Abstract
Increased vascular resistance in the fetoplacental circulation is a characteristic of preeclampsia. However, the potential molecular mechanisms of this condition remain obscure. The current study aimed to determine the direct effect of the peptide antigen corresponding to the second extracellular loop of the angiotensin II type 1 receptor (AT1R-ECII) activating autoantibody (AT1-AA), a novel risk factor in preeclamptic patients, on fetoplacental villus stem blood vessels. Immunohistochemistry revealed that AT1 receptors were localized in the veins and arteries of human placental villi. Among 58 serum samples from preeclamptic patients, 28 (48.28%) were proved AT1-AA-positive by enzyme-linked immunosorbent assay [P < 0.01 vs. 2/51 (3.92%) in the normal pregnancy group]. Total IgGs purified from AT1-AA-positive patients' sera (AT1-AA-IgGs) were added to isolated normal human placental blood vessels. The IgG significantly constricted both the villus veins and arteries in a dose-dependent manner in vitro, which could be blocked by the peptide corresponding to the human AT1R-ECII, anti-human IgG or the AT1 receptor antagonist losartan. Additionally, the venous constriction induced by AT1-AA-IgGs remained unchanged even at the end of the experiment (about half an hour), but the vasoconstriction caused by the AT1 receptor agonist angiotensin II underwent desensitization within three minutes. Collectively, our results demonstrated that AT1-AA in preeclamptic sera can directly constrict fetoplacental villus blood vessels without desensitization via the AT1 receptor in vitro, which might contribute to poor fetoplacental perfusion in preeclampsia. J. Cell. Physiol. 228: 142–148, 2013. © 2012 Wiley Periodicals, Inc.
Preeclampsia, a pregnancy-specific medical condition characterized by hypertension, proteinuria and edema, is a major cause of maternal morbidity and mortality, preterm birth, perinatal death, and intrauterine growth restriction. The sole curative treatment of the syndrome is delivery of the baby; moreover, the etiology and pathogenesis remain controversial (Mutter and Karumanchi, 2008; Uzan et al., 2011).
It is now accepted that a relatively hypoxic or ischemic placenta may be the underlying pathology of preeclampsia (Hladunewich et al., 2007). Poor extravillous trophoblastic invasion due to an abnormal microenvironment or genetic factors may result in inadequate conversion of the uterine arteries into low resistance uteroplacental vessels and thus reducing placental intervillus blood flow. To minimize intervillus/intravillus flow mismatch, localized intravillus vasoconstriction response mediated by autocrine/paracrine will be produced. When the increased vascular resistance becomes widespread, abnormal umbilical blood flow will occur and low fetoplacental perfusion may finally result in intrauterine growth restriction or lower birthweight. However, the mechanisms of the villus vessel vasoconstriction are poorly understood. Known or unknown placental factors working in autocrine/paracrine mode may be one of the important candidates (Sebire and Talbert, 2001; Kaufmann et al., 2003; Maynard and Karumanchi, 2011).
The renin-angiotensin system (RAS) has been implicated in the pathogenesis of preeclampsia. Wallukat et al. (1999) first reported that there was an autoantibody against the second extracellular loop of the angiotensin II type 1 receptor (AT1-AA) in the sera of preeclamptic patients but not in healthy pregnancies, which displayed agonist-like effects. Later animal researches indicated that AT1-AA might be produced by reduced placental perfusion (LaMarca et al., 2009). This autoimmune antibody can cause a shallow placental trophoblast invasion (Xia et al., 2003), enhance intracellular Ca2+ in vascular smooth muscle cells (Thway et al., 2004), stimulate placental and vascular NADPH oxidase (Dechend et al., 2003), and even induce hypertension (Zhou et al., 2008) via interacting with the AT1 receptor, all of which are the pathological features of preeclampsia. AT1-AA is thus considered to be one of the potential causative factors for preeclampsia.
Existing studies have also demonstrated that AT1-AA can pass the placental barrier and be closely related to intrauterine growth restriction and lower birthweight (Irani et al., 2009). However, the relevant mechanisms need further elucidation. We recently reported that AT1-AA from preeclamptic patients can induce significant vasoconstrictive effects in isolated rat thoracic aorta, arteriae cerebri media, and coronary arteries through activation of the AT1 receptor (Yang et al., 2008). Therefore, we aim to discover whether the AT1-AA in preeclamptic sera has direct effects on fetoplacental villus vessels, which may contribute significantly to the poor fetoplacental perfusion.
Materials and Methods
Sera collection
One hundred nine pregnant women were recruited from the First and Second Affiliated Hospital of Shanxi Medical University (Taiyuan, China). All patients gave written consent, and the research protocol was approved by the Institutional Committee for the Protection of Human Subjects of Shanxi Medical University Hospital. The study adhered to the principles of the Declaration of Helsinki. Women with recent or prior endocrine disorders or autoimmune diseases were excluded from the experiment. Fifty-eight women had preeclampsia, defined by a blood pressure of ≥140/90 mmHg after week 20 of pregnancy, combined with proteinuria (protein excretion of at least 0.3 g per 24 h, or a spot urine protein/creatinine ratio ≥ 30 mg/mmol), according to the guidelines of the International Society for the Study of Hypertension in Pregnancy (Brown et al., 2001). For preeclamptic patients, the gestational age at delivery ranged from 36 to 40 weeks (average of 38 weeks). Fifty-one normotensive pregnant women characterized by uncomplicated pregnancies with normal-term deliveries were also studied. The preeclampsia group ranged in age from 26 to 35 years (mean age: 31 years), and the healthy pregnant individuals aged 24–33 years (mean age: 28 years). For serum preparation, fasting blood samples were collected from all the subjects through cubital veins and centrifuged at 3,000 rpm for 30 min. The sera were isolated and stored at −40°C.
Enzyme-linked immunosorbent assay (ELISA)
The peptide corresponding to the sequence of the human AT1R-ECII (165–191, I-H-R-N-V-F-F-I-I-N-T-N-I-T-V-C-A-F-H-Y-E-S-Q-N-S-T-L) was synthesized as antigen by GL Biochem Ltd (Shanghai, China). The AT1-AA titers in the sera of pregnant women were detected by modified ELISA (Liu et al., 1999; Zhang et al., 2010). Briefly, 96-well microtiter plates were coated with 1 µg/ml human AT1R-ECII peptide dissolved in Na2CO3 solution (0.1 mol/L, pH 11.0) and incubated overnight at 4°C. The wells were saturated with 0.1% PMT buffer [0.1% (w/v) albumin bovine V, 0.1% (v/v) Tween 20 in phosphate-buffered saline (PBS-T), pH 7.4] at 37°C for 1 h. After washing the plates with PBS-T three times, 50 µl serum sample dilutions were added to the plates and incubated at 37°C for 1 h. After three washings, biotinylated goat anti-human IgG antibodies (1:3,000; Zhongshan, Beijing, China) were diluted in PMT and also incubated at 37°C for 1 h. After three washings, streptavidin–peroxidase conjugate (1:2,000 dilutions in PMT, Vector, CA) was added to the wells and incubated under the same conditions. Finally, 2, 2-azino-di (3-ethylbenzothiazoline) sulphonic acid (ABTS)-H2O2 (Roche, Basel, Switzerland) substrate buffer was applied and reacted in the dark at room temperature for a half hour. The optical densities (OD) were measured at 405 nm in an ELISA reader (Spectra Max Plus; Molecular Devices, Sunnyvale, CA). Results were also judged by the value of P/N [(specimen OD − blank control OD)/(negative control OD − blank control OD)]. Negative control samples were prepared as described before (Liu et al., 1999). The positivity of the serum sample to AT1-AA was defined as P/N ≥ 2.1, while the negativity was defined as P/N ≤ 1.5.
Purification of the immunoglobulin G fraction
Based on the results of ELISA detection, AT1-AA-positive preeclamptic patients and AT1-AA-negative normal pregnant women were chosen. The total IgGs were isolated from serum samples in the two groups by Mab Trap Kit (Amersham, NJ) according to the previous methods (Zhang et al., 2010), respectively. The purities of extractions were assessed by sodium dodecylsulfonate–polyacrylate gel electrophoresis (SDS–PAGE, Supplementary Fig. S1).
Preparation of placental blood vascular ring
Placental samples were obtained from fifteen normal pregnant women with term deliveries (aged from 25 to 31 years) in the Affiliated Hospital of Shanxi Medical University. The experimental protocol was approved by the Institutional Committee for the Protection of Human Subjects of Shanxi Medical University Hospital. Pregnant women were informed about the purpose and protocol of the study, and written consent was obtained. All of the placental tissues were removed immediately after cesarean section and placed in ice-cold 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer (in mM: NaCl 144.0, KCl 5.8, MgCl2·6H2O 1.2, CaCl2 2.5, glucose 11.0, Hepes 5.0, pH 7.4). The villus stem arteries and veins were then isolated (Supplementary Fig. S2). After clearing the surrounding tissue, the vessels were cut into rings of 3–4 mm in length and suspended on steel hooks in tissue baths containing 10 ml of HEPES buffer bubbled with 100% O2 and maintained at 37°C. The changes in isometric force were recorded by a PowerLab system (AD Instruments, Sydney, Australia). Passive tension was adjusted to 1.0g. When equilibriums were reached, the rings were preconstricted with HEPES-buffer containing 30 mmol/L KCl (in mM: NaCl 119.8, KCl 30.0, MgCl2·6H2O 1.2, CaCl2 2.5, glucose 11.0, Hepes 5.0, pH 7.4). And this constriction value was recorded as the baseline of vascular constriction. The contractile responses of the vascular rings to different drugs were defined as a percentage of the baseline.
The anti-humans IgG antibody, losartan (AT1 receptor antagonist), and PD123319 (AT2 receptor antagonist) were produced by Sigma–Aldrich (Saint Louis).
Immunohistochemical studies
Fetal placental tissues were fixed in 4% paraformaldehyde and embedded in paraffin. The tissues were sliced to 6 µm sections and slices were mounted on slides. After dewaxing and hydrating, the slides were incubated in 3% H2O2 for 20 min at room temperature and then microwave antigen retrieval was carried out. An anti-AT1 receptor antibody (produced in rabbit; Sigma, Saint Louis) was added and reacted overnight at 4°C. Non-immune goat serum was used as a diluent of the primary antibody 1:1,500 to eliminate non-specific staining. Amplification of the primary antibody reaction was achieved using a polymeric conjugate consisting of a large number of secondary antibodies (goat anti-rabbit) bound directly to a dextran backbone containing HRP (Zhongshan) for 25 min at 37°C, followed by diaminobenzidine (DAB) color reagent for staining. Between each step the sections were washed in PBS for 5 min. Finally, routine hematoxylin staining was performed for morphological examination of the cell nucleus. To test the specificity of the immunohistochemical staining, the primary antibody was replaced with goat non-immune serum in control experiments.
Statistical analysis
All data are described as mean ± SD. Statistical analysis was performed with SPSS 15.0 software. The positive rates in the two groups were compared with chi-square test. The t-test was applied for comparing two independent sample means, and the one-way ANOVA was used for comparing means of more than two samples. P-value < 0.05 was considered to be statistically significant.
Results
A much higher level of AT1-AA existed in the sera of preeclamptic patients than that in the normal pregnant women
Clinical data of patients who provided serum samples were shown in Table 1. As shown in Figure 1A, the titers of AT1-AA detected by ELISA were markedly increased in the sera of preeclamptic patients compared with that in the normal pregnancy group (OD value, 0.71 ± 0.27 vs. 0.44 ± 0.18, P < 0.01). According to the P/N value, the positive rate of the autoantibody in the preeclamptic group was 48.28% (28/58, Fig. 1B). Unexpectedly, two individuals among 51 normal pregnant women were also AT1-AA-positive (3.92%, Fig. 1B), although there was a significant difference in the positive rate between the two groups (P < 0.01).
| Patients | Normal pregnancy (n = 51) | Preeclampsia (n = 58) |
|---|---|---|
| Maternal age (years) | 28 (24–33) | 31 (26–35) |
| Gestational age at sampling (weeks) | 39 (38–41) | 38 (36–40) |
| Ethnic background | Han | Han |
| SBP (mmHg) | 118 ± 8.4 | 157 ± 10.0* |
| DBP (mmHg) | 76 ± 7.3 | 108 ± 8.1* |
| Proteinuria (mg/day) | 53.6 ± 10.2 | 490 ± 50.7* |
| Platelet (×109/L) | 229.8 ± 53.4 | 162.7 ± 70.3* |
- SBP, systolic blood pressure; DBP, diastolic blood pressure.
- Data are expressed as mean ± SD.
- * P < 0.01 versus normal pregnancy.
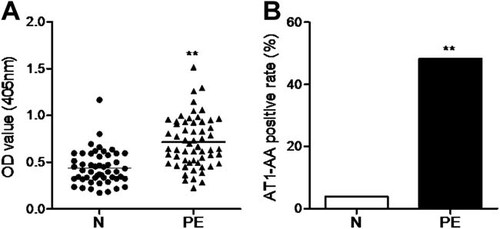
The serum levels of AT1-AA in the preeclampsia group and normal pregnancy group. A: The serum titers of AT1-AA were detected in 58 preeclamptic patients and 51 normal pregnant women using enzyme linked immunosorbent assay. Data are expressed as mean ± SD. **P < 0.01 versus N. B: Then the serum positive rates of AT1-AA in the two groups were calculated. **P < 0.01 versus N. AT1-AA, autoantibody against angiotensin AT1 receptor; N, normal pregnancy group; OD, optical density; PE, preeclampsia group.
AT1 receptor localized in normal human placental villus veins and arteries
Normal human placental villus tissues were collected from several cesarean section deliveries. Related clinical data of pregnant women were summarized in Table 2. The gestational age at delivery ranged from 38 to 41 weeks (average of 39 weeks). At term, besides trophoblast cells, endothelial and smooth muscle cells of both placental villus veins (Fig. 2A) and arteries (Fig. 2B) showed strong staining for the AT1 receptor. The control section in which the primary anti-AT1 receptor antibody was removed showed negligible staining (Fig. 2C).
| Normal pregnancy (n = 15) | Mean ± SD |
|---|---|
| Maternal age (years) | 27 (25–31) |
| Gestational age (weeks) | 39 (38–41) |
| Ethnic background | Han |
| SBP (mmHg) | 114 ± 9.7 |
| DBP (mmHg) | 77 ± 5.9 |
- SBP, systolic blood pressure; DBP, diastolic blood pressure.
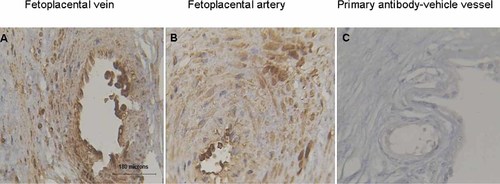
Localization of AT1 receptor in normal human placental villus vein and artery. Immunohistochemistry showed the expressions of AT1 receptor in fetoplacental villus vein (A) and artery (B). Positive immunostaining was defined by the presence of cytoplasmic brown granules. Panel (C) was the control in which the primary anti-AT1 receptor antibody was replaced with goat serum. The scale bars were 180 µm.
AT1-AA-positive IgGs from preeclamptic patient sera constricted human placental villus vessels through AT1 receptor but not angiotensin II type 2 (AT2) receptor in vitro
As shown in Figure 3A 1 µmol/L immunoglobulin G fractions isolated from the sera of AT1-AA-negative normal pregnancies failed to induce any vasoconstriction (Fig. 3A-a and A-b); however, IgGs from AT1-AA-positive preeclamptic sera at the same concentration can significantly constrict normal human placental villus veins (Fig. 3A-c) as well as the villus arteries (Fig. 3A-d) in vitro. The statistical vasoconstrictive values of six independent experiments were summarized in Figure 3B. The contractility of veins and arteries were 14.30 ± 2.70% and 11.19 ± 1.20%, respectively. And there was no significant difference between them (P > 0.05).
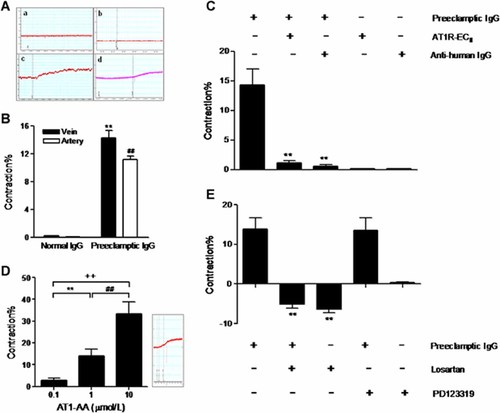
The direct effects of AT1-AA-positive/negative IgGs on normal human fetoplacental blood vessels. AT1-AA-negative IgGs isolated from the sera of normal pregnant women had no effects on the human fetoplacental veins (A-a, B) and arteries (A-b, B), while the AT1-AA-positive IgGs obtained from preeclamptic patient sera can significantly constrict the villus veins (A-c, B) as well as the arteries (A-d, B). n = 6/group. Data are expressed as mean ± SD. **P < 0.01 versus veins in normal IgG group, ##P < 0.01 versus arteries in normal IgG group. Panel (C) showed the vasoconstrictive effects of AT1-AA-positive IgGs on fetoplacental venous rings when it was preincubated with the synthetic ATR1-ECII peptide fragment or anti-human IgG antibody. n = 5–6/group. **P < 0.01 versus preeclamptic IgG group. AT1R-ECII, the second extracellular loop of human AT1 receptor. D: villus venous rings were treated with different concentrations (0.1, 1, and 10 µmol/L) of IgGs from AT1-AA-positive sera of preeclamptic patients. n = 6. **P < 0.01 versus 0.1 µmol/L IgGs group; ##P < 0.01 versus 1 µmol/L IgGs group; ++P < 0.01 versus 0.1 µmol/L IgGs group. E: The angiotensin II receptors involved in the IgGs-induced vasoconstriction. Both the AT1 receptor antagonist losartan and the AT2 receptor antagonist PD123319 were used to determine the receptor which mediated the constriction of villus veins caused by AT1-AA-positive IgGs. n = 5–6/group. **P < 0.01 versus preeclamptic IgG group.
Villus veins were chosen for the following experiment. As illustrated in Figure 3C, when preincubated the AT1-AA-positive IgGs solution (1 µmol/L) with the synthetic peptide corresponding to the second extracellular loop of the human AT1 receptor (AT1R-ECII, 1 µmol/L) or anti-human IgG antibody (5 µmol/L), the vasoconstrictive effects in villus veins (14.30 ± 2.70%) were completely blocked (1.09 ± 0.40%, 0.52 ± 0.37%, respectively, both P < 0.01). However, the receptor peptide or anti-human IgG antibody alone had no effects on villus vessels. Moreover, Figure 3D demonstrated that the addition of purified IgGs from AT1-AA-positive preeclamptic sera at cumulative concentrations of 0.1, 1, and 10 µmol/L significantly constricted villus veins in a dose-dependent fashion, the relative vasoconstrictive values were 2.73 ± 1.10%, 14.00 ± 3.20%, and 33.30 ± 5.60% (all P < 0.01), respectively.
In addition, as displayed in Figure 3E, the vasoconstrictor effect in villus veins caused by 1 µmol/L AT1-AA-positive IgG fractions in the preeclampsia group (13.90 ± 2.88%) can be neutralized by pretreating the vascular rings with 10 µmol/L losartan (AT1 receptor antagonist, −5.12 ± 1.00%, P < 0.01), but not PD123319 (AT2 receptor antagonist) at the same concentration (13.50 ± 3.30%, P > 0.05). Losartan alone can slightly relax the veins (−6.33 ± 0.90%), although there was no significant difference between losartan alone and losartan combined with the IgG group. In addition, PD123319 alone showed no effect on vascular function in vitro.
There was a lack of desensitization of AT1-AA-positive IgGs-mediated vasoconstriction
AT1-AA-positive IgGs and angiotensin II displayed similar vasoconstrictor effects at a concentration of 1 µmol/L (13.90 ± 3.00% and 8.11 ± 1.60%, respectively), both of which can be completely inhibited by 10 µmol/L losartan but not PD123319 at the same concentration (Figs. 3E and 4A). However, the time courses of actions of the autoantibody and the receptor agonist were obviously different. The duration of action of angiotensin II was about 2.93 ± 0.3 min (Fig. 4B-b and C), yet the vasoconstrictor effect of IgGs from AT1-AA-positive patients' sera was maintained until the end of the experiment at about 33.6 ± 3.1 min (Fig. 4B-a and C). There was a significant difference between them (P < 0.01).
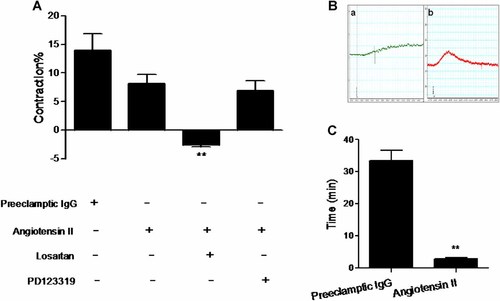
The time courses of angiotensin II and AT1-AA-positive IgGs-induced vasoconstrictions. A: The similar vasoconstrictive effects were also caused by angiotensin II via AT1 receptor in human fetoplacental veins. n = 5–6/group. Data are expressed as mean ± SD. **P < 0.01 versus angiotensin II. However, there was a great difference between AT1-AA-positive IgGs (B-a, C) and angiotensin II (B-b, C) in the length of time of vascular contraction. Both of the two agents' concentrations were 1 µmol/L. n = 6/group. **P < 0.01 versus preeclamptic IgG group.
Discussion
The current study provided direct evidences for the first time that AT1-AA from preeclamptic patients can induce vasoconstrictive effects in the fetoplacental villus veins and arteries, which might contribute to reduced placental perfusion and poor fetal growth.
Preeclampsia is a potentially deadly complication which affects about 5–7% of all pregnancies. Besides the characteristic hypertension, renal impairment, and proteinuria, preeclampsia is also responsible for about 15% of premature births (Roberts and Gammill, 2005). The pathogenesis of preeclampsia is complex because of the numerous genetic, immunological, and environmental factors involved. Furthermore, a relative placental hypoxia/ischemia linked to diffuse maternal vascular dysfunction appears to underlie the clinical features of the disorder (Sebire and Talbert, 2001; Maynard and Karumanchi, 2011).
Angiotensin II is a hormone that constricts blood vessels and causes blood pressure to rise. Increased sensitivity to the vasoconstrictor angiotensin II with unknown mechanisms is a common feature of preeclampsia (Quitterer et al., 2004). Recently, an autoantibody against the AT1 receptor (AT1-AA) identified in the plasma of women with preeclampsia might provide some new ideas about the complicated pathological process. A previous study has shown that AT1-AA might increase angiotensin II sensitivity (Wenzel et al., 2011). Furthermore, AT1-AA itself can induce the production of reactive oxygen species in the vessel and placenta (Dechend et al., 2003), promote coagulation (Dechend et al., 2000), and contribute to hypertension (Yang et al., 2008; Zhou et al., 2008), all of which suggest that the autoantibody might mediate placental and vascular dysfunction.
The placenta provides necessary nutrition, oxygen, and life support for the fetus which needs a stabilized and steadily rising blood flow both from the maternal and from the fetal side. In preeclampsia, the increased resistance to fetal blood flow due to uteroplacental ischemia/hypoxia is associated with retarded fetal growth. Although lacking a neuronal supply, fetal placental vessels can respond to vasoactive agents, such as angiotensin II, 5-HT. The autocrine/paracrine responding to uteroplacental ischaemia/hypoxia will mediate the localized vasoconstriction and is considered to be the main mechanism of the enhanced fetal vascular resistance. Additionally, the major changes in resistance are at the level of the villus stem vessels rather than terminal villus capillaries (Walters and Boura, 1991; Sebire and Talbert, 2001). Therefore, the current research interests are focused on detecting the direct effects of AT1-AA, which is considered to be an important placenta-derived risk factor, on arteries and veins in the fetoplacental stem villi.
Fifty-eight preeclamptic patients were enrolled and the sera levels of AT1-AA were determined via ELISA method. Almost half of the patients were AT1-AA-positive, which was much higher than the positive rate of the antibody (3.92%) in the normal pregnancy group. Then, the total IgGs in the AT1-AA-positive preeclamptic sera were isolated and the vasoconstrictive response to the immunoglobulin fragments was determined in normal human fetoplacental arteries and veins in vitro.
We firstly confirmed the localization of AT1 receptor protein in the endothelial cells and smooth muscle cells of human fetoplacental villus blood vessels using immunohistochemistry. Then vasoconstrictive responses to total IgGs from the AT1-AA-positive preeclampsia group sera in a dose-dependent fashion were observed in the placental villus veins. However, the IgGs from the AT1-AA-negative normal pregnant group sera had no vasoconstrictive effects. Moreover, the vasoconstrictive effects induced by the AT1-AA-positive IgGs can be completely abolished by preincubating the antibody solution with synthetic human AT1R-ECII peptide or anti-humans IgG. All data above demonstrated that it was the immunoglobulin G isotype (IgG) AT1-AA in patients' sera that induced vasoconstriction in villus veins. In addition, AT1-AA-positive IgG fraction had a similar vasoconstrictive effect on artery in the placental villi.
The ultrastructure of smooth muscle cells and extracellular matrices in the media were practically similar between the full-term placenta arteries and veins in the stem villi. However, the villus vein had a larger caliber than the artery, which may serve as capacitance vessels and mainly control the blood flow of fetoplacenta (Tanaka et al., 1999). Therefore, the villus veins were chosen for the following experiments. To further determine the active receptor, losartan and PD123319, the selective AT1 and AT2 receptor antagonists, were used. PD123319 failed to inhibit the venous constriction caused by AT1-AA-positive IgGs at relevant concentrations, whereas losartan can completely eliminate the vasoconstriction, confirming that the effect was mediated via the AT1 receptor but not AT2 receptor.
Another interesting finding of the current study was that the antibody-induced vasoconstriction was maintained for more than half an hour. This should be compared with the angiotensin II-stimulated venous constriction which was also through the AT1 receptor and underwent desensitization within 3 min. We cannot compare the autoantibody with the receptor agonist in vasoconstrictor potency because the immunoglobulin fragments used here were purified total IgGs but not specific AT1-AA. Despite this, there was a significant difference between them in the time course. In contrast to classic agonists, the autoantibodies failed to induce desensitization of the receptor, and then it can lead or contribute to the maintenance of excessive vascular tone. A similar phenomenon has also been found in the long-term sympathetic overactivity caused by the autoantibody against the β1-adrenoceptor (Magnusson et al., 1994; Wallukat et al., 1991; Supplementary Fig. S3). The potential mechanisms for the non-desensitization observed with the autoantibodies are poorly understood. It might be because the antibody-receptor complexes remain at the plasma membrane, or the receptor desensitization process was disturbed. Whatever the case may be, we will place some points of interest on the related mechanisms in future studies.
Taken together, the main observation that AT1-AA can directly constrict fetoplacetal blood vessels in vitro via the AT1 receptor might explain to some extent the molecular mechanism of the increased resistance to fetal perfusion and the subsequent poor intrauterine growth environment. These data strongly suggest that AT1-AA is a risk factor worthy of attention in the pathological process of preeclampsia. Moreover, a recent study by Jensen et al. (2012) has reported that AT1-AA in preeclampsia can be produced by CD19+CD5+ B-1a B cells. These CD19+CD5+ cells seem to be driven by the high levels of human chorionic gonadotropin which are released by the placentas in patients. Therefore, we believe that the production of AT1-AA may originate from the abnormal placenta and AT1-AA will then conversely deteriorate the placental dysfunction, which will create a vicious cycle in preeclampsia. Of course, this requires further study.
Study limitations
It is not understood whether AT1-AA elicits a different contractile response in fetoplacental villus vessels from preeclamptic patients in comparison with normal human fetoplacetal vessels. In addition, the in vivo effects of AT1-AA on fetoplacetal blood vessels need to be further investigated. In the current study, we found that a small number of normal pregnant women also possessed AT1-AA, whether this autoantibody displays similar roles as that of AT1-AA circulating in preeclamptic patients will be another point of interest in our future studies.
Acknowledgements
We are grateful to Lindsey Devillier for correcting the English spelling and grammar.