Glucose transporter 2 expression is down regulated following P2X7 activation in enterocytes†
The authors have no conflict of interest to disclose.
Abstract
With the diabetes epidemic affecting the world population, there is an increasing demand for means to regulate glycemia. Dietary glucose is first absorbed by the intestine before entering the blood stream. Thus, the regulation of glucose absorption by intestinal epithelial cells (IECs) could represent a way to regulate glycemia. Among the molecules involved in glycemia homeostasis, extracellular ATP, a paracrine signaling molecule, was reported to induce insulin secretion from pancreatic β cells by activating P2Y and P2X receptors. In rat's jejunum, P2X7 expression was previously immunolocalized to the apex of villi, where it has been suspected to play a role in apoptosis. However, using an antibody recognizing the receptor extracellular domain and thus most of the P2X7 isoforms, we showed that expression of this receptor is apparent in the top two-thirds of villi. These data suggest a different role for this receptor in IECs. Using the non-cancerous IEC-6 cells and differentiated Caco-2 cells, glucose transport was reduced by more than 30% following P2X7 stimulation. This effect on glucose transport was not due to P2X7-induced cell apoptosis, but rather was the consequence of glucose transporter 2 (Glut2)'s internalization. The signaling pathway leading to P2X7-dependent Glut2 internalization involved the calcium-independent activation of phospholipase Cγ1 (PLCγ1), PKCδ, and PKD1. Although the complete mechanism regulating Glut2 internalization following P2X7 activation is not fully understood, modulation of P2X7 receptor activation could represent an interesting approach to regulate intestinal glucose absorption. J. Cell. Physiol. 228: 120–129, 2013. © 2012 Wiley Periodicals, Inc.
Intestinal epithelial cells (IECs) form a permeable barrier to luminal pathogens while performing their digestive function and absorption of nutrients, such as glucose. To accommodate luminal glucose fluctuations, IECs have the ability to rapidly adapt their absorption capacity (Kellett and Brot-Laroche, 2005). IECs absorb luminal glucose either in an active manner by the sodium-dependent glucose transporter (SGLT1) or through facilitated transport using glucose transport facilitators 2 (Glut2; Kellett and Brot-Laroche, 2005). In the classical model of sugar absorption, Glut2 was thought to be located only at the basolateral membrane where it transports glucose into blood (Cheeseman, 1993). More recently, it was shown that Glut2 is transiently inserted in the apical membrane of IECs by trafficking from an internal pool in response to a number of stimuli, including food intake, sugar content, and hormones (Kellett et al., 2008). In this context, the role of Glut2 in the small intestine is of particular interest since it can transport glucose from the apical membrane of enterocytes to the blood stream on the basal side (Kellett and Brot-Laroche, 2005). Therefore, it is not surprising that IECs absorption of luminal glucose is considered as the first check points in the control of glycemia (Mithieux, 2005).
Extracellular ATP is an endogenous intracellular signaling molecule like hormones and growth factors (Corriden and Insel, 2010). ATP activates G-protein-coupled P2Y2 and 11 receptors and P2X1–7 ligand-gated ion channels (Corriden and Insel, 2010). In IECs, other than the previously described role of P2Y receptors in jejunum electrolyte secretion (Ghanem et al., 2005), the functions of the P2X receptors have not been defined. However, immunolocalization studies have suggested different roles for this family of receptors. In rat's duodenum, P2X1 was localized to the capillary plexus in the intestinal villus, suggesting a role in the uptake and transport of metabolites (Groschel-Stewart et al., 1999). P2X5 immunostained the membranes goblet cells, possibly influencing synthesis and release of mucin (Groschel-Stewart et al., 1999). P2X7 was localized to the membrane of enterocytes and goblet cells at the tip of the villus where cells are undergoing anoikosis, consistent with its involvement in apoptotic events (Groschel-Stewart et al., 1999). More recently, P2X7 function in IECs was associated to an inflammatory amplification loop during neutrophils migration (Cesaro et al., 2010).
The P2X7 receptor is unique among the P2X receptors family. Its elongated carboxy terminal end present in the full-length P2X7A isoform contains several protein–protein and lipid–protein interaction motifs that are associated with multi-protein complexes composed of cytoskeletal proteins, heat shock proteins, phosphatidylinositol 4-kinase (PI4-K), integrin β2, and receptor phosphotyrosine phosphatase-β (Kim et al., 2001). The repeated or prolonged stimulation of P2X7 is associated with the formation of membrane pores that allows bi-directional passage of molecules up to 900 Da in size, which could lead to plasma membrane depolarization and apoptosis (North, 2002). However, we and others have shown that transient activation of P2X7 is associated with its ionic channel properties and activation of PKC and PKD, SAPK, MAPK, and Akt phosphorylation (Humphreys et al., 2000; Bradford and Soltoff, 2002; Gendron et al., 2003; Jacques-Silva et al., 2004). P2X7 stimulation is also associated with phospholipid signaling through the control of target enzymes involved in phospholipid metabolism, such as phospholipases C (PLC) and PLD to generate lipid messengers, such as diacylglycerol (DAG; Sun et al., 1999; Bradford and Soltoff, 2002; Takenouchi et al., 2005). Among the non-apoptotic functions of P2X7, there is its key role in the inflammasome, where its activation leads to IL-1β maturation (Qu et al., 2007). P2X7 activity is also associated to increase lymphoid cells proliferation (Baricordi et al., 1999), the stimulation of human epidermal keratinocytes terminal differentiation (Greig et al., 2003), and the modulation of plasma membrane trafficking in rat thyrocytes (Kochukov and Ritchie, 2004). P2X7A is thus not only involved in pro-apoptotic events but also in normal cell functions. It was suggested that the non-apoptotic effects of P2X7A could be mediated by the formation of heterotrimers composed of the C-terminal end truncated isoform P2X7B and at least one P2X7A subunit (Cheewatrakoolpong et al., 2005; Adinolfi et al., 2010), although P2X7A stimulation can also stimulates non-apoptotic events as stated above (Adinolfi et al., 2010). Finally, there is some evidence for the formation of P2X7 and P2X4 heteromers (Casas-Pruneda et al., 2009; Weinhold et al., 2010) in which the upregulation of P2X4 expression and function could compensate P2X7 depletion (Weinhold et al., 2010).
In this study, we propose a new role for P2X7 in IECs function. We showed that P2X7 is not only expressed at the tip of small intestine villi (Groschel-Stewart et al., 1999), but also its expression extends to the top two-thirds of villus. We have shown that P2X7 activation reduces glucose absorption and transport by IECs by down-regulating Glut2 membrane expression. Hence, we proposed that PKD participates to protein endocytosis.
Materials and Methods
Reagents
DMEM, Hank's balanced salt solution 1× (HBSS), penicillin–streptomycin, HEPES, and fetal bovine serum (FBS) were purchased from Wisent (St. Bruno, QC, Canada). GlutaMax, fura-2/AM, and Hoechst 33342 were from Invitrogen Life Technologies (Burlington, ON, Canada). ATP was from Roche Applied Science (Laval, QC, Canada). Periodate oxidized ATP (oATP), 2′- & 3′-O-(4-benzoylbenzoyl)-ATP (BzATP), BAPTA-AM, and phenylarsine oxide (PAO) were from Sigma–Aldrich (Oakville, ON, Canada). The PKC inhibitors (GF109203X, Gö6983, Gö6976) and the PLC inhibitor (U73122) were acquired from EMD Millipore (Billerica, MA). The selective PKD inhibitor 2,3,4,5-tetrahydro-7-hydroxy-1H-benzofuro[2,3-c]azepin-1-one (CID755673) and N-[1-[[(Cyanoamino)(5-quinolinylamino)methylene]amino]-2,2-dimethylpropyl]-3,4-dimethoxybenzeneacetamide (A740003) were purchased from Tocris Bioscience (Ellisville, MO). Rabbit polyclonal anti-phospho-PKD1/PKCµ (Ser744/748), rabbit polyclonal anti-phospho-PKD1/PKCµ (Ser916), rabbit polyclonal anti-phospho-PKCδ (Thr505), rabbit polyclonal anti-phospho-PLCγ1 (Tyr783), rabbit anti-PKD/PKCµ, rabbit anti-PKCδ, and rabbit anti-PLCγ1 were purchased from Cell Signaling Technology (Pickering, ON, Canada). Mouse monoclonal anti-actin (clone C4), rabbit polyclonal anti-P2X7, and control peptide antigen were obtained from EMD Millipore. Rabbit polyclonal anti-Glut2 and horseradish peroxidise (HRP)-conjugated donkey anti-mouse immunoglobulin G (IgG) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). HRP-conjugated goat anti-rabbit IgG was from GE Healthcare Life Sciences (Baie D'Urfe, QC, Canada), and ECL reagent was from EMD Millipore. Sulfo-NHS-SS-biotin and streptavidin–agarose resin were from Fisher Scientific (Ottawa, ON, Canada).
Cell culture
The rat cell line IEC-6, the human colon carcinoma cell line Caco-2 and human embryonic kidney cell line HEK293T (from the American Type Culture Collection, Manassas, VA) were grown as previously described (Degagne et al., 2009; Langlois and Gendron, 2009; Grbic et al., 2011). Cells were incubated in serum-free medium for 24 h at 37°C before each experiment.
Generation of P2X7 shRNA IEC-6 cell lines
The 29 mer shRNA constructs directed against rat P2X7 were purchased from Origene (Rockville, MD). Retroviruses were produced in HEK293T cells and used for IEC-6 cells infection according to manufacturer recommendations. shRNA efficiency was validated by measuring transcript expression using quantitative real-time PCR.
Dynamic video imaging of cytosolic Ca2+
IEC-6 and Caco-2 cells (5 × 105) were grown on collagen 1-coated glass coverslip in complete DMEM for 48 h. Cells were washed twice with HBSS with calcium and magnesium (HBSS 1×; Wisent bioproducts, St. Bruno, QC, Canada). Cells were incubated with 1 µM fura 2-AM in HBSS for 20 min at room temperature (RT) in the dark. Cells were washed and bathed in fresh HBSS for 20 min at RT to ensure complete hydrolysis of the fura-2/AM. The coverslips were clamped into a Teflon circular open-bottom chamber and was mounted onto the stage of a Olympus IX71 microscope fitted with a MetaFluor digital imaging and photometry system (Olympus, Markham, ON, Canada). Fluorescence from isolated fura 2-loaded cells was monitored by videomicroscopy using alternating excitation wavelengths of 334 and 380 nm and recording emitted fluorescence at 510 nm. All experiments were performed at RT. The data are expressed as the intracellular free Ca2+ concentration (nM) calculated from the 334/380 fluorescence ratio according to Grynkiewicz et al. (1985).
Extraction of membrane proteins
Treated cells were washed twice with ice-cold PBS and incubated for 90 min at 4°C in 1 ml of ice-cold PBS containing 2 mg/ml sulfo-NHS-SS-biotin. After biotinylation, cells were washed three times with ice-cold PBS and quenched with 10 mM glycine ice-cold PBS solution. Cells were lysed in Triton buffer [40 mM Tris (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.2 mM sodium orthovanadate, 40 mM β-glycerophosphate, 0.1 mM PMSF, and protease inhibitor mixture]. An aliquot of 25 µg of each cell lysate was kept as the total protein fraction. The remaining lysates were precipitated overnight at 4°C with streptavidin–agarose resin with constant agitation. Biotinylated protein–streptavidin–agarose complexes were washed three times with 1 ml of Triton buffer. Beads were then resuspended in 40 µl Laemmli 2× buffer and processed for Western blot analysis.
Immunoblot analysis
After treatment and stimulation, cells were harvested and used for Western blot analysis as previously described (Grbic et al., 2008). Immunodetection was performed using anti-phospho-PKD1/PKCµ (Ser744/748; 1:500), anti-phospho-PKD1/PKCµ (Ser916; 1:500), anti-phospho-PKCδ (Thr505; 1:500), anti-phospho-PLCγ1 (Tyr783; 1:500), or anti-Glut2 antibodies (1:500). After extensive washing with PBS-T, the blots were incubated for 1 h at RT with a HRP-conjugated secondary antibody and immunostained bands revealed with the Millipore chemiluminescence system and Hyperfilm™ ECL (GE Healthcare Life Sciences) according to the manufacturer's instruction. For signal normalization, membranes were stripped of antibody and re-probed with anti-PKD1/PKCµ (1:500), anti-PKCδ (1:500), anti-PLCγ1 (1:500), or with anti-actin (1:1,000) antibodies.
Detection of Glut2 membrane expression by flow cytometry
IEC-6 cells were treated with or without inhibitors (oATP or CID755673), stimulated for 5 min with 100 µM BzATP and media replaced by fresh serum-free media. Cells were harvested at different times using trypsin as indicated in figures. Cells were rapidly washed with ice-cold PBS and fixed using 3% paraformaldehyde (PFA) for 30 min. PFA was washed out of the fixed cells using PBS and non-specific antigen sites blocked using 5% BSA in PBS for 1 h with gentle agitation. Cells were rinsed and 1 µg of anti-Glut2 primary antibody was added to the cell suspension for 1 h with agitation. After washing, 2 µg of Alexa Fluor 488 F(ab')2 goat anti-rabbit IgG was added for 30 min. Glut2 surface expression was then evaluated using a FACSCalibur™ flow cytometer and CellQuest Pro software (BD Bioscience, Mississauga, ON, Canada).
Indirect immunofluorescence
Rat's jejunum tissues were harvested and fixed in 4% PFA overnight at 4°C, embedded in paraffin, cut into 5-µm sections, and applied to Super-frost/Plus slides (Fisher Scientific). Deparaffinized and rehydrated slides were processed as previously described (Grbic et al., 2008). P2X7 immunolocalization was performed using rabbit anti-P2X7 antibody as the primary antibodies, incubated with the sections overnight at 4°C. Slides were washed in phosphate-buffered saline (PBS) and then incubated for 2 h with Alexa Fluor 568 F(ab')2 goat anti-rabbit IgG (H + L; Invitrogen) as the secondary antibody. Similarly, IEC-6 cells grown on sterile glass coverslips were washed twice with ice-cold PBS after treatment and then fixed with 1% PFA for 30 min at 4°C. Cells were washed with PBS, quenched with 100 mM glycine for 10 min, washed, and then blocked with PBS containing 2% bovine serum albumin (BSA) for 20 min. Alternatively, cells were washed with PBS, quenched with 100 mM glycine for 10 min, and then permeabilized with PBS containing 0.1% Triton X-100 for 10 min at RT and processed as described above. Cells were subsequently immunostained overnight at 4°C with the rabbit anti-Glut2 as the primary antibody and for 30 min with the Alexa Fluor 568 F(ab')2 goat anti-rabbit IgG (H + L) or Alexa Fluor 488 F(ab')2 donkey anti-rabbit IgG as the secondary antibodies. Nuclei were stained with Hoechst 33342. Non-immune controls (normal IgG antibody) were included in all experiments. Slides were washed in PBS, mounted, and images were captured on a Leica DM2500 microscope using a Hamamatsu ORCA-R2 digital camera.
In vitro glucose absorption
For the glucose absorption assays, IEC-6 cells were seeded in 100-mm2 petri dishes. After reaching confluence, cells were stimulated for 5 min with 100 µM BzATP and media replaced for FBS-free DMEM supplemented with 200 nCi of 3-O-[methyl-14C]-D-glucose (Perkin Elmer, Woodbridge, ON, Canada). After 3 h, cells were extensively washed using warm PBS and harvested using trypsin. Cells were directly lysed in 5 ml of BCS scintillation cocktail (GE Healthcare Life Sciences). 3-O-[methyl-14C]-D-glucose incorporation was determined using a Wallac 1409 DSA liquid scintillation counter and Wallac LSC software (PerkinElmer).
In vitro glucose transport
For the in vitro glucose transport assays, Caco-2 cells (200,000 cells/well) were seeded on polycarbonate membrane transwell supports coated with collagen I. Cells were grown 15 days post-confluence as previously described (Langlois and Gendron, 2009). The transepithelial electric resistance (TEER) of the filter-grown Caco-2 monolayer was measured using an EVOM volt ohmmeter (EVOM, World Precision Instruments, Sarasota, FL) with a pair of STX-2 chopstick electrodes to ensure polarized monolayer integrity. Wells with a TEER value smaller than 1,000 ohms were rejected. Caco-2 cells were treated with the P2X7 antagonist A740003 (100 µM) for 30 min at 37°C prior to receptor stimulation with 100 µM BzATP for 5 min. Following stimulation, the cell culture media of the lower compartment was replaced by fresh DMEM, whereas the upper part was changed for DMEM supplemented with 200 nCi of 3-O-[methyl-14C]-D-glucose. Aliquots (50 µl) were collected at 0, 5, 10, 20, 40, and 90 min after the addition of the glucose analogs and placed in 5 ml of BCS scintillation cocktail (GE Healthcare Life Sciences). Radioactivity was determined as described above.
Results
P2X7 receptor is expressed by IECs
Indirect immunofluorescence analysis showed that P2X7 receptor expression was not only localized to the apex of rat jejunum villi, as previously reported (Groschel-Stewart et al., 1999), but also it extended to the top two-thirds of the villus (Fig. 1A). Presence of a functional P2X7 receptor in IEC-6 (Fig. 1B) and Caco-2 (Fig. 1C) cells was determined by measuring the variation of intracellular calcium concentration (Δ[Ca2+]i) in response to BzATP stimulation. Addition of 100 µM BzATP to adherent cells loaded with fura-2 showed an increase in intracellular calcium concentration (Fig. 1B,C). Addition of 100 µM A740003, a P2X7 selective antagonist, prior to BzATP stimulation significantly reduced by 50% the Δ[Ca2+]i in both IEC-6 cells (Fig. 1B) and Caco-2 cells (Fig. 1C). Although PPADS (100 µM), a general P2 receptor antagonist, also significantly reduces the Δ[Ca2+]i when compared to non-stimulated control cells, the inhibition was not statistically different than the inhibitory effect induced by the P2X7 selective antagonist A740003 (Fig. 1B,C). We next determined if P2X7 stimulation with 100 µM BzATP increases the expression of the apoptosis marker annexin V at the surface of IEC-6 cells and cleaved caspase-3. Receptor activation did not increase the number of annexin V positive cells (Supplemental Fig. S1A). On the other hand, staurosporine (STS)-treated IEC-6 cells led to a significant increase in the number of apoptotic cells as compared to non-stimulated control cells (Ctrl; Supplemental Fig. S1A). In agreement with the annexin V results, cleaved caspase-3 could only be detected in STS-treated cells and not in BzATP-stimulated IEC-6 cells (Supplemental Fig. S1B). These results showed that a functional P2X7 receptor is present on IECs. It also appears that P2X7 is the main effectors involved in the response to BzATP stimulation. In IEC-6 cells, P2X7 stimulation using BzATP up to 30 min did not induce an apoptotic response, suggesting that the function of this receptor extend far beyond its role in apoptosis.
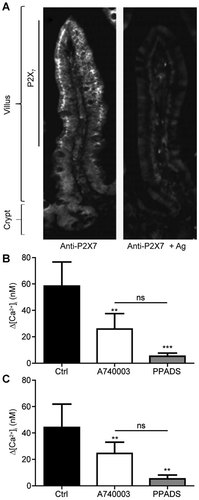
P2X7 receptor is expressed by IECs. A: Localization of the P2X7 receptor to the top two-thirds of rat's jejunum villi (left panel) using immunofluorescence. Control with preabsorbed anti-P2X7 antibody to the immunizing peptide is presented on the right panel. Original magnification is 20×. Presence of a functional P2X7 in (B) IEC-6 cells or (C) Caco-2 cells was determined by measuring the variation of intracellular calcium concentration (Δ[Ca2+]i). IEC-6 and Caco-2 cells were stimulated with 100 µM BzATP in the presence or not of 100 µM of the selective P2X7 receptor antagonist A740003 or 100 µM PPADS, a general P2 receptors antagonist. Presented is a typical graph of (Δ[Ca2+]i of three separate sets of experiments. Results are presented as the mean ± SEM and statistical significance was one-way ANOVA with post-test, where **P < 0.01 and ***P < 0.001 as compared to non-stimulated control (Ctrl) cells and ns: non-significant versus A740003 treated cells as indicated on figure panels.
Stimulation of P2X7 receptor in normal rat IEC-6 cells down-regulates Glut2 cell-surface expression
In resting IECs, Glut2 is expressed at the cell surface or in intracellular storage vesicles (Kellett et al., 2008). In accordance with the reported role of P2X7 on membrane internalization and protein endocytosis (Kochukov and Ritchie, 2004, 2005); we determined if P2X7 receptor stimulation could regulate Glut2 expression at the plasma membrane of IEC-6 cells (Fig. 2). Cell-surface proteins were isolated from IEC-6 cells using biotin and streptavidin, and protein extracts were analyzed by Western blots to determine Glut2 expression at the cell surface. As previously reported (Kellett and Helliwell, 2000), we observed the expression of Glut2 at the cell surface of non-stimulated IEC-6 cells (Fig. 2A). However, a reduction of Glut2 cell-surface expression was observed 5 min following a 5 min treatment of IEC-6 cells with 100 µM BzATP, a potent P2X7 receptor agonist (Fig. 2A). Glut2 was further internalized 10, 15, and 30 min after the BzATP treatment (Fig. 2A). Contrary to a previous report (Hou et al., 2009), the decrease in Glut2 cell-surface expression was not associated with its degradation, as total Glut2 expression remained the same in total protein extracts (Fig. 2A). Taking advantage that the Glut2 antibody was directed against an extracellular epitope, we could observe Glut2 expression at the cell surface of unstimulated and non-permealized IEC-6 cells (Fig. 2B). Glut2 protein expression was characterized by a punctuated pattern of staining at the cell surface. A similar staining pattern was observed on cells treated with the P2X7 receptor antagonist A740003 (Fig. 2C). Thirty minutes after the initial 5 min BzATP stimulation, the fluorescence staining on the non-permeabilized cells is strongly reduced and corresponds to a loss of Glut2 protein expression at the cell surface (Fig. 2D). The internalizing effect of BzATP was blocked by the addition of A740003 prior to cell stimulation (Fig. 2E) and PPADS (Supplemental Fig. S2). In fact, following P2X7 stimulation, Glut2 expression was mainly found in intracellular vesicles (Supplemental Fig. S3). We validated these observations by FACS analysis and showed that Glut2 membrane expression was reduced after 15 and 30 min following the initial P2X7 receptor stimulation (Fig. 2G). The loss of Glut2 expression at the cell surface was prevented by the addition of the P2X7 receptor antagonist oATP prior to receptor stimulation (Fig. 2H). To circumvent potential oATP non-specific effect, we developed an IEC-6 cell line with reduced P2X7 expression. Infected IEC-6 cells generally displayed a significant reduction in P2X7 gene expression as compared to wild-type (wt) control cells and IEC-6 cells infected with genome-integrated control non-target (shNT) shRNA lentivirus (Supplemental Fig. S4). Reduction of receptor expression also prevented the reduction in Glut2 cell-surface expression (Fig. 2I). These results are thus suggesting that P2X7 regulates Glut2 internalization.
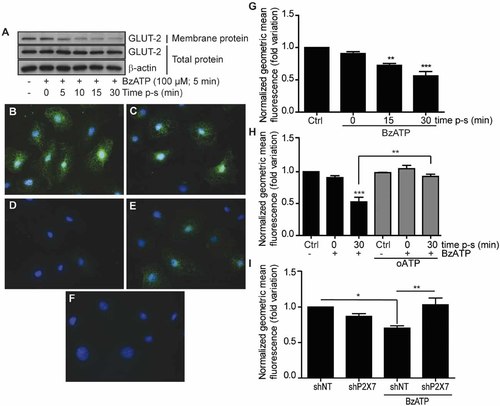
P2X7 receptor activation on normal IEC-6 rat cells with BzATP down-regulates Glut2 cell-surface expression. A: Cell-surface proteins were biotinylated with membrane-impermeant NH-S-S-biotin and immunoprecipitated using streptavidin–agarose. Western blot analysis shows a reduction in Glut2 cell-surface expression with time following P2X7 receptor activation (100 µM BzATP, 5 min at 37°C), whereas total Glut2 expression remained unchanged. β-Actin antibody was used as a control for equal loading and to monitor protein integrity. Data are representative of three separate sets of experiments. B: Glut2 expression was detected by immunofluorescence analysis at the cell surface in DMSO (vehicle)-treated cells using non-permeabilized IEC-6 cells and in cells treated only with the selective P2X7 antagonist A740003 (C). D: Glut2 cell-surface expression was strongly reduced 15 min following the initial 5 min stimulation (15 min post-stimulation; p-s) of IEC-6 cells with 100 µM BzATP at 37°C. E: Addition of 100 µM A740003 prior to BzATP stimulation blocks the internalization of Glut2. F: Non-immune staining for Glut2 immunolocalization using non-permeabilizaed IEC-6 cells. Nuclei were stained with Hoechts 33342. The immunofluorescence data (panels B–F) are representative of three to four separate sets of experiments and the original magnification was of 20×. G: Flow cytometric analysis confirmed the Western blot data. Results are the means ± SEM of three separate experiments done in duplicate. Statistical significance was determined by an unpaired t-test, where **P ≤ 0.01 and ***P ≤ 0.001 when compared to control unstimulated cells (Ctrl). H: The addition the P2X7 receptor antagonist, oATP, at 100 µM for 2 h prior to IEC-6 cells stimulation abolished the down-regulation of Glut2 membrane expression observed. Results are the means ± SEM of three separate experiments done in duplicate. Statistical significance was determined by an unpaired t-test where **P ≤ 0.01 and ***P ≤ 0.001. I: Reduction in P2X7 receptor expression using shRNA inhibited the down-regulation of Glut2 membrane expression as compared to non-targeting scramble shRNA (shNT). Results are the means ± SEM of three separate experiments done in duplicate. Statistical significance was determined by an unpaired t-test, where *P ≤ 0.05 and **P ≤ 0.01.
Glucose absorption and transport are reduced in P2X7 stimulated IEC-6 and Caco-2 cells
Because P2X7 activation decreases Glut2 cell-surface expression, we validated these observations by measuring glucose absorption and transepithelial transport using IEC-6 cells and polarized and differentiated Caco-2 cells, respectively (Fig. 3). In accordance with the observations on Glut2 expression, P2X7 activation reduced the absorption of 3-O-[methyl-14C]-D-glucose by IEC-6 cells by more than 35% when compared to unstimulated cells (Fig. 3A). In enterocytes, Glut2 allowed the passage of glucose from the apical side and its release in the blood stream at the basal side. In this context, we performed glucose transport assays using polarized and differentiated Caco-2 cells which display an enterocyte-like phenotype (Beaulieu and Quaroni, 1991; Gendron et al., 2006). As shown in Figure 3B, the rate of glucose transport after 90 min is significantly reduced by about 30% as compared to control, whereas the overall rate of transport is reduced by more than 20% (Fig. 3C). As for the modulation of Glut2 expression at the plasma membrane, the addition of A740003 (100 µM) prior to P2X7 stimulation restore the rate of glucose transport back to control levels (Fig. 3B,C). The partial inhibition of glucose absorption could be explained by the presence of SGLT1 that remains unaffected following P2X7 activation in IEC-6 cells (data not shown), and that P2X7 stimulation does not lead to complete Glut2 internalization. We then investigated the mechanism linking P2X7 receptor activation to Glut2 internalization.
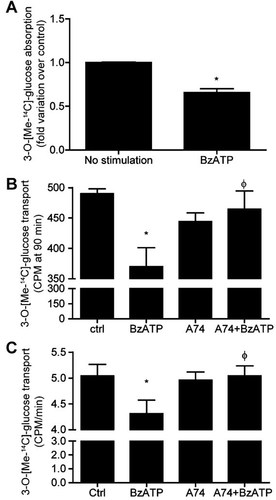
Glucose absorption and transport is reduced in P2X7 stimulated IECs. A: Absorption of the metabolically stable D-glucose analog, 3-O-[methyl-14C]-D-glucose, is reduced by 35% in cells stimulated with 100 µM BzATP at 37°C. Results are presented as the mean ± SEM of three separate experiments done in triplicate. Statistical significance was determined by an unpaired t-test, where *P ≤ 0.05 when compared to unstimulated cells. B: The transepithelial transport of 3-O-[methyl-14C]-D-glucose is significantly reduced after 90 min following the initial 5 min stimulation of Caco-2 cells with 100 µM BzATP. This effect was blocked by the addition of 100 µM A740003 prior to cell stimulation with BzATP. C: The overall rate of glucose transport is reduced by BzATP treatment and blocked by A740003 added prior to cell activation. Results are presented as the mean ± SEM of three separate experiments done in triplicate. Statistical significance was determined by an unpaired t-test, where *P ≤ 0.05 when compared to unstimulated cells (ctrl) and ϕP < 0.05 when compared to BzATP stimulated cells.
P2X7 stimulates PKD phosphorylation to regulate Glut2 internalization in normal rat intestinal epithelial IEC-6 cells
Phosphorylation of PKD in response to P2X7 stimulation has previously been described in rat's parotid acinar salivary cells (Bradford and Soltoff, 2002), but never in IECs. Activation of P2X7 using 100 µM BzATP (Fig. 4A) or 1 mM ATP (Supplemental Fig. S5) stimulated PKD catalytic domain activation loop phosphorylation on Ser744/748 and on autophosphorylation residue on Ser916 in a time-dependent manner. The P2X7-mediated PKD phosphorylation was strongly reduced in the presence of the selective P2X7 receptor antagonist A740003 (Fig. 4B) or in the presence of shP2X7 (Fig. 4C). PKD activity is generally associated with the regulation of vesicular transport between the trans-Golgi network (TGN) and cell membrane (Van Lint et al., 2002). However, in this study the selective inhibition of PKD activity using CID755673 prevented P2X7-mediated internalization of Glut2 (Fig. 4D). These results suggest that PKD could regulate P2X7-induced Glut2 internalization in IECs.
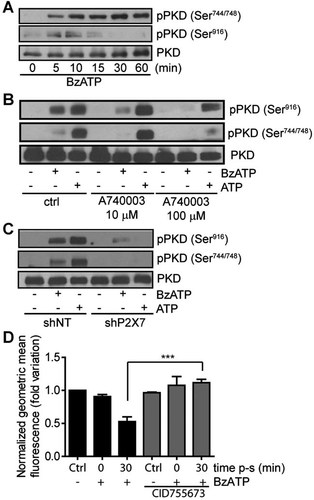
P2X7 receptor stimulates PKD1/PKCµ phosphorylation. A: IEC-6 normal rat IECs incubated in serum-free DMEM were stimulated with 100 µM BzATP in a time-dependent manner as indicated on figure. PKD1 phosphorylation on Ser744/748 and Ser916 were determined by Western blot analysis. B: Addition of 10 or 100 µM A74000330 min prior to the stimulation of IEC-6 cells with 100 µM BzATP for 5 min reduced PKD1 phosphorylation. Antibody recognizing the non-phosphorylated form of PKD1/PKCµ was used as a control for equal loading and to monitor protein integrity. C: Reduction of P2X7 expression using shRNA abolished receptor induced PKD1 phosphorylation as compared to control shNT. Presented Western blot results are representative of three separate sets of experiments. D: Addition of the selective PKD inhibitor CID755673 (546 nM, 20 min) prior to P2X7 receptor stimulation with BzATP (100 µM, 5 min) inhibited P2X7-mediated Glut2 internalization as determined by flow cytometry analysis. Results are the means ± SEM of three separate experiments done in duplicate. Statistical significance was determined by an unpaired t-test, where ***P ≤ 0.001.
The P2X7 receptor stimulates PKCδ activation in intestinal epithelial cells
As previously reported (Bradford and Soltoff, 2002; Gendron et al., 2003), we also observed phosphorylation of PKCδ on Thr505 following the addition of BzATP to IEC-6 cells (Fig. 5). PKCδ phosphorylation was detected after only 5 min of P2X7 stimulation and was maintained for up to 15 min, after which the phosphorylation is rapidly decreasing (Fig. 5A). Addition of oATP prior to P2X7 stimulation inhibited PKCδ phosphorylation (Fig. 5B). Given the nature of PKCδ, it was not surprising to find that inhibition of PLC using U73122 (Fig. 5C) reduced P2X7 stimulation of PKCδ phosphorylation. Numerous enzymes and different pathways can synthesize PIP2; one route comes from the phosphorylation of phosphatidylinositol to phosphatidylinositol 4-phosphate (PI4P) by PI4-K, and from the phosphorylation of PI4P to PIP2 by phosphatidylinositol 4-phosphate 5 kinases (PIP5K). We thus added a PI4-K inhibitor, PAO (20 µM), prior to the stimulation of IEC-6 cells with 100 µM BzATP or 1 mM ATP (Fig. 5D). Cells treated with PAO displayed a significant reduction in PKCδ phosphorylation. Finally, we identified PLCγ1 as one of the potential lipases that could be involved in the hydrolysis of PIP2 to generate DAG. P2X7 stimulation of IEC-6 cells led to a time-dependent increase of PLCγ1 phosphorylation on Tyr783 residue (Fig. 5E).
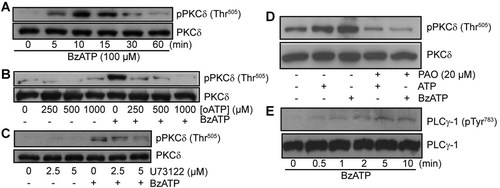
P2X7 receptor-mediated PKCδ phosphorylation on Thr505. A: IEC-6 cells were stimulated with 100 µM BzATP in a time-dependent manner and phosphorylation status of PKCδ-Thr505 was analyzed by Western blots. B: Addition of oATP (0–1 mM) 2 h prior to P2X7 receptor stimulation with 100 µM BzATP for 5 min at 37°C led to a reduction in PKCδ phosphorylation. C: IEC-6 cells were treated by the addition of U73122 (0–10 µM) 30 min prior to P2X7 stimulation with 100 µM BzATP for 5 min. D: Addition of phenylarsine-oxide (PAO, 20 µM) to inhibits PI4-K activity reduced the phosphorylation of PKCδ on Thr505 mediated by P2X7 activation (100 µM BzATP or 1 mM ATP, 5 min at 37°C) in IEC-6 cells. E: Stimulation of IEC-6 cells by 100 µM in a time-dependent manner (0–10 min) led to PLCγ1 phosphorylation on Tyr783 residue. Antibodies recognizing the non-phosphorylated form of PKCδ or PLCγ1 were used as a control for equal loading. Results are representative of three to four separate sets of experiments.
The phosphorylation of PKD is downstream of PKCδ
It is well known that PKD activation can be regulated by different PKC isoforms. Having established that PKD and PKCδ were both activated by P2X7, we next determined if PKCδ was located upstream of PKD. First, we showed that PKD phosphorylation on Ser747/748 was independent on the influx of calcium triggers by P2X7 activation and channel opening using calcium-free HBSS (Fig. 6A) as well as by chelating intracellular calcium using BAPTA-loaded cells (Fig. 6B). These results were an indication that PKD activation involves a calcium-independent PKC, such as PKCδ. Previous reports have shown that GF109203x and Gö6983 could inhibit P2X7-mediated PKCδ phosphorylation (Bradford and Soltoff, 2002; Gendron et al., 2003). Based on these observations, the addition of both GF109203x (Fig. 6C) and Gö6983 (Fig. 6D), prior to P2X7 stimulation by BzATP, lead to a strong inhibition of PKD phosphorylation on serine residues 744 and 748. On the other hand, the presence of Gö6976, a PKC inhibitor that does not inhibit PKCδ (Martiny-Baron et al., 1993), did not reduce PKD phosphorylation (Fig. 6E). These results suggest that PKDδ activity is required to phosphorylate and activate PKD in IECs.
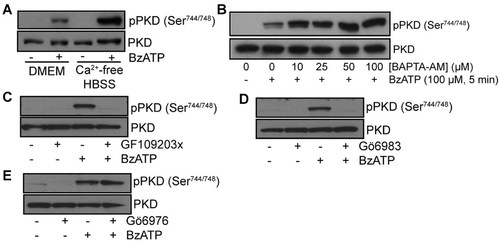
The phosphorylation of PKD/PKCµ on Ser744/748 by P2X7 is downstream of PKCδ activation. IEC-6 cells were grown in serum-free DMEM for 24 h or in calcium-free HBSS for 1 h before the stimulation of P2X7 with 100 µM BzATP for 5 min at 37°C. A: The absence of calcium or (B) the chelation of intracellular calcium using BAPTA-AM did not inhibit PKD1/PKCµ phosphorylation. Addition of different PKC inhibitors prior to P2X7 activation by BzATP (100 µM, 5 min at 37°C) led to a reduction of PKD phosphorylation on Ser744/748 in the presence of (C) GF109203X (2.5 µM) or (D) Gö6983 (5 µM), but not of (E) Gö6976 (5 µM). Antibody recognizing the non-phosphorylated form of PKD/PKCµ was used as a control for equal loading. Results are representative of three separate sets of experiments.
Discussion
P2X7 nucleotide receptors have multiple functions ranging from the control of electrolyte transport to the modulation of cell proliferation, apoptosis, and cytokine release (Baricordi et al., 1999; North, 2002; Wildman and King, 2008; Adinolfi et al., 2010). In the small intestine, this receptor was first localized at the apex of villi (Groschel-Stewart et al., 1999). This particular localization lead the authors to speculate about a role in IECs apoptosis (Groschel-Stewart et al., 1999). However, a closer look at the micrographs presented by Groschel-Stewart et al. (1999), a weak staining along villi and not only at the villi tips and goblet cells can be observed. This particular staining suggests that P2X7 could also be involved in functions other than apoptosis and mucin release (Groschel-Stewart et al., 1999). In fact, recent findings showed that P2X7 is associated to an inflammatory amplification loop during neutrophil migration (Cesaro et al., 2010). In this study, using an antibody recognizing the extracellular loop of P2X7, we showed that receptor expression extends to the top two-thirds of rat jejunum villi (Fig. 1A), suggesting that P2X7 may have broader biological functions in IECs. In fact, the use of this antibody could have lead to the recognition, not only of the full-length P2X7A receptor, but also of other naturally occurring truncated isoforms, such as the P2X7B variant. This P2X7B variant is associated with the positive effects mediated by this receptor in various tissues (Adinolfi et al., 2010). Our results suggest that P2X7A and/or P2X7B isoforms are present along jejunum villi. Although the P2X7B isoform is denuded of a C-terminal domain, the presence of P2X7 heterotrimers formed of at least one P2X7A sub-unit could provide the necessary C-terminal domain that couples this receptor to a spectrum of downstream effectors important for P2X7 intracellular signaling pathways independent on Ca2+ influx. This calcium independent signaling pathway identified in this study is thus ruling out the possible involvement of other BzATP-sensitive P2X receptors, which are mediating their effect by acting as calcium-gated channels. Hence, as shown on Figure 4C, inhibition of P2X7 expression using interfering RNA (shRNA) abrogate the PKD stimulating effect of BzATP and ATP, thus showing that P2X7 is most probably the principal P2X receptors involved in the phosphorylation of PKD and the internalization of Glut2.
Extracellular ATP acting on both P2X and P2Y receptors can stimulate insulin release by pancreatic β cells, thus reducing the level of blood glucose (Petit et al., 2009). In addition to the pancreas, the regulation of glucose homeostasis also involves the intestine which is characterized by its high uptake capacity and glycolytic activity (Mithieux, 2005). To accommodate the large quantities of glucose found in the lumen in a postprandial situation, the low-affinity and high-capacity transporter Glut2 is required (Kellett and Helliwell, 2000). The regulation of Glut2 function or its expression at the level of the IECs could provide an avenue for the regulation of glucose absorption. It has been proposed that insulin provides such a protective mechanism by stimulating the internalization and vesicular storage of Glut2 in enterocytes of healthy but not of insulin-resistant mice, thus reducing glucose uptake (Tobin et al., 2008). However, beside insulin and glucose, only a few other molecules, such as flavonoids or glucocorticoids, have been reported to regulate Glut2 function or expression as means of regulating glucose absorption (Shepherd et al., 2004; Kwon et al., 2007). In this study, we showed that P2X7 receptor could modulate the absorption of glucose by IECs and its transepithelial transport from the apical to the basal cell compartment by controlling the expression of Glut2 at the cell surface. However, unlike the effect of insulin, ATP-dependent internalization of Glut2 leads to the formation of Glut2 storage vesicles and not to its rapid degradation by lysosomes, as observed in β cells (Hou et al., 2009). The different fates of internalized Glut2 in IECs versus β cells could be explained by the different roles of Glut2 in these cells. In the pancreas, Glut2 is part of the glucose sensing mechanism (Leturque et al., 2009), whereas in IECs, Glut2 functions are to facilitate glucose entry in response to variations in glucose levels (Kellett and Helliwell, 2000; Leturque et al., 2009). Thus, storage of Glut2 in intracellular vesicles would allow its rapid mobilization. It thus appears that the internalization and processing of Glut2 could be independent on the cell type and the physiological conditions.
Although PKA-dependent phosphorylation of the Glut2 intracellular domains have been proposed as a mechanism to regulate its function (Thorens et al., 1996), the mechanism leading to Glut2 internalization is not understood. In this study, we propose that P2X7-dependent internalization of Glut2 involves a PKCδ–PKD signaling pathway independent on calcium influx. It is well accepted that P2X7 activation by ATP leads to a massive influx of extracellular calcium, which is associated with an array of physiological effects (North, 2002). There are also several studies showing that this receptor can stimulate numerous signaling pathways independent on calcium (Amstrup and Novak, 2003; Garcia-Marcos et al., 2006). Among these different effectors, we found enzymes involved in phospholipid signaling, such as PI4-K (Kim et al., 2001; Garcia-Marcos et al., 2006). Once activated, PI4-K generates the lipid precursor (PI4P) necessary for the formation of DAG and subsequent activation of novel PKC molecules, such as PKCδ. DAG synthesis is generally associated with the catalytic activity of PLCβ; however in this study, we propose PLCγ1 as an alternative for the production of DAG in IECs as a result of P2X7 stimulation. PLCγ1 activation generally occurs via receptor tyrosine kinase (RTK), but non-RTK receptors can also activate this enzyme. Although the stimulation of PKCδ and PKD1 by P2X7 has been described previously for other cell types (Bradford and Soltoff, 2002; Gendron et al., 2003; Carrasquero et al., 2010), we are the first to report a link between this P2X7-dependent signaling pathway and the regulation of Glut2 membrane expression.
PKD is a particular serine/threonine kinase activated by the transient phosphorylation of Ser744 and Ser748 found in the activation loop. This activating phosphorylation is followed by the sustain autophosphorylation of Ser916. This autophosphorylation reaction creates docking sites for PKD-binding partners and influence PKD localization within the cell (Nishikawa et al., 1998; Hausser et al., 1999). Although PKD is generally associated with TGN vesicle trafficking (Liljedahl et al., 2001), a large fraction of PKD1 resides in the cytosol where it could be recruited to the plasma membrane following PLC activation (Van Lint et al., 2002). Numerous substrates not associated with TGN trafficking were identified as E-cadherin (Jaggi et al., 2005), β-catenin (Du et al., 2009), vanilloid receptor type 1 (Wang et al., 2004), plasma membrane Na+/H+ exchanger (Haworth et al., 1999), and cardiac troponin I (cTnI; Haworth et al., 2004). PKD1-dependent phosphorylation of the vanilloid receptor type 1 at its N-terminus enhances receptor function (Wang et al., 2004), whereas PKD-dependent phosphorylation of the plasma membrane Na+/H+ exchanger reduces activity (Haworth et al., 1999). Thus, depending on the context, localization, and cell type, PKD-dependent phosphorylation could either act as a positive or negative regulator of protein functions (Mikhalap et al., 2008). Of particular interest is the phosphorylation of cTnI by PKD1, occurring at PKA sites (Haworth et al., 2004). It could be that PKD1-dependent internalization of Glut2 uses a similar strategy to phosphorylate Glut2 at the PKA sites and stimulates its internalization. However, additional experiments will be needed to confirm this possibility.
Considering the obesity and diabetes epidemic, regulation of blood sugar levels represents an increasing challenge. The data presented in this study represent an interesting avenue to regulate glycemia. Contrary to most type II diabetes therapies, we proposed modulating glucose entry into cells by regulating the expression of Glut2 at the surface of enterocytes. We provided evidences that Glut2 membrane expression could be down-regulated from IECs plasma membrane following P2X7 activation. As illustrated in this study, Glut2 internalization was not due to the apoptotic properties of the P2X7 receptor. We also demonstrated that the internalization of Glut2 was dependent on the activity of PKCδ and PKD in a calcium-independent manner. Although more experiments are required to fully elucidate the contribution of PKD1 to regulation of Glut2 expression, the data provided are supportive of a new role for P2X7 in the intestine and suggest a novel function for PKD1 in IECs.
Acknowledgements
This research was supported by the Natural Sciences and Engineering Research Council of Canada discovery grant (#327128-06) to F.P.G. F.P.G. is member of the FRSQ-funded Centre de Recherche Clinique Étienne-Le Bel.