to several stresses, including the tumor cell transformation. In particular, recent studies suggested that autophagy functions as a pro-death mechanism within different tumor contexts. It is, however, widely reported that autophagy represents both a survival mechanism or contributes directly to cell death fate. This interplay of the autophagy functions has been observed in many types of cancers and, in some cases, autophagy has been demonstrated to both promote and inhibit antitumor drug resistance. From a therapeutical point of view, the effects of the modulation of the tumor cell autophagic status, in response to ionizing radiations, are presently of particular relevance in oncology. Accordingly, this review also provides a perspective view on future works for exploring the modulation of autophagic indices in tumor cells as a novel molecular-based adjuvant strategy, in order to improve radiotherapy and chemotherapy effects in cancer patients. J. Cell. Physiol. 228: 1–8, 2013. © 2012 Wiley Periodicals, Inc.
Autophagy
The term autophagy, which derives from Greek and means self (auto) eating (phagy), refers to a catabolic pathway able to promote lysosomal degradation of cytoplasmic components and organelles. Macroautophagy is a specific form of autophagy that involves autophagosomes (Maiuri et al., 2007), double-membrane vesicles that progressively engulf cytoplasmic constituents (including protein aggregates as well as old, damaged, and supernumerary organelles) and deliver them to lysosomes for degradation (Fig. 1). Membranes from endoplasmic reticulum, Golgi apparatus, and mitochondria organelles can also contribute to the formation of autophagosomes (Juhasz and Neufeld, 2006). Once they are sealed, autophagosomes fuse with lysosomes to generate the so-called auto(phago)lysosomes, and this is coincident with the acidification of the luminal microenvironment and the activation of lysosomal hydrolases, that degrade both the autophagosomal inner membrane and its cargo (Lum et al., 2005a). Although baseline autophagy contributes to the maintenance of cellular homeostasis, autophagic flow is upregulated in response to many adverse conditions, including nutrient or growth factor deprivation, accumulation of unfolded proteins and intracellular microbial infections. Thus, autophagy frequently exerts cytoprotective functions by acting as a stress response mechanism (Kroemer and Levine, 2008). The critical role of autophagy in maintaining cell viability upon shortage of external nutritional sources was first documented in yeast (Ohsumi, 2001; Levine and Klionsky, 2004), and later, a similar cytoprotective role of autophagy-dependent production of metabolites during nutrient starvation or growth factor deprivation, was also demonstrated in mammalian cells lacking essential autophagy-related (ATG) genes (Boya et al., 2005; Degenhardt et al., 2006; Lum et al., 2005b). Mice deficient of known autophagic signaling molecules die either during embryonic development or within 1 day after the birth, indicating that autophagy can also prolong the life of multicellular organisms (Qu et al., 2003; Yue et al., 2003; Kuma et al., 2004; Komatsu et al., 2005). Autophagy is also involved in removing damaged or over-activated and thereby potentially dangerous organelles (mitochondria, endoplasmic reticulum, peroxisomes, and lysosomes) as well as cytotoxic protein aggregates from the cell, promoting overall survival (Ravikumar et al., 2002; Rodriguez-Enriquez et al., 2004; Bernales et al., 2006; Iwata et al., 2006; Høyer-Hansen and Jaattela, 2007; Ostenfeld et al., 2008). This autophagy-mediated removal of intracellular protein aggregates, as well as of pathogens, has been suggested to prolong the life of individuals exposed to neurodegenerative processes and infectious diseases (Ravikumar et al., 2002, 2004; Qu et al., 2003; Kouroku et al., 2007). However, autophagy has also been implicated in cell death known as “autophagic or type II programmed cell death” (Schweichel and Merker, 1973; Clarke, 1990; Kroemer et al., 2005). It should be noted that this definition does not distinguish whether autophagy directly contributes to death or whether it is a failed effort to preserve cell viability. Due to these reasons, the definition of autophagy-dependent cell death is preferred (Klionsky et al., 2008). Recent identification of the ATG family of genes, controlling autophagosome formation, has allowed the confirmation of the role of autophagy in cell death signaling. Studies based on ATG genes depletion have shown autophagy-dependent death of cultured cells exposed, for example, to a variety of treatments, for example, endoplasmic reticulum and oxidative stresses, caspases inhibition, growth factor deprivation, interferon-gamma and anti-cancer drug administration, p53 activation, oncogene activation, radiation administration (Yu et al., 2004b; Pyo et al., 2005; Crighton et al., 2006; Djavaheri Mergny et al., 2006; Kim et al., 2006a; Li et al., 2006; Reef et al., 2006; Shimizu et al., 2004; Xu et al., 2006; Yu et al., 2006; Ding et al., 2007).
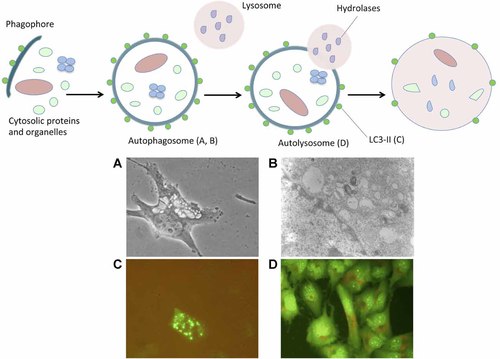
Schematic representation of the autophagy process. Autophagy is initiated by the generation of the phagophore, an isolation membrane that likewise derives from the endoplasmic reticulum. This phagophore surrounds the material destined to degradation, and eventually forms a double-membrane vesicle known as autophagosome. Autophagosomes mature by fusing with lysosomes or late endosomes and hence generate auto(phago)lysosomes. Finally, the luminal content of the auto(phago)lysosome is catabolized by acidic hydrolases, resulting in the generation of metabolic substrates that are re-exported into the cytosol via permeases of the auto(phago)lysosomal membrane. A Light microscopy at 40× resolution morphological examination (A), a transmission electron microscopy visualization of doubled-membrane vesicles (B), an exogeneous LC3B-GFP expression at autophagosomes after transient transfection using fluorescence microscope (C) and an orange-acridine fluorescent staining of acidic vesicles (D) of human glioma T98G cells after autophagy induction by Rapamycin administration (unpublished data). Autophagy induction and autophagy detection assays were performed as reported (Barbieri et al., 2011; Palumbo et al., 2012).
How Autophagy Occurs in Cancer
Upregulation of autophagy has been observed in many types of cancer and it has been demonstrated to promote both cell survival and cell death. However, to date it is still unknown how autophagy switches between these two cellular fates; this shift probably depends, to a large extent, on the nature and duration of the induced cellular stress/treatment as well as on the involved tumor type. For example, autophagy protects HeLa cervix carcinoma cells against starvation, but it contributes to the death of the same cells following treatment with interferon-gamma (Boya et al., 2005; Pyo et al., 2005); differently, Ding et al. (2007) showed that the autophagy induction by Tunicamycin, an agent that blocks the synthesis of all N-linked glycoproteins and causes cell cycle arrest in G1 phase, enhanced the survival of colon cancer cells but, in a different context, it contributed to the killing of immortalized murine embryonic fibroblasts. More recently, the resistance of Paclitaxel (antineoplastic drug) has been associated with profound changes in cell death response with a depletion of multiple apoptotic factors in breast cancer, thus promoting a switch from apoptosis to autophagy as the principal mechanism of drug-induced cytotoxicity (Ajabnoor et al., 2012). However, in a different cancer type, that is, in osteosarcoma, a recent study of Huang et al. (2012) demonstrated that high mobility group box 1 protein (HMGB1)-mediated autophagy played an important role to confer resistance to chemotherapeutics. Inhibition of both HMGB1 and autophagy increased the drug sensitivity of osteosarcoma cells in vitro and in vivo (Huang et al., 2012). The final outcome might also depend on the activity level of autophagy: a moderate induction supports cell survival while massive and/or prolonged induction leads the cell to literally “eat itself,” thus to death. Furthermore, other results indicated that autophagosome formation was not per se the main pro-death determinant, but the final fusion with lysosomes to form autolysosomes was required to induce effective cell death processes (Trincheri et al., 2008). Thus, it was suggested that the autolysosomes themselves might play a role in the autophagic cell death pathway by leaking lysosomal hydrolases into the cytosol, in conditions where they can induce either apoptosis- or necrosis-like cell death, depending on the amount of the leakage (Kroemer and Jäättelä, 2005). To this regard, Andrographolide (Andro), a diterpenoid lactone isolated from an herbal plant Andrographis paniculata, was recently demonstrated to suppress the autophagic flux, inhibiting autophagosome maturation not by affecting the lysosomal function, but by impairing autophagosome–lysosome fusion (Zhou et al., 2012). In this work, Andro was capable to sensitize cisplatin-induced cell killing of human cancer cells via apoptosis induction and through the suppression of autophagy. Furthermore, Li et al. (2012) demonstrated that a cancer-associated gene, LAPTM4B, that plays an important role in lysosomal functions, was critical for autophagic maturation. Its amplification and overexpression promoted autophagy, rendering tumor cells resistant to metabolic and genotoxic stresses and thus resulting in an increase of tumor growth. Studies on apoptosis defective cells suggested that autophagy, as well as lysosomal membrane permeabilization, emerged as a cell death mechanism upon treatment with various anti-cancer drugs, once the primary cell death pathway was inhibited (Fehrenbacher et al., 2004). Upregulation of autophagy was also detected in Bax/Bak(−/−) cells and inhibition of pro-apoptotic proteins with consequential induction of autophagy sensitized cancer cells to therapy (Kim et al., 2006b). Cancer cells that accumulate defects in their apoptotic machinery may be more prone to succumb by autophagy than their normal counterparts. Importantly, differences in the upstream signaling pathways activating the autophagic machinery are likely to contribute to the cell fate. In particular, the induction of autophagy via pathways that inhibit the potent survival kinase Akt sensitized cells to death (Martelli et al., 2007). In addition, upregulation of XIAP-associated factor 1 (XAF1) induced apoptosis and inhibited tumor growth in gastric cancer cells. In a recent study, the transduction of an adenovirus XAF1-mediated vector, induced autophagy through upregulation of Beclin 1 protein expression and by inhibition of Akt/p70S6K pathway, revealing a new mechanism of XAF1 in tumor suppression (Sun et al., 2011). The tubulin inhibitor MG-2477 (3-cyclopropylmethyl-7-phenyl-3H-pyrrolo[3,2-f]quinolin-9(6H)-one) inhibited the in vitro growth of several cancer cell lines, blocking tubulin polymerization and arresting cells in metaphase (Viola et al., 2012). Treatment of human adenocarcinoma A549 cells with MG-2477 caused cells to arrest in G2/M phase of the cell cycle, which was accomplished by autophagy induction, in addition to the reduction of the phosphorylation status of mTOR downstream targets, that is, p70 ribosomal S6 kinase and 4E-BP1. Overexpression of Akt, upstream of mTOR, decreased MG-2477 induced autophagy, indicating that Akt was involved in autophagy modulation. Moreover, the therapeutic potential of the Akt inhibitor Triciribine was tested in T-ALL (T-cell acute lymphoblastic leukaemia) cell lines (Evangelisti et al., 2011). Triciribine caused a dose-dependent dephosphorylation of Akt1/Akt2 and of mTOR complex 1 downstream targets. Triciribine-induced autophagy could be interpreted as a defensive mechanism, because the autophagy inhibitor Chloroquine increased Triciribine-induced apoptosis. In addition, autophagy can facilitate the caspase-dependent execution of the cell in conditions where autophagic and apoptotic proteases are simultaneously activated (Berry and Baehrecke, 2007). And finally, the different signaling pathways may result in different cargo-specificity of the autophagosomes. For example, selective autophagic degradation of catalase has been reported in fibroblasts treated with the pan-caspase inhibitor zVAD-fmk, but not in the same cells during starvation-induced autophagy (Yu et al., 2004a; Yu et al., 2006). Specific removal of catalase then contributed to zVAD-fmk-induced autophagic cell death via the accumulation of reactive oxygen species, membrane lipid oxidation, and loss of membrane integrity.
Autophagy Expression as a Prognostic Factor in Cancer
During the last few years, autophagy expression was investigated as a prognostic factor in cancer. In detail, the expression of Beclin 1 transcript and protein were examined in 212 primary human brain tumors (Miracco et al., 2007). In most high-grade astrocytic, ependymal neoplasms and atypical meningiomas, it was found a decrease of cytoplasmic protein expression that was, instead, high in the majority of low-grade tumors and in medulloblastomas. The expression level of Beclin 1 mRNA was significantly lower in glioblastomas than in low grade and in all glial tumors when compared to all meningiomas. These data suggested a possible differences of Beclin 1 involvement and its role among the different histotypes of brain neoplasms. The prognostic role of Beclin 1 expression was also investigated by immunochemistry in high-grade glioma patients (Pirtoli et al., 2009), founding that high Beclin-1 cytoplasmic expression protein (BPCE score) positively correlated with apoptosis, and negatively with cell proliferation. High BPCE was also significantly correlated with survival both at the univariate and multivariate analysis, with high KPS values, and with the accomplishment of an optimal postoperative therapy. Furthermore, among patients showing a MGMT methylated gene, survival was significantly higher in cases with a higher BPCE score. In hepatocellular carcinoma (HCC), it was reported a decreased basal expression of autophagic genes and their corresponding autophagic activity under conditions of starvation; in particular, autophagy defect well correlated with the highly malignant phenotype (Shi et al., 2009). Moreover, in colorectal tumor cells, perinuclear LC3A accumulation was found as a marker of good prognosis, presumably reflecting a basal autophagic activity (Giatromanolaki et al., 2010). An abnormal or excessive autophagic response, as indicated by increased numbers of SLS (“stone-like” intracellular structures) was linked to metastasis and poor prognosis. Therefore, significative increase of SLSs was strongly correlated with a poor outcome also in non-small cell lung carcinoma, suggesting possibly that autophagy functions as a survival tool in cancer cells (Karpathiou et al., 2011). To this regard, it was recently reported that high SLS counts were associated with tumors of extremely poor prognosis. In contrast, a basal level of autophagic activity, as exemplified by the diffuse cytoplasmic and the cytoplasmic/juxta-nuclear patterns, had no impact on prognosis (Sivridis et al., 2011). Furthermore, in another study (Miracco et al., 2010), it was assessed that the expression of Beclin 1 and LC3 in cutaneous melanocytic lesions correlates with conventional histopathologic prognostic factors. Both genes were expressed in all the investigated conditions: Beclin 1 cytoplasmic protein and mRNA, as well as LC3 mRNA, significantly decreased with tumor progression. The lowest expression of LC3 II protein was observed in melanoma metastases (53.3% of cases). Beclin 1 expression was also investigated in primary intrahepatic cholangiocarcinoma (Dong et al., 2011). Immunopositivity for Beclin 1 was found in 72.2% (78 of 108) samples and low Beclin 1 expression was significantly associated with lymph node metastasis. In survival analysis, low Beclin 1 expression was associated with worse overall survival and disease-free survival. In conclusion, in certain cancer types, the assessment of autophagic activity, particularly in the form of SLS, might be useful for evaluating tumor aggressiveness and grade, thus being a potential tool in therapy.
Autophagy and Ionizing Radiation in Cancer
Ionizing radiation (IR) is presently considered a useful component of the antineoplastic treatment. However, some malignancies are relatively resistant to radiation treatment, while others are more responsive. A variety of approaches have been utilized in the efforts to enhance radiation sensitivity. The “classical” radiobiological approach was based on radiation dose fractionation, in order to devise optimal treatment schedules that could optimize tumor cell kill while sparing normal tissues from radiation damage (Jones et al., 1995); to this regard, it is of the utmost importance to identify IR sensitivity to experimental doses that correspond to clinical doses per fraction, in the perspective of assessing an optimal dose–response curve. Recent studies have identified autophagy as a cell death pathway that may mediate IR sensitivity. To this regard, although radiotherapy (RT) is the most efficacious strategy in non-resectable non-small cell lung cancer, IR-resistant clones may lead to treatment failure. Importantly, compared with radiation alone, the alkaloid Berberine, at 5 and 10 mM concentrations, in combination with IR showed significant enhancement on radiation-induced clonogenic inhibition in A549 cells, as well as a substantial shrinkage of the tumor volume in a mouse model (Peng et al., 2008). Administration of Berberine enhanced the cytotoxicity of radiation in both in vivo and in vitro models of lung cancer, resulting in the induction of autophagy. Moreover, autophagy has been reported to be increased in irradiated cancer cells resistant to various apoptotic stimuli, and its induction via mTOR inhibition enhanced radiosensitization in apoptosis-inhibited H460 lung cancer cells and in a derived xenograft model, suggesting that combined inhibition of apoptosis and mTOR enhance radiation favorable response (Kim et al., 2008). Accordingly, it has been demonstrated that knock-down of the pro-apoptotic Bak and Bax proteins resulted in an increase in autophagic cell death and in lung cancer radiosensitivity (Moretti et al., 2007). To further explore the potential of apoptosis inhibition as a strategy to sensitize lung cancer for therapy, M867, a novel caspase-3 inhibitor, was tested in combination with IR (Kim et al., 2008). M867 enhanced the cytotoxic effects of IR in lung cancer cells, accomplished by autophagy cell death activation, even in presence of apoptosis inhibition. Furthermore, in the same tumor, several studies have shown evidence in favor of a strategy directed to target epidermal growth factor receptor (EGFR) signaling cascade proteins, to enhance the overall antitumor activity of radiation. Targeting EGFR-associated downstream signaling radiosensitized a panel of non-small cell lung cancer cell lines (Choi et al., 2010). Although apoptosis was the primary mode of death when cells were subjected to LY294002, a PI3K inhibitor, or to AKT inhibitor VIII, cells pre-treated with Rapamycin showed co-hexistence of apoptosis and autophagy death pathways. To this regard, simultaneous up-regulation of apoptosis, using the Bcl-2 inhibitor ABT-737, and autophagy, through mTOR inhibition by Rapamycin, was used to enhance radiosensitivity of H460 cells and to obtain a growth delay in a xenograft model (Kim et al., 2009). Also, zinc ions supplementation showed anti-apoptotic effects in cell culture, and thus the zinc ionophore PCI-5002 radiosensitized lung cancer cells by inducing autophagic cell death (Kim et al., 2011). In prostate cancer models, administration of the mTOR inhibitor RAD001, as well as the blockage of apoptosis by caspases inhibition and by Bax/Bak small-interfering RNA treatments, were able to enhance radiation-induced mortality and induced autophagy cell death (Cao et al., 2006). New treatments employing oncolytic adenoviruses are promising anti-prostate cancer agents, and their efficacy can be improved by combining them with conventional therapies such as IR. In particular, the combination of oncolytic adenovirus with IR significantly increased in vivo antitumor efficacy compared to radiation alone: in this experimental setup, microarray analysis showed dysregulation in cell cycle and mTOR pathway and a concomitant increase in autophagocytosis (Gewirtz et al., 2009). In breast cancer MCF-7 cells, radiation-induced inhibition of Rapamycin-sensitive pathway caused changes in mitochondria metabolism, activation of autophagy, and an overall decrease in cell survival (Paglin et al., 2005); in addition, autophagic cell death rate was promoted by vitamin D and its analog EB 1089 administration, resulting in enhanced IR effects (Gewirtz et al., 2009). On the other hand, autophagy was found to contribute to resistance of MDA-MB-231 and HBL-100 breast cancer cell lines to IR (Chaachouay et al., 2011). Endocrine therapy was found to interfere with autophagy (Berardi et al., 2011), and it must be taken into account in hormone-dependent cancer, as many breast cancers. Induction of autophagy was able to enhance IR sensitivity also in colon cancer cells; in particular, BCG/CWS (a novel antitumor immunotherapy compound) had a radiosensitizing effect through the induction of autophagic cell death (Yuk et al., 2010). The role of autophagy was also investigated in the treatment of pancreatic cancer with Gemcitabine, a nucleoside analog used in chemotherapy, combined with IR: as a result, in these cells, autophagy activation induced a significant antiproliferative effect (Mukubou et al., 2010). In HCC cells, while apoptosis was in a steady-state level, autophagy was considerably increased after high-linear energy transfer (LET) IR and augmented by the in vitro addition of Oxaliplatin, a third generation platinum drug (Altmeyer et al., 2010). Moreover, considerable autophagy and only limited apoptosis took place in the tumor xenografts after high-LET irradiation, confirming previous in vitro results, and suggesting that autophagy may act as a predominant mode of cell death, conferring a significant efficacy of high-LET radiation treatment (Altmeyer et al., 2011). However, the relative resistance of liver cancer cells to IR and to chemotherapeutic agents seemed sometimes due to a specific autophagic cellular response. In fact, IR-induced autophagy provided a self-protective mechanism against IR in HepG2 cells, and the addition of 3-methyleadenine (3-MA), a specific inhibitor of autophagosome formation, enhanced the cytotoxicity of IR in cell lines and suppressed tumor growth in animal models (Tseng et al., 2011). Also, in esophageal cancer, 3-MA combined with radiation significantly decreased cell viability, compared with radiation alone, accompainied by a reduction of the autophagic status (Chen et al., 2011).
Autophagy and Ionizing Radiation in Glioblastoma
Glioblastoma is the most aggressive primary brain tumor, and these patients have a poor clinical outcome, with a median survival of 12–15 months. To date, post-operative RT is effective in improving survival in patients affected by glioblastoma (Salazar et al., 1979; Walker et al., 1979). Combination of RT with concurrent and sequential administration of Temozolomide (TMZ) improved prognosis to a significant, but limited extent, as a further adjuvant therapy after surgery (Stupp et al., 2005). However, resistance of glioblastoma to most antineoplastic agents, including IR, is a major challenge for tumor research. During the past two decades, new putative cell death modalities have been investigated to this regard. In particular, the interest of molecular oncologists was directed toward the identification of novel potential targets effective in regulating the autophagy process (Barbieri et al., 2011). In fact, some data suggest that autophagy might be involved in high-grade glioma prognosis and response to therapy (Miracco et al., 2007; Pirtoli et al., 2009). Most recently, it was demonstrated that the autophagic process was involved in the modulation of viability and survival effects in human glioma cell lines after IR, combined or not with TMZ (Palumbo et al., 2012). In particular, T98G, but not U373MG cells showed a high radiosensitivity, especially at low and intermediate doses, associated with a constitutive autophagy activation status; a Rapamycin-mediated additional autophagy activation, resulted in a further radiosensitivity of T98G cells, and was able to promote radiosensitivity also in U373MG cells. Moreover, autophagy inhibition by siRNA against BECN1 or ATG-7 genes totally prevented decrease in viability after both IR and IR/TMZ treatments only in the radiosensitive T98G cells, confirming an additive effect of autophagy in inducing cytotoxicity after IR.
The mechanisms underlying neoplastic glial cell growth inhibition after administration of IR, however, remain largely unknown. The response of glioblastoma cells to IR and the elucidation of the factors that correlate with the radiosensitivity of these tumors were characterized in a variety of studies. Recent in vitro and in vivo experiments, identified autophagy as the major non-apoptotic cell death effector, also activated after IR, alone or combined with TMZ administration (Zhuang et al., 2009). It has been demonstrated the evidence of a major susceptibility of glioblastoma cells to autophagy-associated cell death after IR, rather than to apoptosis (Zhuang et al., 2011). In particular, six human cell lines were subjected to increasing doses of radiation, demonstrating a dose-dependent suppression of cell proliferation with transient increase in the expression of the cyclin-dependent kinase inhibitors (CDKIs) p21 and p27 (Yao et al., 2003). While apoptosis did not occur after radiation in any cell lines, autophagic changes were observed, regardless of the relative radiosensitivity of the cell line. However, it has been demonstrated that autophagy inhibitors, 3-Methyladenine and Bafilomycin A1, radiosensitized U373-MG cells (Ito et al., 2005). In particular, gamma H2AX foci, that show the extent of DNA double-strand breaks, were more pronounced and prolonged in the cells treated with IR and autophagy inhibitors than in those treated with IR alone, suggesting that the inhibition of autophagy tuned radiosensitization parameters of malignant glioma cells.
Regarding the condition of cellular radiosensitivity, DNA-dependent protein kinase (DNA-PK) plays a major role in the repair of DNA double-strand breaks induced by IR. Lack of DNA-PK caused defective DNA double-strand break repair and radiosensitization (Plumb et al., 1999). In human malignant glioma M059J and M059K cells, the role of DNA-PK in IR-induced apoptotic and autophagic cell death was investigated (Daido et al., 2005). Low-dose IR induced massive autophagic cell death in M059J cells, that lacked the catalytic subunit of DNA-PK (DNA-PKcs), while most of M059K cells, in which the catalytic subunit was expressed at costitutive levels, survived and proliferated, although a small portion of the cells underwent apoptosis. Furthermore, antisense oligonucleotides against DNA-PKcs radiosensitized T98G and U373-MG cells by inducing autophagy. Moreover, it is well established that autophagy was mainly regulated by the mammalian target of Rapamycin (mTOR) pathway (Klionsky and Emr, 2000; Meijer and Codogno, 2004). The Akt/mTOR pathway also mediated oncogenesis and radioresistance (Kim et al., 2006b). To this regard, treatment with the Akt inhibitor (1L-6-hydroxymethyl-chiro-inositol 2(R)-2-O-methyl-3-O-octadecylcarbonate) successfully inhibited Akt activity and reduced cell viability in U87-MG glioma cells, radiosensitizing the cells through autophagy induction (Fujiwara et al., 2007). Furthermore, xenografts experiments were performed in nude mice subcutaneously transplanted with U-87-MG cells, demonstrating that Oxaliplatin, a coordination complex that is used in cancer chemotherapy, combined with high-LET radiations, caused a marked reduction of tumor growth via autophagy, compared with irradiation alone (Benzina et al., 2008). Autophagy was also investigated in U118-MG glioblastoma cells after IR combined with administration of arsenic trioxide, an antineoplastic drug used to treat a specific type of acute promyelocytic leukemia (Chiu et al., 2010). The enhanced combined cytotoxic effect was based on induction of autophagy, characterized by the presence of cytoplasmic acidic vesicles. Recent studies also suggested that a small subpopulation of malignant glioma cells, the so-called glioma stem cells or glioma-initiating cells (GICs), have true tumorigenic potential and confer glioma radioresistance (Bao et al., 2006). Induction of autophagy by IR in CD133+ glioma stem cells did not allow a significant decrease in cell viability, while Bafilomycin A1 treatment and the silencing of ATG5 and BECN1 genes sensitized CD133+ cells to gamma-radiation, and significantly decreased the viability of the irradiated cells (Lomonaco et al., 2009). Although autophagy activation conferred radio-resistance in these cells, contributing to their malignant progression, in a recent study it was reported that Rapamycin induced differentiation of GICs and increased their sensitivity to radiation by activating autophagy (Zhuang et al., 2011). In detail, transient in vitro exposure to Rapamycin and radiation abolished the capacity of transplanted GICs to establish intracerebral glioblastoma. Most importantly, in vivo combination of Rapamycin and IR effectively blocked tumor growth and its associated mortality, that occurred in mice after intracerebral grafting of human GICs. In GICs isolated from human glioblastoma, Rapamycin administration after IR activated their autophagy status and triggered the differentiation cascade, followed by a reduction in proliferation, cell viability, clonogenic ability, and increased expression of neural differentiation markers. Thus, it has been suggested that autophagy played an essential role in the regulation of self-renewal, differentiation, tumorigenic potential, and radiosensitization of GICs. Furthermore, in order to radiosensitize GICs cells, plasmids encoding short-hairpin RNA (shRNA) targeting DNA-PKcs were successfully transfected into these cells (Zhuang et al., 2011). Moreover, it has been demonstrated that Cilengitide, an integrin inhibitor, induced cell detachment and decreased viability via autophagy induction, followed by cell apoptosis (Lomonaco et al., 2011). In addition, Cilengitide decreased the cell renewal of glioma stem-like cells (GSCs). Inhibition of autophagy decreased the cytotoxic effect of Cilengitide, and pre-treatment of both GSCs and glioma cells with Cilengitide prior to IR resulted in a larger increase in autophagy and in a more significant decrease in cell survival. IR induced massive autophagic cell death also in DNA-PKcs-RNAi transfected cells, but only occasional apoptotic cells were detected among GICs. Specific inhibition of DNA-PKcs in GICs induced autophagy and radiosensitized the cells, suggesting that such radiation-induced autophagy might enhance the effect of glioblastoma therapies. Moreover, the use of gene therapy for malignant gliomas has recently becoming promising, as it allows in situ delivery and selectively targets brain tumor cells while sparing the adjacent normal brain tissue. Viral vectors that deliver genes to malignant glioma cells have been investigated, as well as other cell-based gene delivery strategies. Pro-apoptotic effects of embryonic stem cell (ESC)-mediated mda-7/IL-24 delivery to malignant glioma cell lines were recently tested and compared with conventional IR administrations (Germano et al., 2010). The combination of IR and gene transfer resulted in synergistic effects on tumor cell death with a mechanisms that involved activation of both apoptosis and autophagy. Finally, the combined effects of autophagy modulation with current IR treatment are summarized in Table 1.
| Glioma cell lines | Treatment | Autophagy activation | Cell survival/death | Ref. |
|---|---|---|---|---|
| T98G | IR (0.35; 1.2; 2; 5; 7 Gy) | + | Death |
Palumbo et al. (2012) |
| IR (0; 0.35; 1.2; 2; 5; 7 Gy) + Rapamycin (0.5–0.75.1 µM) | ++ | Death |
Palumbo et al. (2012) |
|
| IR (0.35; 1.2; 2; 5; 7 Gy) + siRNA ATG-7/BECN1 | − | Survival |
Palumbo et al. (2012) |
|
| IR (2; 4; 6 Gy) + AO DNA-PKcs | ++ | Death |
Daido et al. (2005) |
|
| U373-MG | IR (0.35; 1.2; 2; 5; 7 Gy) | − | Survival |
Palumbo et al. (2012) |
| IR (0.35; 1.2; 2; 5; 7 Gy) + Rapamycin (0.5–0.75.1 µM) | + | Death |
Palumbo et al. (2012) |
|
| IR (0.35; 1.2; 2; 5; 7 Gy) + siRNA ATG-7/BECN1 | − | Death |
Palumbo et al. (2012) |
|
| IR (5; 10 Gy) + Baffilomycin A1 (2 nM)/3-methyleadenine (2 mM) | − | Death |
Ito et al. (2005) |
|
| IR (2; 4; 6 Gy) + AO DNA-PKcs | + | Death |
Daido et al. (2005) |
|
| M059K | IR (2; 4; 6 Gy) | − | Survival |
Daido et al. (2005) |
| IR (2; 4; 6 Gy) + AO DNA-PKcs | + | Death |
Daido et al. (2005) |
|
| M059J (DNA-PKcs−/−) | IR (2; 4; 6 Gy) | + | Death |
Daido et al. (2005) |
| U87-MG | IR (2; 5; 10 Gy) | + | Death |
Fujiwara et al. (2007) |
| IR (2; 5; 10 Gy) + Akt-inhibitor (20–40 µM) | ++ | Death |
Fujiwara et al. (2007) |
|
| High-LET radiation (2; 4; 8 Gy) | − | Survival |
Benzina et al. (2008) |
|
| High-LET radiation (2; 4; 8 Gy) + Oxaliplatin (3 µM) | + | Death |
Benzina et al. (2008) |
|
| U118-MG | IR (1; 2; 4; 5; 8 Gy) | − | Survival |
Chiu et al. (2010) |
| IR (1; 2; 4; 5; 8 Gy) + Arsenic trioxide (2 µM) | + | Death |
Chiu et al. (2010) |
|
| GICs CD133+ | IR (2; 3; 5; 9 Gy) | + | Survival |
Bao et al. (2006) |
| IR (5 Gy) | + | Survival |
Lomonaco et al. (2009) |
|
| IR (5 Gy) + Baffilomycin A1 (10 nM)/3-methyleadenine (1 mM) | − | Death |
Lomonaco et al. (2009) |
|
| IR (5 Gy) + siRNA ATG5/BECN | − | Death |
Lomonaco et al. (2009) |
|
| IR (2; 4; 6 Gy) + Rapamycin (200 nM) | ++ | Death |
Zhuang et al. (2011) |
|
| IR (6 Gy) + siRNA DNA-PKcs | ++ | Death |
Zhuang et al. (2011) |
|
| IR (1; 2 Gy) + Cilengitide (10–25 µg/ml) | ++ | Death |
Lomonaco et al. (2011) |
- Autophagy activation after ionizing radiation (IR) was reported according with the correlated references; −: absence of autophagy activation; +: autophagy activation; ++: high autophagy activation. The process was evaluated using the following autophagic markers: Atg-5/7, p62, LC3-I/II, autophagosome formation and GFP-LC3 accumulation in the autophagosome membrane. Cell survival or death was assessed by viability and clonogenic assays.
Conclusion
The autophagic response of cancer cells to antineoplastic therapy, including IR, is controversial. It can originate a protective mechanism against the treatment itself by removing proteins and organelles that are damaged, or, alternatively, produce an effective cell-death process. Thus, autophagy seems to play a pivotal role between survival and death processes: these processes, in fact, might be cell and tissue specific and highly dependent on the expression profile of genes and proteins regulating apoptosis. In principle, most cancers have certain defects in their apoptotic pathway, whereas therapeutic targeting of autophagy pathways might yield better clinical outcomes for patients undergoing RT and cytotoxic drug therapy. As reported here, most experimental data suggest that radiation-induced autophagy in cancer cell lines is related to cell death mechanisms and that autophagy-inducing agents may act especially as radio-sensitizers. The fact that radio-resistant tumors, such as glioblastoma, are resistant to apoptotic stimuli, but highly susceptible to autophagy modulation by IR, may stimulate oncologic research in exploring this novel opportunity. Radiobiology research should focus on the differential effect of fractionation on the induction of autophagy in different tumors and on the manipulation of this process with triggering agents. The interplay between apoptosis and autophagy may be also exploited to improve cancer therapy. Combining autophagy inducers with inhibition of anti-apoptotic pathways, such as BCL-2 silencing, might prove of particular clinical interest in radiation treatment of cancer. Furthermore, future milestones in oncology might relate with the identification of patient-specific cell-death molecular signatures (i.e., the expression profiles of pro-apoptotic and pro-autophagic genes/proteins), to design a more effective patient-specific therapy.
Acknowledgements
We are grateful to Profs. Luigi Pirtoli and Clelia Miracco.