P2Y2 receptor promotes intestinal microtubule stabilization and mucosal re-epithelization in experimental colitis†
FP Gendron is a consultant for Vertez Pharmaceuticals Inc.
Abstract
P2Y2 receptor expression is increased in intestinal epithelial cells (IECs) during inflammatory bowel diseases (IBDs). In this context, P2Y2 stimulates PGE2 release by IECs, suggesting a role in wound healing. For this study, we have used the non-cancerous IEC-6 cell line. IEC-6 cell migration was determined using Boyden chambers and the single-edged razor blade model of wounding. The receptor was activated using ATP, UTP, or 2-thioUTP. Pharmacological inhibitors, a blocking peptide, a neutralizing antibody and interfering RNAs were used to characterize the signaling events. Focal adhesions and microtubule (MT) dynamics were determined by immunofluorescence using anti-vinculin and anti-acetylated-α-tubulin antibodies, respectively. In vivo, the dextran sodium sulfate mouse model of colitis was used to characterize the effects of P2Y2 agonist 2-thioUTP on remission. We showed that P2Y2 increased cell migration and wound closure by recruiting Go protein with the cooperation of integrin αv. Following P2Y2 activation, we demonstrated that GSK3β activity was inhibited in response to Akt activation. This leads to MT stabilization and increased number of focal adhesions. In vivo, P2Y2 activation stimulates remission, as illustrated by a reduction in the disease activity index values and histological scores as compared to control mice. These findings highlight a novel function for this receptor in IECs. They also illustrate that P2Y receptors could be targeted for the development of innovative therapies for the treatment of IBDs. J. Cell. Physiol. 228: 99–109, 2013. © 2012 Wiley Periodicals, Inc.
Crohn's disease and ulcerative colitis are chronic and relapsing inflammatory bowel diseases (IBDs) that can affect the entire length of the intestine (Abraham and Cho, 2009). IBDs are the consequence of intestinal epithelial cells (IECs) and immune cells dysfunction resulting in impaired or abnormal mucosal immune responses (Laukoetter et al., 2008). The regenerative capacity of the mucosal surface epithelium is essential to the maintenance of the intestinal mucosal barrier integrity even after extensive damages (Sturm and Dignass, 2008). However, the inflammatory burst associated with the relapsing episodes observed in IBDs, impaired the regenerative capacity of the IEC monolayer. As a consequence, it is possible to observed crypt abscess formation, uncontrolled bacterial infiltration, exacerbated inflammatory responses, and loss of tissue architecture and functions (Sartor and Sandborn, 2004; Sturm and Dignass, 2008). Consequently, to significantly increase or obtain complete remission the regeneration of a functional epithelium is a pre-requisite (Ardizzone et al., 2011).
The regeneration of the intestinal epithelial lining requires the coordinate and overlapping action of three wound healing processes. First, IECs adjacent to the injured surface migrate into the wound to cover the denuded area. Those epithelial cells that migrate into the wound depolarize, form pseudopodia-like structures, reorganize their cytoskeleton, and repolarize after wound closure. This restitution process is independent of cell proliferation (Sturm and Dignass, 2008). Intestinal epithelial restitution occurs within minutes to hours both in vivo and in vitro. Second, epithelial cell proliferation is necessary to replace and maintain the IEC monolayer. Third, maturation and differentiation of undifferentiated epithelial cells is needed to re-establish the polarized monolayer of differentiated enterocytes and to maintain intestinal function. These events are regulated by a number of structurally distinct regulatory factors, including cytokines, growth factors, adhesion molecules, and phospholipids (Taupin and Podolsky, 2003; Okamoto and Watanabe, 2005).
We and others have shown that extracellular adenosine 5′-triphosphate (ATP) and uridine 5′-triphosphate (UTP) have cytokine-like properties by activating the P2Y2 receptor (la Sala et al., 2003; Ben Yebdri et al., 2009; Langlois and Gendron, 2009; Grbic et al., 2011). Nucleotides are released in the extracellular environment of IECs under normal physiological conditions, but are massively liberated following inflammation and injury (Di Virgilio et al., 2001; Luttikhuizen et al., 2004; Grbic et al., 2008). The exact level of endogenous P2Y2 agonists in the colonic lumen or mucosa under normal or inflammatory conditions is unknown. Nonetheless, a recent study using a novel ATP biosensor reported that colonic mucosa release ATP at a concentration ranging from 0.8 to 1 µM, under normal conditions (Patel et al., 2011). Given that UTP release from non-secretory cells, like colonocytes, follows the same pattern of ATP release, it was proposed that both ATP and UTP are released with a similar time course (Lazarowski and Boucher, 2001). Under normal in vivo conditions, the colonic mucosa is also exposed to mechanical stress that could be induced by the passage of feces. This mechanical stress could stimulate the release of ATP and UTP by a 10- to 20-fold increase (Lazarowski and Harden, 1999; Lazarowski and Boucher, 2001; Lazarowski et al., 2003). Endogenous extracellular ATP and UTP could also originate from intestinal bacteria, apoptotic cells, and/or from luminal immune cells (Gordon, 1986; Di Virgilio et al., 2001; Luttikhuizen et al., 2004; Ivanova et al., 2006). As a result of inflammation, activated leukocytes and platelets as well as the acidic and hypoxic microenvironments provided by inflammation will favor the release of extracellular ATP and UTP (Gordon, 1986; Di Virgilio et al., 2001; Luttikhuizen et al., 2004) as well as UDP (Grbic et al., 2008). Furthermore, we showed that P2Y2 expression was increased in the colon of IBD patients and in an animal model of intestinal inflammation (Grbic et al., 2008). The reported up-regulation of P2Y2 expression was in part due to an increased expression in IECs (Degagne et al., 2009). It was reported that P2Y2 agonists can also negatively regulate immunity and inflammation (Di Virgilio et al., 2009), and could thus affect both mucosal inflammation and the healing process (Dignass et al., 1998; Braun et al., 2006; Crooke et al., 2009; Boucher et al., 2010). In fact, the contribution of adenine nucleotides to intestinal wound healing has been previously reported (Dignass et al., 1998). As of yet, the identity of the specific P2Y receptor involved and the signaling events associated with intestinal wound healing have never been investigated.
Signaling through the Gq-coupled P2Y2 involves the hydrolysis of phosphatidylinositol-4, 5-bisphosphate (PIP2) by phospholipase C, thus generating inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 increases the concentration of intracellular calcium and triggers calcium-dependent cell responses, such as the activation of protein kinase C (PKC; Erb et al., 2006). PKC stimulation of MAPK activity regulates various cellular processes, notably migration and proliferation. In addition to MAPK, P2Y2 can stimulate PI3K/Akt activity through a PLC/IP3/Ca2+ and PKC pathway (Bilbao et al., 2010). P2Y2 is also coupled to integrins αvβ3 and αvβ5 via a RGD integrin-binding domain found in its first extracellular loop (Erb et al., 2001; Bagchi et al., 2005). Association of P2Y2 to the αv integrin subunit is necessary for the recruitment of Go and the initiation of Go-mediated signaling events leading to cell migration (Bagchi et al., 2005).
The participation of the actin cytoskeleton in cell migration has been well described. It provides a protrusion force at the cell front and a contractile force in the cell body (Gardel et al., 2010). In contrast, the role of microtubules (MT) is far more diverse and the tight regulation of MT dynamics is required for proper directed cell migration (Wen et al., 2004; Etienne-Manneville, 2010). Hence, the regulation of MT dynamics are essential to cytoskeleton and focal adhesion organization (Blikslager et al., 2007; Gardel et al., 2010). One key component of MT is α-tubulin (α-Tub), which is stabilized through protein acetylation (Creppe et al., 2009). Consequently, detection of the acetylated form of α-Tub is an indication of MT stabilization (Creppe et al., 2009; Etienne-Manneville, 2010). MT dynamics is regulated by numerous effectors including, but not limited to, GSK3β and PI3K/Akt. The PI3K/Akt pathway is important for MT dynamics as well as being a key element for cell migration and proliferation (Sun et al., 2009), whereas GSK3β regulates MT dynamics by inhibiting the activation of MT-associated proteins like CLIP, EB1, and APC that are responsible for MT elongation and stabilization (Wen et al., 2004; Hur et al., 2011).
In this study, we have investigated the role of P2Y2 in intestinal epithelial wound healing processes. We showed that P2Y2 activation induces migration of a wounded monolayer of IECs. Furthermore, we demonstrated that IECs migration induced by the P2Y2 receptor requires αv integrin subunit and Go protein as well as the subsequent activation of the PI3K/Akt signaling pathway, which leads to the elongation and stabilization of α-Tub via inactivation of GSK3β. Furthermore, animal studies demonstrated that activation of purinergic receptors during the recuperation phase following acute intestinal inflammation strongly enhanced the wound healing process.
Materials and Methods
Materials
Dulbecco's modified Eagle's medium (DMEM), penicillin–streptomycin, HEPES and fetal bovine serum (FBS) were purchased from Wisent (St. Bruno, QC, Canada). GlutaMax®, Alexa Fluor 488 goat anti-rabbit IgG (H + L) and Fluo-4/AM were from Invitrogen (Burlington, ON, Canada). ATP was from Roche Applied Science (Laval, QC, Canada). UTP, suramin, and PPADS were purchased from Sigma–Aldrich (St. Louis, MO) and 2-thioUTP was from Tocris Bioscience (Ellisville, MO). The PI3K inhibitor LY294002, H-Arg-Gly-Asp-Ser-OH (RGDS) peptide and control were acquired from EMD chemicals (Mississauga, ON, Canada). The rabbit anti-phospho-Akt (Ser473), anti-phospho-GSK-3β (Ser9), anti-Ac-α-Tub (Lys40), anti-integrin αv, anti-Akt antibodies, and rabbit monoclonal anti-GSK3β antibody were purchased from Cell Signaling Technology (Pickering, ON, Canada). Integrin αv neutralizing antibody and normal mouse IgG were purchased from Santa Cruz Biotechnologies (Santa Cruz, CA). The rabbit anti-P2Y2 receptor antibody, mouse monoclonal anti-actin (clone C4), DAPI, and anti-ECL reagent were purchased from Millipore (Mississauga, ON, Canada). Horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG and HRP-conjugated goat anti-rabbit IgG were from GE Healthcare Biosciences Corp (Piscataway, NJ). Dextran sulfate sodium (DSS; Mr 36,000–50,000) was obtained from MP Biomedicals (Solon, OH). Pertussis toxin (PTX) was purchased from Enzo Life Sciences (Burlington, ON, Canada).
Cell culture
The non-cancerous rat IEC-6 cell line (ATCC #CRL-1592) was grown as previously described (Degagne et al., 2009; Langlois and Gendron, 2009). Cells were incubated in serum-free medium at 37°C, 24 h prior to experiments.
Generation of shP2Y2 cell lines
The 29mer shRNA constructs against rat P2Y2R, empty control vector (EV) and control shRNA scramble sequence (shNT) were purchased from Origene (Burlington, ON). Retroviruses were produced and used for infection of IEC-6 cells according to Origene recommendations [HuSH shRNA Plasmid Panels (29-mer), Application Guide]. Receptor expression was validated by Western blot.
Cytosolic [Ca2+] measurement
IEC-6 cells (10 × 106 cells grown in 100-mm2 dishes) were detached by a brief trypsin/EDTA treatment, re-suspended in complete culture medium, and washed by centrifugation for 3 min at 100 × g before being incubated with 1 µM Fluo-4/AM in 4.5 ml HBSS with Ca2+ and Mg2+ (Wisent, St-Bruno, QC) for 25 min at 37°C. After a wash by centrifugation, cells were re-suspended in HBSS containing Ca2+ and Mg2+ and incubated for 25 min at 37°C to ensure complete hydrolysis of the Fluo-4/AM. Cells were then centrifuged again and re-suspended in 16 ml of HBSS with Ca2+ and Mg2+ and 2 ml of cells suspension was gently stirred in a quartz cuvette while [Ca2+]i was monitored on a RF-5301 PC Shimadzu spectrofluorometer (Man-Tech, Guelph, ON) with excitation and emission wavelengths of 488 and 520 nm, respectively. Change in intracellular Fluo-4 fluorescence intensity (F) was acquired using the Panorama fluorescence 1.1 software. At the end of each recording, maximal (Fmax) and minimal (Fmin) fluorescence were determined by adding successively 0.1% Triton X-100 and 50 mM EDTA to cell suspensions. The following equation was used to relate the fluorescence intensity to Ca2+ levels: [Ca2+] = Kd(F − Fmin)/(Fmax − F). Kd is the Ca2+ dissociation constant of Fluo-4 (345 nM; Grynkiewicz et al., 1985).
Migration assay
IEC-6 cells or IEC-6 cell populations for shP2Y2 (IEC-6/shY2), shNT (IEC-6/shNT) or EV (IEC-6/EV) were grown to confluence and serum-deprived 24 h before assays. Briefly, cells were trypsinized and resuspended in 600 µl of migrating buffer (0.05% gelatine and 25 mM HEPES in DMEM). The upper chamber of an 8 µm Transwell was loaded with 175,000 cells. The lower chamber was filled with 600 µl of migrating buffer supplemented or not with P2Y2R agonists, with or without P2 receptor antagonists suramin or PPADS. Antagonists were added 30 min prior to cell stimulation with 100 µM ATP or UTP, or 10 µM 2-thioUTP. After a 6 h incubation period, the upper and lower chambers were washed using PBS. The remaining cells in the upper chamber were removed with a cotton swab. Cells present beneath the membrane were fixed with ice-cold methanol. The filter was then removed and placed on a microscope slide. Migrating epithelial cells were counted in four random fields using a Leica DM2500 microscope equipped with a Hamamatsu ORCA-R2 digital camera under bright field illumination.
Wounding assay
Cells were grown to confluence in 100-mm2 dishes. They were incubated in serum-free medium for 24 h, wounded with a single-edged razor blade and incubated in the presence or absence of 100 µM ATP or UTP, or 10 µM 2-thioUTP for 24 h. To assess the signaling pathways involved, IEC-6 cell monolayers were pre-treated for 30 min with 10 µg/ml anti-integrin αv neutralizing antibody, 10 µg/ml normal mouse IgG, or10 µM of LY294002 wounded and stimulated in the presence or absence of ATP or UTP. In the assays in which PTX was used, PTX was added at a concentration of 250 ng/ml for 16 h prior to wounding and nucleotide stimulation. Photomicrographs were taken at four locations per wound, and the area of migrating cells was measured using ImageJ software.
Western blotting
After treatments, IEC-6 cells were lysed and samples processed for protein immunoblotting, as previously described (Grbic et al., 2008). Western blots were performed using a 1/1,000 dilution of rabbit anti-phospho-Akt (Ser473), anti-phospho-GSK-3β (Ser9), anti-acetyl-α-Tub K40, and anti-P2Y2 anti-integrin αv. Specific protein bands on membranes were detected using a 1:10,000 dilution of HRP-conjugated anti-rabbit as the secondary antibody and visualized on autoradiographic films using the Millipore chemiluminescence system. For normalization of signals, membranes were stripped of antibodies (Grbic et al., 2008) and reprobed with a 1/1,500 dilution of rabbit anti-Akt, a 1/5,000 dilution of mouse anti-GSK3β or a 1/10,000 dilution of mouse monoclonal anti-β-actin followed by a 1/10,000 dilution of HRP-conjugated anti-rabbit IgG or HRP-conjugated anti-mouse IgG as the secondary Ab, respectively.
Indirect immunofluorescence
IEC-6 cells grown on sterile glass coverslips were washed twice with ice-cold PBS after treatments and fixed with 3% paraformaldehyde for 30 min at 4°C. Cells were washed with PBS, quenched with 100 mM glycine for 10 min, and permeabilized with PBS containing 0.1% Triton X-100 for 10 min at RT. Cells were washed and then blocked with PBS containing 2% BSA for 20 min at RT. They were then immunostained for 2 h with the rabbit anti-acetyl-α-Tub as the primary antibody, followed by the Alexa Fluor 488 goat anti-rabbit IgG (H + L) as the secondary antibody for 1 h. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI). Staining for the focal adhesion proteins vinculin and F-actin using phalloidin was realized using the Actin Cytoskeleton and Focal Adhesion Staining Kit (Millipore) as recommended by the manufacturer. Negative controls (normal IgG antibody) were included in all experiments. Slides were washed in PBS, mounted, and images were captured on Leica DM2500 microscope using a Hamamatsu ORCA-R2 digital camera.
Mouse model of IBD and 2-thioUTP injections
Adult CD-1 mice (30–35 g) were purchased from Charles River Laboratories (St. Constant, QC, Canada). Colitis-like disease was induced by adding 4% (w/v) DSS to the drinking water for 7 days as previously described (Grbic et al., 2008, 2011). After a 24 h recovery period, mice received daily rectal enemas of a 2-thioUTP solution at a dose of 2 µg/g of body weight versus saline. The dose of 2-thioUTP used was based on a recent article by Grbic et al. (2011) in which UDP was given by enemas at a dose of 100 µg/g of body weight to stimulate, in vivo, the P2Y6 receptor. The reported EC50 for 2-thioUTP toward P2Y2 receptor is ranging from 35 to 50 nM (El-Tayeb et al., 2006) as compared to previously reported average EC50 values ranging from 1.5 to 5.8 µM for ATP and UTP (Velazquez et al., 2000). Thus, 2-thioUTP is more potent for the P2Y2 receptor by about a 90-fold factor as compared to ATP or UTP. ATP has been previously used in clinical trial as a cancer treatment at doses ranging from 24 (Beijer et al., 2010) to 135 µg/g (Agteresch et al., 2000), and even up to 228 µg/g (Haskell et al., 1998) for an average dose of about 129 µg/g. Given the potency and selectivity of 2-thioUTP as compared to ATP we decided to use a dose of 2 µg/g, which reflect the difference in potency between P2Y2 receptor natural agonists and 2-thioUTP and also similar to the recently reported level of nucleotide secreted by colonic mucosa in vitro (Patel et al., 2011). The 2-thioUTP solution was prepared in normal saline and sterilized by filtration (0.22 µm filter pores). The 2-thioUTP solution or saline was injected in the distal colon up to the first curvature leading to the transverse colon as we previously described (Grbic et al., 2011). Colitis was assessed by clinical and histological scoring, as previously described (Cooper et al., 1993; Dieleman et al., 1998; Grbic et al., 2008, 2011). Spleen was harvested to measure bacterial translocation. All procedures were approved by the Université de Sherbrooke Animal Care Committee and performed according to the Canadian Guidelines for Care and Use of Experimental Animals.
Bacterial translocation
Enteric bacterial translocation to spleen was assessed in CD-1 mice that were treated with 2-thioUTP after receiving 4% (w/v) DSS in their drinking water for 7 days. The harvested tissues were removed under strictly aseptic conditions and were homogenized in sterile PBS. Homogenates were plated on tryptic soy agar under aerobic conditions and were allowed to grow for 24 h at 37°C. CFUs were counted and expressed as the Log10CFU/organ.
Histological analysis
Mice colon were fixed in 4% paraformaldehyde overnight at 4°C, embedded in paraffin, cut into 5-µm sections, and applied to Superfrost/Plus slides (Fisher Scientific, Ottawa, ON, Canada), as previously described (Grbic et al., 2008). Hematoxylin and eosin (H&E) staining was performed by the QTRN/CToDE intestinal phenotyping platform from the Université de Sherbrooke. Images were captured on a Leica DMLB2 microscope using a Leica DC300 camera.
Statistical analysis
Results are expressed as the means ± standard error of the mean (SE). Statistical significances were determined using ANOVA tests or as indicated in the figure legends. The numbers of replicates for each experiment is presented in the figure legends.
Results
P2Y2 agonists stimulate IECs migration in vitro
First, we determined the optimal concentrations of ATP, UTP, or 2-thioUTP that were required to stimulate P2Y2 receptor activity and cell migration. As shown in Supplemental Figure S1A, the most efficient concentration of ATP or UTP stimulating P2Y2 activation was 100 µM as determined by measuring the variation of intracellular calcium concentration in IEC-6 cells loaded with Fluo-4, whereas 10 µM 2-thioUTP was the most efficient analog concentration stimulating IEC-6 cells migration following wounding (Supplemental Fig. S1B). We showed that the addition of 100 µM ATP (Fig. 1A), UTP (Fig. 1B), or 10 µM 2-thioUTP (Fig. 1C) to the bottom compartment of Boyden chambers induced IEC-6 cell migration across the membrane delineating the upper chamber from the lower compartment. Migration was significantly increased by more than 20–30% as compared to control. Addition of suramin, a general P2 receptor antagonist, prior to P2Y2 activation reduced IEC-6 cell migration back to control levels (Fig. 1A–C). Addition of PPADS, a P2 receptor antagonist not affecting P2Y2 (Grbic et al., 2008), prior to cell stimulation had no effect on receptor-mediated IEC-6 cell migration. To circumvent the absence of a specific P2Y2 antagonist, a stable IEC-6 cell population in which P2Y2 expression was knocked down by retroviral infection was generated. Cell lines were generated with the empty vector (EV), a non-targeting scrambled shRNA (shNT), or a shRNA targeting the receptor. Neither the EV nor the shNT constructs affected the expression of P2Y2 (Fig. 1D). However, the expression of shP2Y2-08 showed a 60% decrease in receptor expression and was used in subsequent experiments (Fig. 1D). The migration of IEC-6/shP2Y2 cells in response to agonists was brought back to non-stimulated control levels (Fig. 1E), thus clearly suggesting a role for P2Y2 in IECs migration.
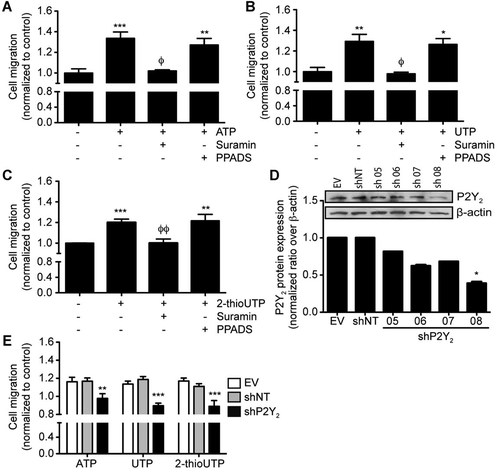
P2Y2 agonists increase IEC-6 cell migration. IEC-6 cells were seeded in the upper compartment of Boyden's chamber and 100 µM ATP (A), UTP (B), or 10 µM of 2-thioUTP (C) was added to the lower chamber with or without 100 µM of P2Y receptor antagonists, suramin, or PPADS. The number of migrating cells was measured in four different optical fields and normalized to control. Results represent means ± SE of three separate experiments. Statistical significance was determined by one-way ANOVA in which *P < 0.05, **P < 0.01, and ***P < 0.001 as compared to unstimulated cells, and ϕP < 0.05 and ϕϕP < 0.01 versus nucleotide stimulated cells. D: P2Y2 expression in the shP2Y2 cell lines generated was detected by immunoblot analysis. E: EV, shNT, and shP2Y2 IEC-6 cells were seeded in the upper chamber of Boyden'schamber and stimulated in the lower chamber with 100 µM ATP or UTP, or 10 µM 2-thioUTP, and the number of migrating cells was measured in four different optical fields and normalized to control. Results represent means ± SE of three separate experiments. Statistical significance was determined by one-way ANOVA in which *P < 0.05, **P < 0.01, and ***P < 0.001 as compared to stimulated IEC-6/shNT cells.
Wound healing is increased following P2Y2 activation
The role of P2Y2 in epithelial cell migration was analyzed using the single-edged razor blade model of wounding. Confluent IEC-6 cell monolayer wounded and stimulated for 24 h with P2Y2 agonists ATP, UTP, or 2-thioUTP showed a significant increase by more than 40% in the area covered by IEC-6 cells after wounding, as opposed to unstimulated cells (Fig. 2A, B). Reduction of P2Y2 expression by shRNA significantly decreased the area of migrating cells (Fig. 2C). Our results support our hypothesis that P2Y2 stimulates IECs migration and wound closure.

Agonists of P2Y2 stimulate wound closure of a wounded IEC-6 cell monolayer. A: Photomicrographs of representative wounds 24 h after wounding. IEC-6 cell monolayer was wounded and either left unstimulated (ctrl) or stimulated with 100 µM of ATP, UTP, or 10 µM 2-thioUTP. The original magnification was 5× with scale bars = 435 µm. B: Cell migration was measured 24 h following wounding and stimulation with or without ATP, UTP, and 2-thioUTP. Results represent means ± SE of three separate experiments. Statistical significance was determined by one-way ANOVA in which *P < 0.05, **P < 0.01, and ***P < 0.001 as compared to unstimulated cells. C: EV, shNT, and shP2Y2 IEC-6 cell monolayers were wounded and either left unstimulated (ctrl) or stimulated with 100 µM of ATP or UTP, or 10 µM 2-thioUTP, and the area of migrating cells into the wound after 24 h was measured. Results represent means ± SE of three separate experiments. Statistical significance was determined by one-way ANOVA in which *P < 0.05, **P < 0.01, and ***P < 0.001 as compared to unstimulated cells.
P2Y2-induced migration is attributable to its cross-talk with αv integrin subunit
Association of P2Y2 with the αv integrin subunit is necessary for receptor-dependent recruitment of Go and initiation of Go-mediated signaling events leading to cell migration (Bagchi et al., 2005). This migratory advantage is of particular interest for the IECs located at the margin of the wounded epithelium as it could favor wound closure. As shown in Figure 3A, addition of a RGDS blocking peptide to IEC-6 cells inhibited the cell migration effect mediated by ATP and UTP. The role and expression pattern of αv integrin subunit in IECs is unclear, although it was suggested that it acts as a fibronectin receptor in human IECs (Gagne et al., 2010). In this study, we showed that IEC-6 cells express αv integrin subunit (Fig. 3B), which could participate with P2Y2 to promote IECs migration. As predicted by the literature (Chorna et al., 2007), addition of the anti-αv neutralizing antibody for 24 h significantly decreased the integrin expression by 30% (Fig. 3B). Consequently, the addition of the neutralizing antibody against αv integrin subunit blocks P2Y2-mediated IEC-6 cell migration and wound closure as compared to normal IgG-treated cells (Fig. 3C).
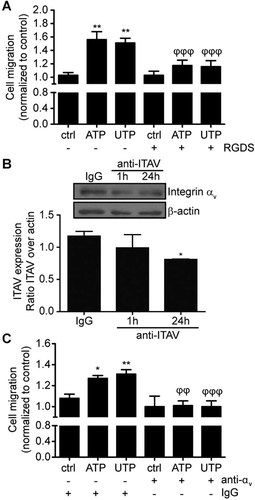
P2Y2 and integrin αv cooperate to increase cell migration. A: IEC-6 cell monolayers were pre-treated with 100 µM of RGDS for 30 min, wounded and either left unstimulated (ctrl) or stimulated with 100 µM ATP or UTP, and the wound area was measured 16 h after wounding. Results represent fold variation of the means ± SE of three separate experiments normalized to control. Statistical significance was determined by one-way ANOVA in which *P < 0.05, **P < 0.01, and ***P < 0.001 as compared to unstimulated cells. B: Integrin αv expression in IEC-6 cells was detected by immunoblot analysis in the presence or not of 10 µg/ml anti-integrin αv blocking antibody (anti-ITAV) or 10 µg/ml control IgG for the indicated time. A typical Western blot is presented with densitometry quantification of three independent experiments. Results (mean ± SE) are presented as the ratio of integrin αv over β-actin expression. Statistical significance was determined by an unpaired t-test, where *P < 0.05 versus IgG-treated cells. C: IEC-6 cell monolayers were pre-treated with 10 µg/ml of anti-integrin αv neutralizing antibody or 10 µg/ml of control IgG for 30 min, wounded and either left unstimulated (ctrl) or stimulated with 100 µM ATP or UTP. The wound area was measured 16 h after wounding. Results represent fold variation of the means ± SE of three separate experiments normalized to control. Statistical significance was determined by one-way ANOVA in which *P < 0.05, **P < 0.01, and ***P < 0.001 as compared to unstimulated cells.
P2Y2 promotes cell migration via stabilization of the elongating microtubule
Regulation of MT dynamics is essential to cytoskeleton and focal adhesion organization (Blikslager et al., 2007; Gardel et al., 2010). Accordingly, we observed that P2Y2 activation by ATP or UTP leads to α-Tub acetylation on Lys 40, as shown by immunofluorescence microscopy (Fig. 4A–C). While the number of F-actin stress fibers is slightly increased (red) in response to ATP or UTP stimulation, nucleotide treatments lead to increased focal adhesions as assessed by staining for the focal adhesion protein vinculin (green; Supplemental Fig. 2A–C).
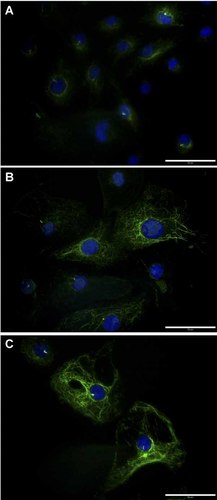
P2Y2 activation stimulates the acetylation of α-tubulin in IEC-6 cells. Sub-confluent IEC-6 cells were treated with or without 100 µM ATP or UTP for 15 min. IEC-6 cells were stimulated for 15 min with PBS (A), 100 µM ATP (B), or 100 µM UTP (C). The acetylated form of α-tubulin (α-Tub) was detected with an anti-acetyl-α-Tub (Lys40) antibody (green filaments). Nuclei (blue) were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Presented results are typical immunofluorescence micrographs of 2–3 independent experiments. The original magnification was set to 40× and scale bars = 50 µm.
P2Y2 mediates cell migration through a PI3K/Akt pathway regulating MT dynamics
MT dynamics is an important element for cell migration and proliferation, and as such it must be tightly regulated. In this context, we showed that P2Y2 activation with 100 µM ATP (Fig. 5A) or UTP (Fig. 5B) increased Akt phosphorylation within 5 min. Inhibition of the PI3K/Akt pathway using 20 µM LY294002 strongly reduced the number of Ac-α-Tub positive MT fibers that were formed in response to 100 µM ATP (Fig. 5C) or UTP (Fig. 5D) when compared to IEC-6 cells stimulated with ATP (Fig. 5E) or UTP (Fig. 5F) alone. The loss of staining for Ac-α-Tub correlated with the inhibition of IEC-6 cell migration induced by ATP and UTP in the presence of LY294002 (Fig. 5H).
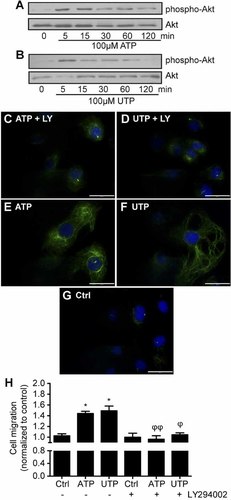
P2Y2 promotes cell migration by activating the PI3K signaling pathway and the stabilization of microtubules. IEC-6 cells were stimulated with 100 µM ATP (A) or UTP (B) for 0 (control), 5, 15, 30, 60, and 120 min. Akt phosphorylation was detected by Western blot analysis. Presented blots are typical of three separate experiments. Indirect immunofluorescence of acetyl-α-tubulin in IEC-6 cells stimulated with 100 µM ATP (C) or UTP (D) for 15 min in the presence of 20 µM LY294002 or in the absence of inhibitor (panels E and F). Unstimulated control IEC-6 cells are presented on panel G. Original magnification of 63× with scale bars = 30 µm. H: IEC-6 cell monolayers were pre-treated with 20 µM of LY294002 for 30 min, wounded and either left unstimulated (ctrl) or stimulated with 100 µM of ATP or UTP, and the wound area was measured 16 h after wounding. Results represent fold variation of the means ± SE of three separate experiments normalized to control. Statistical significance was determined by one-way ANOVA, in which *P < 0.05, **P < 0.01, and ***P < 0.001 as compared to unstimulated cells, and ϕP < 0.05 and ϕϕP < 0.01 versus nucleotide stimulated cells.
P2Y2 stimulates GSK3β phosphorylation downstream of PI3K/Akt
The inhibition of GSK3β activity is strongly associated with MT stabilization and cell migration. As shown in Figure 6, P2Y2 activation with 100 µM ATP (Fig. 6A) or UTP (Fig. 6B) induced the inhibitory phosphorylation of GSK3β on Ser9. These findings suggest that the effect of P2Y2 on MT stabilization is dependent on the inhibition of GSK3β activity. We showed that the addition of the PI3K inhibitor, LY294002, prior to P2Y2 stimulation with ATP or UTP (Fig. 6C) favors the balance toward an active non-phosphorylated GSK3β that will block cell migration. These results showed that GSK3β is activated downstream of the PI3K/Akt pathway.
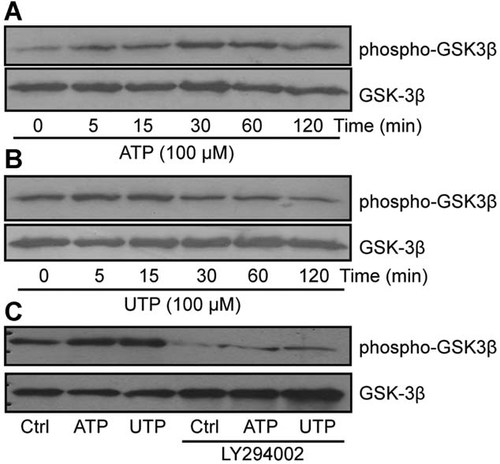
P2Y2 activation induces GSK3β phosphorylation downstream of the PI3K/Akt pathway. Rat intestinal epithelial IEC-6 cells were stimulated with 100 µM ATP (A) or UTP (B) and GSK3β phosphorylation on Ser9 determined by Western blot. Presented blots are typical of three separate experiments. C: IEC-6 cells were pre-treated with 20 µM of LY294002 for 30 min before stimulation with 100 µM ATP or UTP for 5 min. GSK-3β phosphorylation was detected by Western blot analysis. Presented blots are typical of three separate experiments.
The migrating effect mediated by P2Y2 involves a PTX-sensitive G protein
To determine the contribution of Go to P2Y2-mediated migration, we added 250 ng/ml PTX to IEC-6 cells for 16 h before nucleotide stimulation. As shown in Figure 7A, PTX treatments reduced the phosphorylation of Akt on Ser473 and correlated with the inhibition of ATP- and UTP-induced IEC-6 cell migration and wound closure effects (Fig. 7B). As for the inhibition of the PI3K/Akt pathway using LY294002, addition of PTX (250 ng/ml) reduced the number of Ac-α-Tub positive MT fibers that were formed in response to 100 µM ATP (Fig. 7C) or UTP (Fig. 7D) when compared to IEC-6 cells stimulated with ATP (Fig. 7E) or UTP (Fig. 7F) alone.
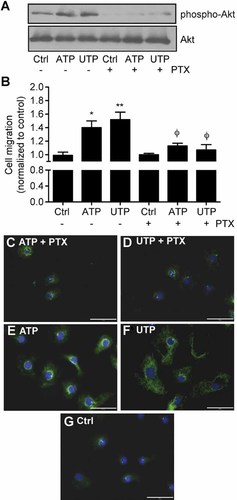
ATP- and UTP-induced wound closure is dependent on a pertussis toxin sensitive G-protein located upstream of Akt. A: Stimulation of P2Y2 with 100 µM ATP or UTP for 5 min phosphorylated Akt as determined by Western blot analysis. Addition of 250 ng/ml of pertussis toxin (PTX) 16 h prior to P2Y2 activation blocked Akt phosphorylation. B: Inhibition of Go-protein with PTX abolished the ATP and UTP healing effects of a wounded IEC-6 cell monolayer. Results represent fold variation of the means ± SE of three separate experiments normalized to control. Statistical significance was determined by one-way ANOVA, in which *P < 0.05 and **P < 0.01 as compared to unstimulated cells and where ϕP < 0.05 versus ATP or UTP stimulated cells without PTX. Indirect immunofluorescence of acetyl-α-tubulin in IEC-6 cells stimulated with 100 µM ATP (C) or UTP (D) for 15 min in the presence of 250 ng/ml PTX or in the absence of inhibitor (panels E and F). Unstimulated control IEC-6 cells are presented on panel G. Original magnification of 40× with scale bars = 50 µm.
2-thioUTP administrations during the remission phase of mice suffering from colitis-like disease improve recovery
Non-inflamed mice treated for 3 days with PBS enemas displayed typical colon architecture with well define crypts and undisturbed surface epithelium (Fig. 8A–A′), as shown by H&E staining. On the contrary, mice suffering from DSS-induced colitis had regions where the epithelium was completely absent (Fig. 8B) and where the crypt architecture was lost (Fig. 8B′). As for the non-inflamed mice treated with PBS enemas, non-inflamed mice treated with 2-thioUTP also displayed normal colon architecture (Fig. 8C–C′). Finally, inflamed mice treated with 2-thioUTP had regained part of their epithelium although crypts and surface epithelium were not as well defined (Fig. 8D–D′). Hence, massive infiltrates of immune cells were still visible in the colon of inflamed mice treated with the P2Y2 agonist (Fig. 8D). Administration of 2-thioUTP during recovery significantly reduced the disease activity index (DAI) value as compared to mice receiving PBS enemas (Fig. 8E). From the H&E staining, we evaluated the overall histological damages following treatment with 4% DSS prior to daily injections of 2-thioUTP for 3 days using the scoring chart described by Jeffers et al. (2002). In Figure 8F, we showed that 2-thioUTP-treated mice had a score significantly lower than control PBS mice. As an indication of IECs barrier function, we determined bacterial translocation in spleen. We observed that bacterial translocation to the spleen (Fig. 8G) was significantly reduced in mice treated with daily injections of 2-thioUTP during recovery as compared with non-treated mice.
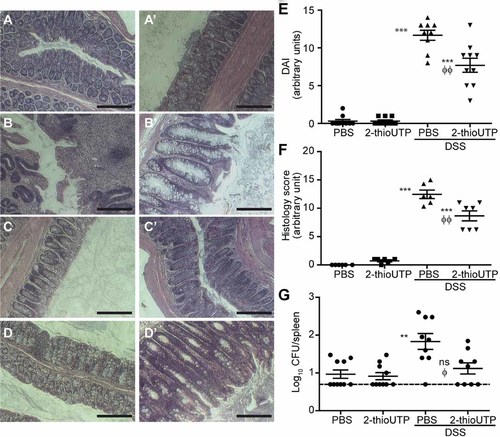
2-thioUTP enemas stimulate recovery in a mouse model of colitis. Colitis-like inflammation was induced with 4% (w/v) DSS in drinking water of mice for 7 days. Twenty-four hours after the end of the treatment, mice received 2-thioUTP (2 µg/g body weight) enemas or control PBS for 3 days. A–D′: Typical H&E staining of two different mice colons are showed after 3 days of recovery: (A–A′) Normal control mice receiving PBS enemas, (B–B′) mice suffering from DSS-induced colitis with PBS enemas, (C–C′) normal mice treated with 2-thioUTP (2 µg/g body weight) enemas, and (D–D′) mice suffering from DSS-induced colitis after the 2-thioUTP treatment. The original magnification was set to 20× with scale bars = 200 µm. E: Disease activity index after 3 days of 2-thioUTP enemas to mice suffering colitis-like symptoms. F: H&E score of colonic tissues of mice receiving 2-thioUTP or not after DSS treatment. G: Bacterial translocation to spleen. Horizontal bars represent the mean ± SE of 6–10 animals. Statistical significance was determined by one-way ANOVA test with multiple comparisons post-test, where ns: non-significant, **P < 0.01 and ***P < 0.001 when compared to normal control mice, and ϕP < 0.05 and ϕϕP < 0.01 versus DSS mice with PBS enemas.
Discussion
IECs constitute a physical barrier to bacteria and other luminal contents that could trigger an uncontrolled immune response (Blikslager et al., 2007). Following wounding, cells located at the wound margin rapidly migrate to seal the epithelial defect and reconstitute the barrier (Sturm and Dignass, 2008). In a recent study, it was shown that inhibition of P2Y2 expression in vivo using siRNA applied to a model of corneal wounding delayed the restitution response to receptor agonists (Crooke et al., 2009). IEC-6 cell is an accepted and validated in vitro model to study epithelial restitution as it gives reproducible wounds. Hence, cell migration is independent of DNA synthesis and there is no proliferation at the wound edge even 24 h after wounding (McCormack et al., 1992). Using this cell model, it was previously reported that ATP stimulation increased the cell migration rate when compared to control (Dignass, 2001). However, no mechanisms or any receptors were identified as being involved in this process. The reported EC50 values for the recombinant P2Y2 receptor range from 1.5 to 5.8 µM (Velazquez et al., 2000) and although, 300 µM of ATP or UTP elicited the maximal variation in intracellular calcium, we chose the 100 µM concentration as the working concentration based on our previous publications (Degagne et al., 2009; Langlois and Gendron, 2009). Our data clearly showed that the most efficient 2-thioUTP-concentration stimulating cell migration was 10 µM. Hence, we showed that P2Y2 is involved in wound closure of an IEC-6 monolayer. In fact, the selective inhibition of P2Y2 expression by shRNA highlighted the important role of this receptor in cell migration and the wound healing processes triggered by ATP and UTP.
P2Y2 cooperates with integrin αv through a RGD motif found in the receptor's first extracellular loop to promote cell migration (Erb et al., 2001). This cooperation results in the recruitment of Go (Bagchi et al., 2005), the clustering of αv integrin and the activation of Mek/Erk pathway dependent on PI3K activity (Chorna et al., 2007). Integrin clustering can be seen as focal adhesions in cells and these cellular entities act as a platform for different signaling pathways involved in cell migration (Welf et al., 2011). Using a RGD blocking peptide and an anti-integrin αv neutralizing antibody, we demonstrated that P2Y2-induced cell migration required the coordinate action of integrin αv and P2Y2. The reduction of αv integrin subunit expression following treatment with the integrin blocking antibody is in accordance with the literature (Chorna et al., 2007). Using U-937 cell line, Chorna et al. (2007) demonstrated that incubation of these cells for 24 h with αv integrin blocking antibody leads to the internalization of this integrin, resulting in a decrease of integrin expression at the cell surface by 35%.
We report here a novel mechanism by which P2Y2 enhances IECs migration that involves the stabilization of MT through the stimulation of α-Tub acetylation. In fact, recent studies have showed that the stabilization of MT requires the coordinate actions of MT-associated proteins to catalyze the acetylation of α-Tub and thus promote cell migration (Gundersen and Bulinski, 1988; Creppe et al., 2009; Etienne-Manneville, 2010). MT stabilization is regulated by numerous factors, but GSK3β seems central to this process. Active GSK3β was reported to inhibit MT stabilization by down-regulating MT-associated protein CRMP2 activity and by negatively regulating Tau (Sun et al., 2009). GSK3β activity is inhibited by phosphorylation of Ser9 residue found in its N-terminal domain. Consequently, inhibition of GSK3β results in Tau and CRMP2 activation, as well as the subsequent elongation and stabilization of MT. We showed that P2Y2 activation leads to an Akt-dependent GSK3β phosphorylation, ensuing acetylation of α-Tub on Lys40 in IEC-6 cells. These findings are not only in accordance with the role of Akt and MT stabilization in cell migration (Creppe et al., 2009; Etienne-Manneville, 2010), but most interestingly propose that P2Y2 is involved in the control of MT dynamics. Furthermore, indirect immunofluorescence for Ac-α-Tub in IEC-6 cells pre-treated with PTX showed a reduction in the acetylation of α-Tub induced by ATP and UTP (Fig. 7). On the other hand, the presence of PTX prior to ATP and UTP stimulation did not influence the number of vinculin positive FA (data not shown). These results are thus suggesting that activation of a PTX-sensitive Go protein is responsible for the increase in the acetylated form of α-Tub.
The maintenance of the intestinal epithelium integrity is a necessity to prevent the translocation of bacteria or entry of luminal molecules that could trigger an excessive and uncontrolled immune response and lost of tissue architecture and function. Here, we reported that following P2Y2 activation there is an increase in the acetylated form of α-Tub and in the number of FA. Both α-Tub acetylation and FA are essential to IECs migration. FA are composed in part of integrin αvβ3 and other signaling molecules, like vinculin, FAK, and Src, that are involved in signaling cascades responsible for the attachment of migrating cells to the extracellular matrix (Hamadi et al., 2009). Contrary to the role of FA, the contribution of MT to IECs migration is not well defined. In the course of cell migration, there is a reorientation of the MT organizing center towards the leading edge that allows the asymmetrical polarization and elongation of MT to the migrating front (Kaverina et al., 1998; Wehrle-Haller and Imhof, 2003). MT are then pulled at their plus ends by plus-end tracking proteins and stabilized by protein like CLIP (Schober et al., 2007). MT at the leading edge can thus deliver important signaling molecules necessary to cell migration. In fact, disruption of α-Tub acetylation results in a significant decreased in the binding and motility of kinesin-1 (Reed et al., 2006). Kinesin-1 is necessary to the transport of cargo towards the MT plus-end. Moreover, it was showed that Necl-5 interacts with PDGF receptor and intregrin αvβ3 at the leading edge of NIH3T3 to promote directional cell migration (Minami et al., 2007, 2010). They have showed that activation of Necl-5 allows the growth and attraction of MTs at the leading edge in an intregrin αvβ3-dependant manner. We believe that a similar process occurs following P2Y2 receptor activation and its subsequent interaction with integrin αv to promote the growth and stabilization of MT and to increase cell migration. In another way, activation of P2Y2 leads to an increase in the number of acetylated α-Tub filaments and the resulting stabilized MT could be necessary to the motor activity of kinesin-1. However, additional experiments will be needed to validate this hypothesis.
Administration of 2-thioUTP during the recovery phase of mice suffering from acute DSS colitis displayed a reduction of the DAI and histological score values as well as a reduction in bacterial translocation to the spleen when compared with untreated mice. Although mice receiving 2-thioUTP enemas still displayed signs of colitis-like disease after 3 days of treatment, our results indicate that treated mice were in significantly better shape than control animals. In this context, we could argue that by increasing the dose of 2-thioUTP or the length of treatment we could favor the healing process. Nonetheless, the observed reduction in bacterial count in spleen are good indicators that 2-thioUTP treatments favored the re-epithelization process, since bacterial translocation can be the result of increased barrier permeability, as observed in IBDs (Berg, 1999).
In this study, we provided evidence that the activation of P2Y2 during the remission phase following acute inflammation could facilitate wound healing. Therefore, not only we have described a novel function for this receptor in IECs, we are also the first to show that P2Y2 could modulate MT dynamics. Regulation of MT dynamics is essential for wound closure (Etienne-Manneville, 2010), but deregulation of this phenomenon is also linked to cancer progression (Etienne-Manneville, 2010). In fact, as proposed by our in vivo experiments, our findings could lead to the development of novel therapies aimed at stimulating intestinal wound healing, which could represent a step forward in the treatment of IBDs (Pineton de Chambrun et al., 2010; Ardizzone et al., 2011).
Acknowledgements
We thank Gabriel Mitchell from the department of Biology from the Université de Sherbrooke for his technical help with the bacterial translocation studies. This research was supported by the Crohn's and Colitis Foundation of Canada Grant in Aid of Research (2009–2012) to F.P.G. F.P.G. is member of the FRSQ-funded Centre de Recherche Clinique Étienne-Le Bel. D.M.G. is a recipient of an Alexander Graham Bell scholarship from the Natural Sciences and Engineering Research Council of Canada.