Distal-less homeobox 5 inhibits adipogenic differentiation through the down-regulation of peroxisome proliferator-activated receptor γ expression†‡
All authors state that they have no conflicts of interest.
The title of this article posted in the online issue did not match the author's accepted title. This article has been reposted to correct the title. The print issue was unaffected.
Abstract
Distal-less homeobox 5 (Dlx5) is a positive regulator of osteoblast differentiation that contains a homeobox domain. Because there are possible reciprocal relationships between osteogenic and adipogenic differentiation of bone marrow mesenchymal stem cells (MSCs), we examined the regulatory role of Dlx5 in adipogenic differentiation in this study. Adipogenic stimuli suppressed the expression levels of Dlx5 mRNA in mouse bone marrow stromal cells. Over-expression of Dlx5 inhibited adipogenic differentiation in human bone marrow MSCs and 3T3-L1 preadipocytic cells whereas knockdown of Dlx5 enhanced adipogenic differentiation in 3T3-L1 cells. Over-expression of Dlx5 suppressed the expression of adipogenic marker genes, including CCAAT/enhancer-binding protein α (C/EBPα) and peroxisome proliferator-activated receptor γ (PPARγ). Dlx5-mediated suppression of adipogenic differentiation was overcome by over-expression of PPARγ but not by that of cAMP response element binding protein (CREB) or C/EBPα. Dlx5 decreased the transcriptional activity of CREB and C/EBPα in a dose-dependent manner. Dlx5 directly bound to CREB and C/EBPα and prevented them from binding to and subsequently transactivating the PPARγ promoter. These results suggest that Dlx5 plays an important regulatory role in fate determination of bone marrow MSCs toward the osteoblast lineage through the inhibition of adipocyte differentiation as well as the direct stimulation of osteoblast differentiation. J. Cell. Physiol. 228: 87–98, 2013. © 2012 Wiley Periodicals, Inc.
Bone marrow mesenchymal stem cells (MSCs) are a multipotent stem cell that can give rise not only to osteoblasts but also to a range of other cell types including adipocytes, chondrocytes, and myoblasts (Bennett et al., 1991; Pittenger et al., 1999). Among these fates, differentiation to the osteoblast and adipocyte lineages has particular relevance to the maintenance of normal bone homeostasis. Because osteoblasts and adipocytes differentiate from a common progenitor, inducers of differentiation toward one lineage may inhibit cell differentiation into another lineage. In age-related osteoporosis, bone mass is reduced, whereas fat accumulation increases in bone marrow (Meunier et al., 1971). One explanation for this phenomenon is that, compared with young MSCs, the MSCs from the older mice show enhanced differentiation potential toward the adipogenic lineage and decreased potential of osteogenic differentiation (Beresford, 1989; Gimble et al., 1995; Moerman et al., 2004). In addition to aging-related osteoporosis, osteopenia induced by glucocorticoids and immobilization also accompany increased fat accumulation in the bone marrow (Minaire et al., 1984; Martin and Zissimos, 1991; Ali et al., 2005; Kitajima et al., 2007). In contrast, mechanical loading-induced increases in bone mass are associated with enhanced osteoblastogenesis at the expense of adipogenesis (David et al., 2007; Sen et al., 2008). These reports indicate that there are the inverse relationships between increased adipogenesis and decreased osteogenesis in the bone marrow.
Several transcription factors have been suggested to be involved in the reciprocal regulation between osteoblast and adipocyte differentiation, including runt related transcription factor 2 (Runx2), β-catenin and peroxisome proliferator-activated receptor γ (PPARγ) (Muruganandan et al., 2009). Runx2 and PPARγ are master transcription factors for osteogenic and adipogenic differentiation, respectively (Ducy et al., 1997; Barak et al., 1999). Osteogenic stimuli, such as mechanical stress, increase the expression and transcriptional activity of Runx2 while suppressing PPARγ expression, resulting in the shift of MSCs fate toward the osteogenic lineage (David et al., 2007; Sen et al., 2008). In contrast, adipogenic stimuli, such as immobilization and an antidiabetic medicine rosiglitazone, show opposite effects; that is, enhancement of PPARγ expression and suppression of Runx2 expression are observed, resulting in enhanced adipogenesis in place of osteogenic differentiation (Minaire et al., 1984; Ali et al., 2005). Canonical Wnt ligands and their down-stream β-catenin signaling have also been reported to be a positive regulator for osteogenesis and a negative regulator for adipogenesis (Cawthorn et al., 2012). Extracellular stimuli or transcription factors that regulate the expression or transcriptional activity of the above-mentioned transcription factors will also function as a reciprocal regulator for fate determination of MSCs.
Distal-less homeobox 5 (Dlx5) is one member of the Distal-less homeobox domain (HD) protein family. Dlx5-null mice exhibit defects in axial, appendicular and craniofacial bone formation (Robledo et al., 2002). Dlx5 is involved in the commitment of mesenchymal progenitors to the osteoblast lineage through the following actions. During osteoblast differentiation from MSCs, Dlx5 is expressed earlier than Runx2 and Osterix and directly up-regulates the expression levels of these transcription factors (Hassan et al., 2006; Holleville et al., 2007; Ulsamer et al., 2008). Furthermore, Dlx5 up-regulates the expression levels of alkaline phosphatase and osteocalcin through its binding to the respective gene promoter (Hassan et al., 2004; Kim et al., 2004). Over-expression of Dlx5 also increases mineralized nodule formation in vitro (Ryoo et al., 1997; Hassan et al., 2004). Given that Dlx5 up-regulates Runx2 expression and Runx2 is a critical regulator for MSCs fate determination toward osteoblasts, it is suggested that Dlx5 may also exert a negative effect on adipogenic differentiation similar to that of Runx2. However, there have been no reports published on the regulatory role of Dlx5 in adipocyte differentiation. Therefore, we explored the regulatory role of Dlx5 in adipocyte differentiation by examining the expression levels of Dlx5 during adipocyte differentiation and the effect of Dlx5 knockdown on adipocyte differentiation. In addition, we over-expressed Dlx5 in human bone marrow MSCs (hBMSCs) and murine 3T3-L1 preadipocytes and compared their effects on adipocyte differentiation. Finally, the regulatory mechanism of Dlx5 to down-regulate PPARγ expression was elucidated.
Materials and Methods
Cell culture and transient transfection
hBMSCs (Lonza; Basel, Switzerland) and murine 3T3-L1 cells (Korean cell line bank; Seoul, Korea) were cultured in Dulbecco's modified Eagle's medium (DMEM; Hyclone; Logan, UT) supplemented with 10% heat-inactivated fetal bovine serum (FBS; BioWhittaker; Walkersville, MD), 100 µg/ml streptomycin and 100 U/ml penicillin. Bone marrow stromal cells were isolated from the tibias and femurs of 6-week-old C57BL/6 mice and cultured in alpha modified Eagle's medium (αMEM, Hyclone) supplemented with 10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin. To induce osteogenic or adipogenic differentiation, the cells were incubated in osteogenic medium (growth medium supplemented with 0.1 µM dexamethasone, 10 mM β-glycerophosphate and 50 µg/ml ascorbic acid) or adipogenic medium (growth medium supplemented with 0.1 µM dexamethasone, 50 µM indomethacin, 0.5 mM isobutylmethylxanthine, and 10 µg/ml insulin) for the indicated periods. Transient transfection of hBMSCs was performed by electroporation using the BioRad Gene Pulser II (Hercules, CA), and transient transfection of 3T3-L1 cells was performed using Lipofectamine™ reagent (Invitrogen; Carlsbad, CA).
Antibodies and reagents
Anti-Dlx5 antibody was purchased from Sigma (St. Louis, MO) and anti-actin antibody and goat anti-rabbit HRP-conjugated IgG were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-CCAAT/enhancer-binding protein α (C/EBPα), anti-C/EBPβ, anti-PPARγ, and anti-cAMP response element binding protein (CREB) antibodies were obtained from Cell Signaling Technology (Danvers, MA). Anti-GST, anti-lipoprotein lipase (LPL) and anti-adipocyte protein 2 (aP2) antibodies were purchased from Abcam (Cambridge, UK), and anti-HA antibody (HA11.3) was from Covance (Princeton, NJ). The easy-BLUE™ and StarTaq™ reagents were ordered from iNtRON Biotechnology (Sungnam, Korea). AccuPower RT PreMix was purchased from Bioneer (Daejeon, Korea), and SYBR premix EX Taq was from Takara (Otsu, Japan). The primers for PCR and 5′-biotinylated oligonucleotides were synthesized by Cosmogenetech (Seoul, Korea). Protein G agarose beads were purchased from Millipore (Billerica, MA). Rosiglitazone was obtained from Cayman Chemical (Ann Arbor, MI). Ascorbic acid, β-glycerophosphate, dexamethasone, isobutylmethylxanthin, insulin, and indomethacin were purchased from Sigma. NE-PER Nuclear and Cytoplasmic Extraction Reagents was obtained from Pierce Biotechnology (Rockford, IL). The Supex reagent for Western blot analysis was ordered from Dyne-Bio (Sungnam, Korea). Dual-Glo luciferase assay kit and glutathione-sepharose beads were purchased from Promega (Madison, WI). Poly(dI-dC) preabsorbed streptavidin-agarose beads were purchased from Thermo Scientific (Rockford, IL). Overnight Express Autoinduction System 1 was purchased from Novagen (Madison, WI).
Plasmid construction
The construction of the HA-tagged Dlx5 expression vector has been described previously (Cho et al., 2009). PPARγ and C/EBPα expression vectors and PPARγ promoter reporter plasmids were kindly provided by Prof. JB Kim from Seoul National University. The constructs for truncated mutants of Dlx5 were prepared by Cosmogenetech using HA-Dlx5 as a template (Fig. 7B). GST-C/EBPα and GST-CREB constructs were prepared by digestion of C/EBPα and CREB expression vectors with EcoRI and XhoI and isolation of DNA fragments and subsequent ligation of fragments into EX-P0067-M12x, a GST expression vector. Nucleotide sequences of all the constructs were confirmed by DNA sequencing.
Reverse transcription-polymerase chain reaction (RT-PCR)
To evaluate mRNA expression, semi-quantitative RT-PCR was performed in the range PCR of linear amplification. Total RNA was extracted using easy-BLUE™ RNA Extraction Reagents. cDNA was synthesized from total RNA using the AccuPower™ RT-PreMix and was subsequently subjected to PCR amplification using StarTaq™ polymerase. The PCR products were electrophoresed in a 1.2% agarose gel and visualized under UV light by ethidium bromide staining. Human and mouse genes, and their primer sequences for PCR, were described in Table 1. Real-time PCR was performed to observe mRNA expression levels of Dlx5 and adipocyte differentiation marker genes using SYBR premix EX Taq in an AB 7500 Fast Real-Time system. Primer sequences used were described in Table 2.
| Gene | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| Human PPARγ | GACCACTCCCACTCCTTTGA | CGACATTCAATTGCCATGAG |
| Human C/EBPα | CACGAAGCACGATCAGTCCAT | GGCACAGAGGCCAGATACAAG |
| Human CREB | ACCAGCAGAGTGGAGATGCT | CTGCCCACTGCTAGTTTGGT |
| Human aP2 | CATCAGTGTGAATGGGGATG | GTGGAAGTGACGCCTTTCAT |
| Human LPL | ACAGGGCGGCCACAAGTTTT | ATGGAGAGCAAAGCCCTGCTC |
| Human GAPDH | CCATCTTCCAGGAGCGAGATC | GCCTTCTCCATGGTGGTGAA |
| Mouse PPARγ | CTTATTTATGATAGGTGTGA | GTGATATGTTTGAACTTGAT |
| Mouse C/EBPα | TCAGACCAGAAAGCTGAGTTGTG | TGGTCCCCGTGTCCTCCTA |
| Mouse CREB | GCCCCTGCCATCACCACT | TCTGCAGGCCCTGTACCC |
| Mouse aP2 | AAAGAAGTGGGAGTGGGCTT | CTCTTGTGGAAGTCACGCCT |
| Mouse LPL | GCGTAGCAGGAAGTCTGACC | CTACAACTCAGGCAGAGCCC |
| Mouse Dlx5 | TCTCTAGGACTGACGCAAACA | GTTACACGCCATAGGGTCGC |
| Mouse GAPDH | TCACCATCTTCCAGGAGCG | CTGCTTACCACCTTCTTGA |
| Gene | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| Human PPARγ | TGACCCAGAAAGCGATTCCT | AAAGTTGGTGGGCCAGAATG |
| Human C/EBPα | CACGAAGCACGATCAGTCCAT | GGCACAGAGGCCAGATACAAG |
| Human CREB | ATGCAGCTGCCACTCAGCCG | TCGTGGGTGCTGTGCGGATC |
| Human aP2 | AGCACCATAACCTTAGATGGGG | CGTGGAAGTGACGCCTTTCA |
| Human LPL | CACGGGCTCAGGAGCATTAC | TAGTTAAACTCCTCCTCCATCCAGTT |
| Human GAPDH | CCATCTTCCAGGAGCGAG | GCCTTCTCCATGGTGGTGA |
| Mouse PPARγ | CCGAAGAACCATCCGATTGAA | GCCCAAACCTGATGGCATT |
| Mouse C/EBPα | CGGGAGAACTCTAACTC | GATGTAGGCGCTGATGT |
| Mouse CREB | AGCTGCCACTCAGCCGGGTA | TGGTGCTCGTGGGTGCTGTG |
| Mouse aP2 | AAAGAAGTGGGAGTGGGCTT | CTCTTGTGGAAGTCACGCCT |
| Mouse LPL | AGAACATTCCCTTCACCCTGC | AGAACATTCCCTTCACCCTGC |
| Mouse Dlx5 | TCTCTAGGACTGACGCAAACA | GTTACACGCCATAGGGTCGC |
| Mouse GAPDH | TCAATGACAACTTTGTCAAGC | CCAGGGTTTCTTACTCCTTGG |
Western blot analysis and immunoprecipitation
Whole-cell lysates were prepared and Western blot analysis was performed as previously described (Lee et al., 2010). For immunoprecipitation, the cells were washed with ice-cold phosphate buffered saline (PBS) and scraped into immunoprecipitation buffer consisting of 20 mM Tris–Cl (pH 7.5), 150 mM NaCl, 1 mM EDTA (pH 8.0), 2% Brij35, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM sodium orthovanadate, 1 mM PMSF, 1 µg/ml aprotinin, 1 µM leupeptin, and 1 µM pepstatin and sonicated briefly. Each sample containing 1 mg of protein was used for immunoprecipitation with the appropriate primary antibodies and protein G agarose beads. The bead pellet was washed five times with immunoprecipitation buffer, denatured by boiling in 2× SDS sample buffer and then subjected to SDS–PAGE and immunoblot analysis.
Dlx5 knock-down by small interfering RNA (siRNA)
Dlx5 siRNA and control siRNA (ON-TARGETplus Non-targeting siRNA #2 L-041957-01-0005) were purchased from Dharmacon (Lafayette, CO). To increase silencing efficiency, Dlx5 ON-TARGETplus SMARTpool siRNA was used, which was a mixture of siRNAs that targets four different sequence of Dlx5. The sequences of each Dlx5 siRNA are as follows: J-041405-09 5′-GCUUUCAGCUGGCCGCUUU-3′, J-041405-10 5′-UCUCUAGGACUGACGCGCAAA-3′, J-041405-11 5′-GCGCAACUGUGGACACCUAU-3′ and J-041405-12 5′-CGCUUUAUUAGAUGGGCUA-3′. Transient transfection of siRNA into 3T3-L1 cells was performed using Dharmafect according to the manufacturer's instructions.
ChIP assay
Cells were cross-linked with 1% formaldehyde, lysed and sonicated to obtain DNA fragments of 200–800 bp. After preclearing with blocked protein G agarose, immunoprecipitation was performed with anti-CREB or anti-C/EBPα antibody (10 µg) or equivalent concentrations of IgG as a negative control. DNA eluted from immune complexes was subjected to PCR amplification targeting the mouse PPARγ promoter region containing the C/EBPα binding elements or cAMP response element (CRE)-like elements. The primer sequences are as follows: C/EBPα-binding element-1 (−576 to −503 bp) f 5′-GTTGGAATTACCAGAGCAGAG-3′ and r 5′-GCCACTGGCGTCTATTTTAC-3′; C/EBPα-binding element-2 (−137 to −21 bp) f 5′-GATGTTTAAGTGATAAGTGC-3′ and r 5′-CTGATCACAATTCCTTAGCC-3′; and CRE-like element (−310 to −154 bp) f 5′-GGTGATTTATTCAACAAGTC-3′ and r 5′-CTGCAGCTGCTACTGATAG-3′.
Luciferase reporter assay
3T3-L1 cells were seeded in a 96-well plate at a density of 1 × 104 cells/well. After overnight culture, the cells were transiently transfected with the indicated plasmids. In each transfection, 0.2 µg of expression vectors (HA-Dlx5, CREB, C/EBPα, PPARγ, or pcDNA3), 0.2 µg of reporters (PPARγ-luc or pGL3-luc) and 0.1 µg of Renilla luciferase plasmid were used when the amount of plasmids was not indicated. After 24 h, the cells were harvested, and luciferase activity was measured using a Dual-Glo luciferase assay kit. Relative luciferase activity was calculated after normalizing the transfection efficiency by Renilla luciferase activity.
GST pull-down assay
3T3-L1 cells were transiently transfected with the expression vectors encoding wild-type or truncated mutants of HA-Dlx5 and incubated for 24 h. Nuclear proteins were then prepared using NE-PER Nuclear and Cytoplasmic Extraction Reagents according to the manufacturer's instructions. GST-CREB, GST-C/EBPα, or GST was expressed in E. coli BL21 DE3 cells with the Overnight Express Autoinduction System 1 according to the manufacturer's instructions. After lysis of the bacteria, GST proteins were captured on glutathione-sepharose beads and washed extensively in lysis buffer before use. Then, 0.5 µg of indicated GST protein and 20 µg of prepared nuclear proteins containing indicated HA-Dlx5 protein were mixed and incubated for 90 min at 4°C. After extensive washing with binding buffer, pull-downed glutathione-sepharose beads were denatured by boiling in 2× SDS sample buffer and then subjected to SDS–PAGE and immunoblot analysis.
Biotin pull-down assay
3T3-L1 cells were transiently transfected with the indicated expression vectors (pcDNA3, HA-Dlx5, CREB, or C/EBPα). After incubation for 24 h, nuclear proteins were prepared. Then, nuclear protein (300 µg) was incubated with the indicated 5′-biotinylated double-stranded oligonucleotides (1 µg) for 20 min at RT, followed by incubation with poly(dI-dC)-preabsorbed streptavidin–agarose beads (30 µl) for 4 h at 4°C. The complex was pulled down, and proteins in the complex were dissociated and analyzed by Western blotting with anti-CREB or anti-C/EBPα antibody. Sequence of biotinylated oligonucleotides was composed of the mouse PPARγ promoter sequence, including C/EBPα-binding element-2 (−100 to −96 bp) or CRE-like element (−238 to 232 bp). Oligonucleotide sequences were as follows: oligonucleotide containing C/EBPα-binding element-2 f 5′-GTCACAATGAAAAAATTGCAGCCCTCCCTG-3′ and oligonucleotide containing CRE-like element f 5′-GGAATAGTTGACAGAATAAAGGCTAG-3′.
Oil Red O and von Kossa staining
Oil red O staining and von Kossa staining were performed to identify accumulation of lipid droplets and matrix mineral deposition, respectively. At the end of the culture period, the cells were fixed with 70% ethanol, washed with PBS and stained with an Oil red O or von Kossa staining solution.
Statistical analysis
All of the quantitative data are represented as the mean ± SD. Statistical significance was analyzed by Student's t-test. A P-value <0.05 was considered statistically significant.
Results
Levels of Dlx5 expression are decreased by adipogenic stimuli
We first examined the pattern of Dlx5 expression in mouse bone marrow stromal cells, which were incubated in growth medium, adipogenic medium or osteogenic medium for 7 days. Similar to the previous studies, osteogenic stimuli significantly enhanced Dlx5 mRNA expression (Fig. 1A). In contrast, incubation under adipogenic conditions significantly diminished the expression levels of Dlx5 mRNA (Fig. 1A). To further examine the time course of Dlx5 expression during adipogenic differentiation, we incubated 3T3-L1 preadipocytic cells in adipogenic medium for 8, 24, or 48 h, and the mRNA levels of Dlx5 and PPARγ were examined by real-time PCR. A dramatic decrease in Dlx5 expression levels was observed even after an 8-h-incubation, and levels further decreased in a time-dependent manner (Fig. 1B). Conversely, the expression levels of PPARγ significantly increased in a time-dependent manner (Fig. 1B). These results indicate that adipogenic stimuli are a strong repressor for Dlx5 expression.
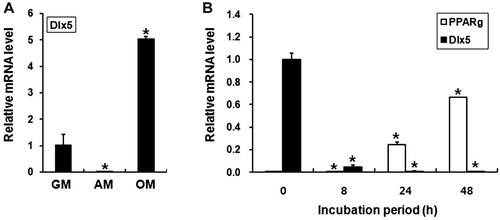
Adipogenic stimuli suppress the expression levels of Dlx5 mRNA. A: Mouse bone marrow stromal cells were incubated in growth medium (GM), adipogenic medium (AM) or osteogenic medium (OM) for 7 days, and mRNA levels of Dlx5 were determined by real-time PCR. B: 3T3-L1 cells were cultured in adipogenic medium for the indicated periods. Then, real-time PCR for Dlx5 and PPARγ was performed. Expression levels of Dlx5 and PPARγ mRNA were presented after normalization to GAPDH. Data are represented as the mean ± SD of the triplicates (*P < 0.05).
Over-expression of Dlx5 inhibits adipogenic differentiation of hBMSCs and 3T3-L1 cells
We next examined the effect of Dlx5 over-expression on adipogenic differentiation. hBMSCs were transiently transfected with an HA-Dlx5 expression plasmid and incubated in adipogenic medium for 21 days. At the end of culture period, adipogenic differentiation was verified by Oil red O staining (Fig. 2A, lower panel). Over-expression of Dlx5 suppressed the accumulation of lipid droplets. The anti-adipogenic activity of Dlx5 was also demonstrated in RT-PCR results of adipogenic marker genes (Fig. 2B). Among the genes examined, the levels of C/EBPα, PPARγ, aP2, and LPL mRNA were significantly down-regulated by Dlx5 over-expression but that of CREB mRNA was unchanged. Expression of Dlx5 was still observed in 21-day cultured Dlx5-transfected cells. As a control, HA-Dlx5-expressing hBMSCs were also incubated in the osteogenic medium for 21 days, and osteogenic induction was verified by von Kossa staining (Fig. 2A, upper panel). Consistent with previous reports, Dlx5 over-expression enhanced matrix mineralization (Hassan et al., 2006; Holleville et al., 2007; Ulsamer et al., 2008). These results indicate that Dlx5 inhibits adipogenic differentiation while stimulating osteogenic differentiation. To further confirm that Dlx5 over-expression shows anti-adipogenic effects, 3T3-L1 cells were transiently transfected with the HA-Dlx5 expression plasmid and incubated under adipogenic conditions for 3 days. Oil red O staining showed that lipid droplet accumulation was strongly suppressed by Dlx5 over-expression (Fig. 2C). The results from RT-PCR and Western blot analysis demonstrated that over-expression of Dlx5 decreased the expression levels of C/EBPα, PPARγ, aP2, and LPL, but not that of CREB (Fig. 2D,E). Over-expression of Dlx5 was confirmed by RT-PCR and Western blot analysis.

Over-expression of Dlx5 inhibits adipogenic differentiation of hBMSCs and 3T3-L1 cells. A,B: hBMSCs were transfected with the HA-Dlx5 expression vector or pcDNA and cultured in osteogenic medium (A, upper panel) or adipogenic medium (A, lower panel and B) for 21 days. Then differentiation was confirmed by von Kossa (A, upper panel) and Oil red O (A, lower panel) staining or by semi-quantitative RT-PCR (B, left panel) and real-time PCR (B, right panel) of adipogenic marker genes. C–E: 3T3-L1 cells were transiently transfected with the HA-Dlx5 expression vector or pcDNA and cultured in adipogenic medium for 3 (C) or 2 (D,E) days. Then, differentiation was confirmed by Oil red O staining (C), semi-quantitative RT-PCR (D, left panel), real-time PCR (D, right panel) and Western blot analysis (E). All of the experiments were repeated at least twice and the representative images were presented. Real-time PCR data are represented as the mean ± SD of two independent experiments (*P < 0.05, compared to pcDNA-transfected cells). Expression levels of adipogenic genes were presented after normalization to GAPDH. (A,C, left panels, 100×).
Knock-down of Dlx5 enhances adipogenic differentiation of 3T3-L1 cells
To further examine the regulatory role of endogenous Dlx5 in adipocyte differentiation, knockdown of Dlx5 was induced by siRNA in 3T3-L1 cells. Adipogenic induction of these cells for 2 days showed that adipogenic differentiation was enhanced by Dlx5 knockdown. Oil red O staining demonstrated that Dlx5 knockdown enhanced accumulation of lipid droplets (Fig. 3A). Total RNA and protein samples were prepared from 3T3-L1 cells incubated in adipogenic medium for 24 h, and RT-PCR and Western blot analysis were performed. RT-PCR and Western blot results demonstrated that, compared with the cells transfected with non-targeting control siRNA, the expression levels of Dlx5 were significantly diminished in the cells transfected with Dlx5 siRNA (Fig. 3B,C). RT-PCR and Western blot analyses showed that the expression levels of C/EBPα, PPARγ, aP2, and LPL after a 24 h-incubation under adipogenic conditions were much higher in Dlx5-silenced cells compared with control cells (Fig. 3B,C). Similar to the results from the over-expression studies, expression of CREB was not significantly altered by Dlx5 knockdown. When the incubation period was prolonged, the stimulatory effect of Dlx5 knockdown on adipogenic differentiation was minimized (data not shown). These results suggest that Dlx5 knockdown advances the adipogenic differentiation of 3T3-L1 cells.
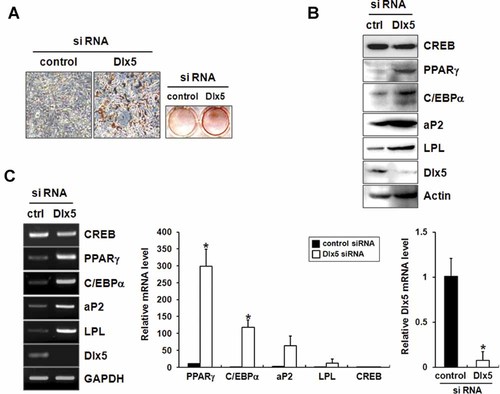
Knock-down of Dlx5 by siRNA enhances adipogenic differentiation of 3T3-L1 cells. For cellular knockdown of Dlx5, 3T3-L1 cells were transfected with Dlx5 siRNA or non-targeting control (ctrl) siRNA. Then, the cells were incubated in adipogenic medium for 48 h (A) or 24 h (B,C). At the end of the culture period, Oil red O staining (A), Western blot analysis (B), semi-quantitative RT-PCR (C, left panel) and real-time PCR (C, right panel) were performed. Knock-down efficiency of Dlx5 siRNA was verified at the mRNA and protein level (B,C). All of the experiments were repeated at least twice and the representative images were presented (A,B). Real-time PCR data are represented as the mean ± SD of two independent experiments (*P < 0.05, compared to control siRNA-transfected cells). Expression levels of adipogenic genes were presented after normalization to GAPDH. (A, left panel, 100×).
Over-expression of PPARγ, but not that of CREB or C/EBPα, overcomes the anti-adipogenic activity of Dlx5
Because the above results showed that Dlx5 over-expression suppressed the expression of PPARγ, a critical transcription factor for adipogenic differentiation, we next examined whether over-expression of PPARγ can rescue the adipogenic differentiation of 3T3-L1 cells in the presence of Dlx5 over-expression. 3T3-L1 cells were co-transfected with PPARγ and HA-Dlx5 expression vectors and incubated under adipogenic conditions for 3 days. Oil red O staining results showed that Dlx5 over-expression did not inhibit lipid droplet accumulation in the presence of PPARγ over-expression (Fig. 4A). Furthermore, the inhibitory effect of Dlx5 on PPARγ and C/EBPα expression was not observed in the presence of exogenous PPARγ over-expression (Fig. 4B, left panel). We also examined the effect of Dlx5 over-expression in the presence of rosiglitazone, an activating ligand for PPARγ. Rosiglitazone increased lipid droplet accumulation in 3T3-L1 cells (Fig. 4C). Similar to the PPARγ over-expression, rosiglitazone blocked the inhibitory effect of Dlx5 on lipid accumulation and adipogenic marker gene expression, including PPARγ and C/EBPα (Fig. 4C,D).
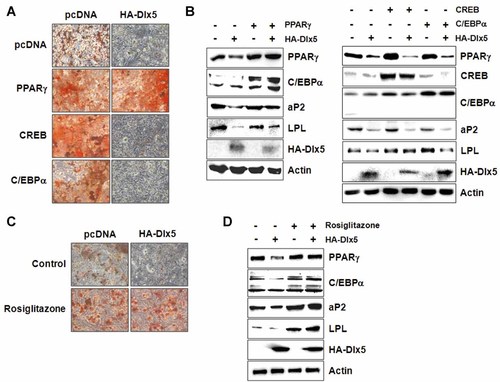
PPARγ over-expression, but not that of CREB or C/EBPα, rescues Dlx5-inhibited adipogenic differentiation. 3T3-L1 cells were transiently transfected with the indicated expression vectors and cultured in adipogenic medium for 3 (A,C) or 2 (B,D) days. When indicated, the cells were incubated in the presence of 500 µM rosiglitazone (C,D). At the end of the culture period, Oil red O staining (A,C) or Western blot analysis (B,D) was performed. All of the experiments were repeated at least twice and the representative images were presented. (A,C, 100×).
Because C/EBPα and CREB are known to be transcriptional activators for PPARγ, we also examined whether over-expression of these transcription factors can block the inhibitory effect of Dlx5 on PPARγ expression (Darlington et al., 1998; Fox et al., 2006). 3T3-L1 cells were co-transfected with C/EBPα, CREB and HA-Dlx5 expression vectors and incubated in adipogenic medium for 3 days. Oil Red O staining results showed that Dlx5 over-expression still suppressed adipocyte differentiation in the presence of CREB or C/EBPα over-expression (Fig. 4A). Western blot analysis demonstrated that Dlx5 inhibition of PPARγ expression was significant even in the presence of CREB or C/EBPα over-expression (Fig. 4B, right panel). These results suggest that Dlx5 exerts anti-adipogenic activity through the down-regulation of PPARγ expression, and that Dlx5 inhibition of C/EBPα expression may be mediated through the down-regulation of PPARγ expression.
Dlx5 inhibits CREB- and C/EBPα-induced PPARγ promoter activity but not PPARγ-induced activity
To determine whether Dlx5 directly regulates PPARγ transcription, luciferase reporter assays were performed using a reporter construct containing the 1-kb promoter region from the mouse PPARγ gene (PPARγ-luc). Based on the computer-aided analysis of transcription factor binding elements, putative HD binding motif was not found within the 1-kb PPARγ promoter region. However, putative binding elements for C/EBPα (ATTGC) and CREB (TGACAGA) were present in the promoter region (Darlington et al., 1998; Fox et al., 2006). Therefore, 3T3-L1 cells were co-transfected with PPARγ-luc and the indicated expression plasmids (0.025–0.2 µg/well of HA-Dlx5 and 0.2 µg/well of C/EBPα or CREB) and incubated in growth medium for 24 h. Renilla luciferase was used as a control for normalization of transfection efficiency. As expected, PPARγ promoter activity was significantly up-regulated by C/EBPα and CREB over-expression (Fig. 5A,B). When C/EBPα and HA-Dlx5 were co-expressed, Dlx5 significantly inhibited C/EBPα-induced PPARγ promoter activity in a dose-dependent manner (Fig. 5A). When CREB and HA-Dlx5 were co-expressed, Dlx5 also suppressed CREB-induced reporter activity in a dose-dependent manner (Fig. 5B). These results were consistent with the results from Western blot analysis (Fig. 4B, right panel), confirming that Dlx5 regulates PPARγ expression at the transcription level. Because PPARγ can auto-regulate its transcription, we also examined whether Dlx5 inhibits PPARγ-induced PPARγ promoter activity. Similar to the result from Western blot analysis (Fig. 4B, left panel), Dlx5 over-expression did not exert any regulatory effect on PPARγ-induced PPARγ promoter activity (Fig. 5C). Given that rosiglitazone activates the transcriptional activity of PPARγ and that Dlx5 over-expression did not inhibit the expression level of PPARγ in the presence of rosiglitazone, these results are also consistent with the Western blot result obtained from rosiglitazone-treated cells (Fig. 4D). These indicate that Dlx5 inhibition of PPARγ promoter activity is a transcription factor-specific event. To further examine the inhibitory effect of endogenous Dlx5 on PPARγ transcription, we performed reporter assays in Dlx5 knock-downed cells, which were incubated in growth medium (Fig. 5D,E). Compared with the cells transfected with control siRNA, the Dlx5-silenced cells showed significantly higher basal PPARγ promoter activity. In addition, Dlx5 knockdown further enhanced C/EBPα- and CREB-induced PPARγ promoter activity. Given that there was no HD-binding element in the 1-kb region of the PPARγ promoter, these results suggest that Dlx5 down-regulates PPARγ transcription through the inhibition of transcriptional activity of CREB and C/EBPα.
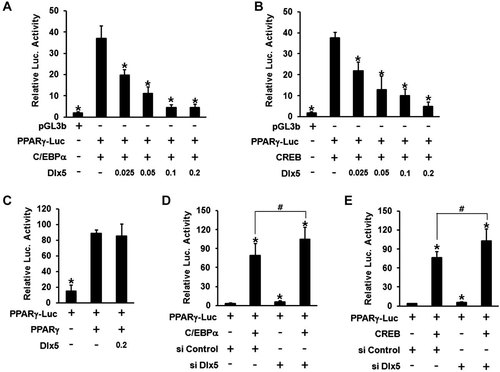
Dlx5 over-expression suppresses C/EBPα- and CREB-induced PPARγ promoter activity, but not PPARγ-induced PPARγ promoter activity. A–C: 3T3-L1 cells were co-transfected with the indicated expression vectors and PPARγ promoter reporter (PPARγ-Luc). In each transfection, 0.2 µg of expression vectors and reporters and 0.1 µg the Renilla luciferase plasmid were used when the amount of DNA was not specified. Luciferase reporter assays were performed after a 24 h-incubation in growth medium. Data represent mean ± SD of quadruplicate (*P < 0.05, compared to C/EBPα-, CREB- or PPARγ-induced PPARγ-luc activity in the absence of Dlx5 over-expression). D,E: 3T3-L1 cells were transfected with control siRNA or Dlx5 siRNA and incubated overnight. The cells were then co-transfected with the indicated expression vectors and PPARγ-Luc and incubated for an additional 24-h, followed by luciferase reporter assays. Data represent mean ± SD of sextuplicate (*P < 0.05, compared to PPARγ-luc activity in control siRNA-transfected cells; #P < 0.05).
Dlx5 inhibits the binding of C/EBPα and CREB to the PPARγ promoter
Given that Dlx5 exerts a negative regulatory effect on the transcriptional activity of CREB and C/EBPα and that in silico analysis did not prove the presence of putative Dlx5-binding motifs on the PPARγ promoter, it is hypothesized that Dlx5 may inhibit the transcriptional activity of CREB or C/EBPα through the regulation of their binding to the PPARγ promoter. The in silico search for CREB- and C/EBPα-binding elements within the 1-kb mouse PPARγ promoter region using the Transcription Element Search System demonstrated that a CRE-like element resides at −238 to −232 bp and three putative C/EBPα-binding elements are present at −541 to −537, −528 to −524 and −100 to −96 bp (Fig. 6A). To clarify the functional binding elements for CREB and C/EBPα, ChIP assays were performed. 3T3-L1 cells were transfected with CREB, C/EBPα or HA-Dlx5 and incubated for 24 h. Nuclear extracts were used for immunoprecipitation with anti-CREB, anti-C/EBPα or control IgG antibodies, and PCR was performed to amplify the promoter regions as follows: CRE-like element (−310 to −151 bp), C/EBPα-binding element-1 (−576 to −503 bp) and C/EBPα-binding element-2 (−137 to −21 bp). Because the putative C/EBPα-binding elements at −541 to −537 and −528 to −524 bp were proximately located, PCR was performed to amplify the region encompassing both elements (Fig. 6A,B, C/EBPα-1). PCR results demonstrated that over-expression of C/EBPα and CREB induced its binding to the C/EBPα-binding element-2 and the CRE-like element, respectively, whereas C/EBPα-binding element-1 was not functional in 3T3-L1 cells (Fig. 6B,C). PCR products were not detected in the samples immunoprecipitated with isotype-matched control IgG, indicating the specificity of PCR amplification. Consistent with the results from Western blot and reporter assays, Dlx5 diminished the DNA binding of C/EBPα and CREB in a dose-dependent manner (Fig. 6B,C). These results support the hypothesis that Dlx5 down-regulates PPARγ transcription by diminishing DNA binding of C/EBPα and CREB.
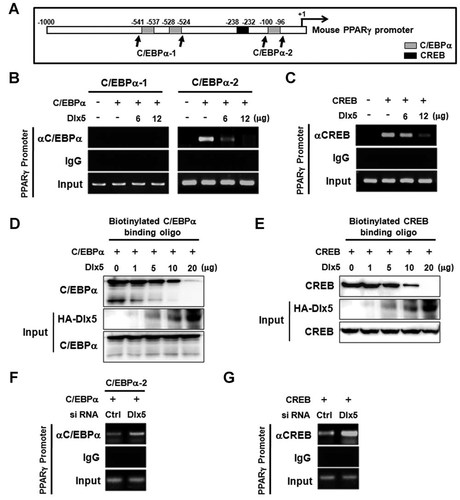
Dlx5 prevents C/EBPα and CREB from binding the PPARγ promoter. A: Schematic illustration of putative binding elements for C/EBPα and CREB on the 1-kb mouse PPARγ promoter. B,C: 3T3-L1 cells were transfected with the indicated expression vectors and incubated for 24 h in growth medium. Then, ChIP assays were performed using anti-C/EBPα (B), anti-CREB (C) or normal IgG (B,C) antibodies and the PPARγ promoter region encompassing the indicated binding elements was amplified by PCR. D,E: 3T3-L1 cells were transfected with the indicated expression vectors and incubated for 24 h. The inhibitory effect of Dlx5 on the DNA binding of C/EBPα and CREB was then confirmed in vitro by biotin pull-down assay using nuclear proteins and biotinylated double-stranded oligonucleotides of which sequence included binding element for C/EBPα (−100 to −96 bp) or CREB (−238 to −232 bp). Then, Western blot analysis was performed to detect proteins bound to biotinylated probes. Input represents 10% of the nuclear proteins used in the binding reaction. F,G: 3T3-L1 cells were transfected with control siRNA or Dlx5 siRNA, incubated overnight and transfected with C/EBPα (F) or CREB (G) expression vectors. After an additional 24 h-incubation in growth medium, ChIP assays were performed as described above. All of the experiments were repeated at least twice and the representative images were presented.
To further verify that Dlx5 regulates DNA-binding affinity of C/EBPα and CREB, an in vitro biotin pull-down assay was performed. Biotinylated double-stranded oligonucleotides containing the C/EBPα-binding element-2 or the CRE-like element were incubated with nuclear proteins prepared from 3T3-L1 cells over-expressing HA-Dlx5, C/EBPα, and CREB. The complexes were pulled down with streptavidin–agarose beads, and bound proteins were detected by Western blot analysis. Consistent with the results from ChIP assays, Dlx5 diminished DNA-binding affinity of C/EBPα and CREB in a dose-dependent manner (Fig. 6D,E). These results further confirmed that Dlx5 down-regulates PPARγ transcription through the inhibition of DNA binding of C/EBPα and CREB.
To further demonstrate that endogenous Dlx5 suppresses the binding of C/EBPα and CREB to the PPARγ promoter, we performed ChIP assays using the nuclear extracts from the cells transfected with control siRNA or Dlx5 siRNA (Fig. 6F,G). Compared to control siRNA, Dlx5 siRNA enhanced the binding of over-expressed C/EBPα and CREB to the respective binding elements in the PPARγ promoter.
The HD and HD/C-terminal domain of Dlx5 is involved in its interaction with C/EBPα and CREB, respectively
The above results implicate the possibility of physical interactions of Dlx5 with C/EBPα and CREB. To determine these physical interactions, co-immunoprecipitation was performed. 3T3-L1 cells were transfected with CREB, C/EBPα and HA-Dlx5. After a 24-h-incubation, whole-cell lysates were prepared, and immunoprecipitation was performed with anti-CREB, anti-C/EBPα, or control IgG antibodies. Western blot analysis with anti-HA antibody was then performed to detect Dlx5 proteins. As expected, Dlx5 was co-immunoprecipitated with both C/EBPα and CREB (Fig. 7A). In the samples immunoprecipitated with control IgG, there were no bands for the CREB, C/EBPα, or HA-Dlx5 proteins, suggesting the specificity of immunoprecipitation (Fig. 7A).
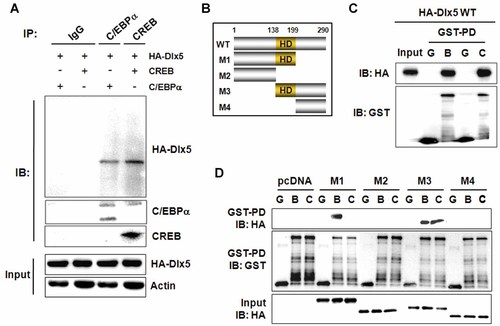
The HD of Dlx5 is involved in its interaction with C/EBPα, whereas both the HD and C-terminal domain of Dlx5 are necessary for binding with CREB. A: 3T3-LI cells were transfected with the indicated expression vectors and incubated for 24 h. Whole-cell lysates were prepared and a co-immunoprecipitation assay was performed. B: Illustration of full-length (WT) and truncated mutants (M1, M2, M3 and M4) of Dlx5 proteins. C,D: HEK293T cells were transiently transfected with expression vectors for full-length Dlx5 (C) or for its truncated mutants (D) for 24 h, and nuclear extracts were prepared. Then, GST pull-down assays were performed using GST, GST-C/EBPα, or GST-CREB, and bound proteins were detected by Western blot analysis. Both full-length and truncated Dlx5 proteins were tagged with HA. All of the experiments were repeated at least twice and the representative images were presented. GST-PD, GST pull-down; G, GST; B, GST-C/EBPα; C, GST-CREB.
To further verify the direct interactions of Dlx5 with CREB and C/EBPα, in vitro GST pull-down assays were performed. Purified GST-CREB or GST-C/EBPα was incubated with nuclear proteins prepared from HEK293T cells over-expressing HA-Dlx5 and pulled down with glutathione-sepharose beads. Immunoblot analysis with anti-HA antibody demonstrated that Dlx5 bound to both GST-CREB and GST-C/EBPα, further confirming co-immunoprecipitation results (Fig. 7C). Absence of an HA-Dlx5 band in the samples reacted with GST protein demonstrates the specificity of the GST pull-down assay.
To determine the Dlx5 protein domains involved in the interactions with CREB and C/EBPα, HA-tagged truncated Dlx5 mutants were prepared, as illustrated in Fig. 7B. The M1 mutant includes N-terminal domain and HD (Δ199–290 aa). The M2 mutant contains only the N-terminal domain (Δ138–290 aa). The M3 mutant is composed of the HD and C-terminal domain (Δ1–137 aa). Finally, the M4 contains only the C-terminal domain (Δ1–198 aa). GST pull-down assays showed that GST-C/EBPα bound to M1 and M3, whereas GST-CREB bound only to M3 (Fig. 7D). These results indicate that the HD of Dlx5 is sufficient for its interaction with C/EBPα, whereas both the HD and C-terminal domain of Dlx5 is required for interaction with CREB.
Discussion
In this study, it is demonstrated that (1) Dlx5 exerts a negative regulatory effect on the adipogenic differentiation of hBMSCs and 3T3-L1 preadipocytes; (2) Dlx5 expression is down-regulated during adipogenic differentiation, and knock-down of Dlx5 enhances adipogenic differentiation of 3T3-L1 cells; (3) Dlx5 down-regulates PPARγ transcription through the physical interactions of Dlx5 with CREB and C/EBPα proteins; and (4) the HD is required for the interaction of Dlx5 with C/EBPα, and both the HD and C-terminal domain of Dlx5 are necessary for the binding of Dlx5 and CREB.
When preadipocytic cells were incubated under adipogenic conditions, the adipogenic differentiation cascade begins with the expression of C/EBPβ within several hours, whereas CREB has already been expressed in preadipocytes before exposure to the adipogenic stimuli, and its expression is maintained during adipogenic differentiation to mature adipocytes (Klemm et al., 2001; Tang et al., 2003). C/EBPβ then promotes the expression of PPARγ and C/EBPα (Wu et al., 1996; Hamm et al., 2001). CREB, C/EBPα and PPARγ also act as a transcriptional activator for PPARγ expression and PPARγ transactivates C/EBPα in adipocytes (Hamm et al., 2001; Fox et al., 2006; Deng et al., 2011). In the present study, over-expression of Dlx5 suppressed the expression levels of PPARγ and C/EBPα, but not that of CREB. Given that PPARγ is a transcriptional activator for C/EBPα and that over-expression of PPARγ or rosiglitazone treatment blocked the inhibitory effect of Dlx5 on C/EBPα expression in this study, Dlx5 appears to indirectly down-regulate C/EBPα expression through the inhibition of PPARγ expression and transcriptional activity. Because only 1-kb of the mouse PPARγ promoter was examined in this study, it is not clear whether these protein–protein interactions are the only way that Dlx5 exerts a negative regulatory effect on PPARγ expression. However, at least in the region examined in this study, the main mechanism of action of Dlx5 appears to be antagonizing the binding of C/EBPα and CREB on its target gene promoter.
As for the regulatory mechanism of Dlx5 for target gene expression, previous studies have shown that Dlx5 is a transcriptional activator for its target genes through the direct binding of Dlx5 to the respective promoter (Hassan et al., 2004, 2006; Kim et al., 2004). It has also been demonstrated that Dlx5 hetero-dimerizes with msh homeobox 2 (Msx2), another HD protein, antagonizing DNA binding of Msx2 and thereby indirectly stimulating the transcription of the alkaline phosphatase gene (Kim et al., 2004). In addition, another study showed that Dlx5 indirectly transactivates the steroidogenic acute regulatory protein gene through its interaction with and subsequent enhancement of the transcriptional activity of GATA binding protein 4 (Nishida et al., 2008). In this study, we demonstrated an inhibitory role of Dlx5 for PPARγ expression through protein-protein interactions with CREB and C/EBPα. This is the first report that Dlx5 can function as a transcriptional repressor to regulate downstream target gene expression. These results suggest that protein-protein interactions are essential for specifying the transcriptional activities of Dlx5.
Dlx5 protein is composed of N-terminal, homeobox and C-terminal domains. Both the N- and C-terminal domains have proline-rich regions, which are implicated in transcriptional activation by binding with p300 and TATA box binding protein (Tanese et al., 1991; de Caestecker et al., 2000). These reports indicate that the N- or C-terminal domains function as transcriptional activation domains. In addition to its DNA-binding function, it has been reported that the HD is also involved in binding to other HD proteins and GATA binding protein 4 (Zhang et al., 1997; Nishida et al., 2008). Similar to these reports, the HD was shown to be essential for C/EBPα binding in this study. However, the HD region alone was not sufficient for Dlx5 binding to CREB, rather the C-terminal domain is also necessary. Given that all the Dlx family proteins share HD, it is suggested that a variance in the N- or C-terminal domain amino acid sequence contributes to differences in the binding partner proteins with which each Dlx protein interacts. Among the HD proteins, Msx2 has been shown to exert an anti-adipogenic effect. Cheng et al. have shown that Msx2 down-regulates PPARγ expression via the interaction with C/EBPα, whereas Ichida et al. demonstrated that Msx2 down-regulates the transcriptional activity of PPARγ, C/EBPβ and C/EBPδ (Cheng et al., 2003; Ichida et al., 2004). Cheng et al. did not elucidate the corresponding domain involved in the interaction of Msx2 with C/EBPα in their study (Cheng et al., 2003). Given that Msx2 shares an HD with Dlx5, our result that the HD region of Dlx5 is involved in the interaction with C/EBPα is consistent with the result from Cheng et al. Because HD family proteins do not share regions of high similarity outside of the HD, there should also be a difference between the protein partners of Msx2 and those of Dlx5.
Accumulated evidence from human patients and animal model systems indicates that there are reciprocal regulations between osteoblast and adipocyte differentiation from bone marrow MSCs (Muruganandan et al., 2009). The molecular mechanism underlying this reciprocal relationship has not yet been well elucidated, but several reports suggest the involvement of key transcription factors, including Runx2 and PPARγ. Runx2-deficient calvarial cells completely lack the ability to differentiate into mature osteoblasts but show strong differentiation potential to adipocytes (Kobayashi et al., 2000). In contrast, PPARγ insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors (Akune et al., 2004). Runx2 suppresses PPARγ expression and vice versa (Akune et al., 2004; Shockley et al., 2009). Furthermore, a recent report has shown that a subset of osteoblasts expressing high endogenous levels of PPARγ switches a fate to adipocytes in a rat calvarial cell culture model, suggesting that Runx2 and PPARγ are master regulators for lineage determination of osteoblasts and adipocytes, respectively (Yoshiko et al., 2010). Dlx5 is known to be involved in bone morphogenetic protein 2-induced commitment of mesenchymal progenitors to the osteogenic lineage through the up-regulation of Runx2 expression (Lee et al., 2005; Hassan et al., 2006). Considering these reports with the results from the current study together, it can be suggested that Dlx5 plays a role as an osteoblast lineage determinant through the up-regulation of Runx2 and the down-regulation of PPARγ. In support of this function, levels of Dlx5 expression were rapidly reduced by adipogenic stimuli but highly increased by osteogenic stimuli. The limitation of our study is that we obtained the large part of data using a 3T3-L1 preadipocyte cell line. However, although 3T3-L1 cells are not an authentic MSC model system, the expression of Dlx5 and other osteoblast marker genes, such as Runx2, alkaline phosphatase and bone sialoprotein, was induced by osteogenic medium supplemented with bone morphogenetic protein 2 in 3T3-L1 cells (data not shown). Therefore, it is likely that the expression level of Dlx5 may also regulate the differentiation lineage of 3T3-L1 cells toward adipocytic or osteoblastic cells.
In conclusion, we demonstrated in this study that Dlx5 exerts an anti-adipogenic effect through the down-regulation of PPARγ expression and that sequestration of CREB and C/EBPα is involved in the Dlx5 down-regulation of PPARγ expression. These results suggest that Dlx5 plays an important regulatory role in fate determination of bone marrow MSCs through the inhibition of adipogenesis as well as the stimulation of osteoblastogenesis.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2009-0068779).