Establishment of keratinocyte colonies from small-sized cervical intraepithelial neoplasia specimens
Abstract
The size of human cervical intraepithelial neoplasia (CIN) biopsies is usually very small and standard methods do not allow an adequate number of keratinocytes to be isolated for culturing purposes. In this study, a new approach to establish keratinocyte cultures from small CIN a tissue fragments was developed. Neoplastic specimens and corresponding normal tissues, which were used as controls, were digested with collagenase. Tissue-derived fibroblasts and keratinocytes were co-cultured in calcium and serum medium. Single keratinocyte colonies from primary cultures were expanded using a culture medium optimized in our laboratory. Primary keratinocyte colonies, as well as expanded colonies, were tested for epithelial and cervical markers such as 5, 14, 17, and 19 keratins, and p63 by immunofluorescence. Our results indicate that a variable number of primary keratinocyte colonies could be detected in neoplastic cultures, depending on the grade of cervical lesions from which the colonies originated. Single colonies, when cultured with our new medium, grew at a high rate with uniform size and morphology for some passages. Epithelial and p63 markers were expressed in keratinocyte colonies, as well as in expanded colonies. In conclusion, our study reports a rapid and easy culturing system which enables keratinocyte colonies from minute cervical tumor tissues to be obtained. Moreover, using the new culture medium, keratinocyte colonies can be expanded at a high proliferative rate. J. Cell. Physiol. 227: 3787–3795, 2012. © 2012 Wiley Periodicals, Inc.
The uterine ectocervical epithelium is pluristratified and consists of a single basal layer of stem cells, intermediate layers of transit amplifying cells, and a superficial layer of differentiated cells (Reid and Coppleson, 1976). The major cell component of normal stratified squamous esocervical epithelium is the keratinocyte. On leaving the intermediate layers, keratinocytes cease to divide and undergo an ordered pattern of stratification and differentiation (Reid and Coppleson, 1976). Basal keratinocytes of the cervical epithelium are natural host cells for human oncogenic Papillomavirus (HPV) types which perturb squamous differentiation and enhance proliferation of infected keratinocytes (zur Hausen, 1996; Hamid et al., 2009). Clinically HPV infection is diagnosed as cervical intraepithelial neoplasia (CIN) which are subdivided into low (CIN1), moderate (CIN2), and high (CIN3) grades (Reid and Coppleson, 1976; Mitchell et al., 1996). It is well established that 90% of CIN is associated with oncogenic HPV infections (Mitchell et al., 1996; zur Hausen, 1996; Muñoz et al., 2003) and long latency period of viral infection is needed for CIN progression toward cervical cancer.
Given the dynamic nature of normal and CIN keratinocytes of the ectocervical epithelium, cultures of these cells may provide interesting findings on mechanisms that control the proliferative, differentiative, and transforming process of normal and neoplastic cervical keratinocytes.
In vitro epithelial cultures from ectocervical CIN epithelium consist of keratinocyte colonies. Studies on CIN keratinocyte cultures, as well as CIN keratinocytes, are poorly available due to the numerous technical and methodological problems associated with the in vitro culturing of these cells. The main problem is related to the size of tumor biopsies which are usually very small and do not allow the isolation of an adequate number of CIN keratinocytes for culturing purposes. To date, Stanley's protocol is the only published method which describes the techniques for isolating and culturing CIN keratinocytes from neoplastic cervical biopsies in detail (Stanley, 2002). Specifically, whole cervical tissue is micro-dissected and keratinocytes migrated from the explants are rescued and subsequently seeded onto a feeder layer of inactivated murine fibroblasts. The major problem in these cultures is that keratinocytes are rescued at a low number and frequently associated with contaminant human fibroblasts which rapidly overgrow the epithelial cells in culture (Stanley, 2002).
The aim of this study was to establish a method for culturing CIN keratinocytes starting from small pieces of CIN1, CIN2, and CIN3 tissues. It is well established that human and murine fibroblasts co-cultured with epithelial cells enhance keratinocyte proliferation and colony-forming efficiency (Okigaki et al., 1980; Barrandon and Green, 1987; Coleman et al., 1993; Hubert et al., 1999; Stanley, 2002). Moreover, cervical and skin keratinocytes which have been immortalized/transformed with oncogenic HPV types are resistant to calcium- and serum- induced terminal differentiation and grow-forming colonies (Sherman and Schlegel, 1996; Pei et al., 1998; Alfandari et al., 1999; Sherman et al., 2002). On these grounds, we reasoned that: (i) the enzymatic digestion of a whole specimen was needed to obtain the maximum number of CIN keratinocytes from small tissue fragments; (ii) cervical fibroblasts co-cultured with CIN keratinocytes may contribute to enhancing keratinocyte proliferation and colony-forming; (iii) naturally HPV-infected keratinocytes cultured in calcium and serum medium conditions may retain resistance to terminal differentiation and grow-forming colonies.
CIN tissue fragments were digested with collagenase and derived fibroblasts and keratinocytes were co-cultured in DMEM-F12 added with 10% fetal bovine serum (FBS). Small tissue fragments from normal uterine cervix (NUC) surrounding each CIN biopsy were used as controls. CIN and NUC primary cultures were sub-cultured for several passages. Single CIN and NUC primary colonies were independently grown in a medium formulated in our laboratory. The cultured keratinocytes were characterized by immunofluorescence for the expression of epithelial and cervical markers.
Materials and Methods
Cervical uterine specimens
Small tissue fragments (2–3 mm3) were taken from nine CIN biopsies, three from each CIN1, CIN2, and CIN3 dysplastic grade. Nine corresponding NUC tissue fragments from each CIN specimen were also provided. Each patient underwent electrosurgical conization under colposcopic examination using both 5% acetic acid and Lugol's iodine solution application. It is well established that acetic acid stains pathological tissue a white color and does not react with normal tissue; on the contrary, Lugol's iodine only stains normal tissue brown (Stafl and Wilbanks, 1991). CIN and NUC specimens were selected and divided by the gynecologist during surgery.
The CIN specimens were classified by pathologists according to international criteria (Horvat et al., 2008).
Specimens were obtained with the informed written consent of patients.
HPV detection and genotyping
A small tissue fraction from each CIN and NUC sample was excised for DNA extraction. DNA isolation was carried out according to standard procedures as described before (Martini et al., 2004). DNA was first tested for suitability to PCR analysis with β-globin primers and then amplified for HPV sequences with the general primers GP5-GP6 which enable the detection of HPV -6b, -11, -16, -18, -31, -33 genotypes (Martini et al., 2004). HPV -6b, -11, -16, -18 DNAs (-6b, -11, -18 cloned into pBR322 vector, and -16 cloned into pUC19 vector; ATCC, Manassasas, VA; Di Luca et al., 1986; Martini et al., 2004; Comar et al., 2011) and HPV -31, -33 DNAs (cloned into pT713 vector; Bethesda Research Laboratories, Gaithersburg, MD), kindly provided by Prof. Pozzi, Department of Biotechnology, University of Siena, Italy, were used as controls. The oligomer-resembling sequences span a fragment of 139–145 base pairs (Table 1). PCR analysis was carried out with 500 ng human genomic DNA, as previously reported (Martini et al., 2004). PCR products were electrophoretically separated on 2.5% agarose gel. To further assess the specificity of the PCR products, a restriction endonuclease analysis of HPV sequences was performed by RsaI digestion. Two RsaI units and the restriction enzyme buffer were directly added to a 10 µl sample of the PCR amplified product, as recommended by the supplier (Roche Diagnostics, Milan, Italy). DNA digestion was at 37°C for 2 h. The digested DNA product was electrophoretically separated on 20% acrylamide gel. The DNA fragment size, generated by the RsaI digestion of the HPV-PCR products is reported in Table 1.
| HPV type | Total length (bp) | Length of RsaI restriction fragments (bp) |
|---|---|---|
| HPV-6b | 139 | 30 |
| 42 | ||
| 67 | ||
| HPV-11 | 139 | 30 |
| 109 | ||
| HPV-16 | 142 | 30 |
| 42 | ||
| 70 | ||
| HPV-18 | 145 | 30 |
| 38 | ||
| 77 | ||
| HPV-31 | 142 | 30 |
| 112 | ||
| HPV-33 | 139 | 30 |
| 39 | ||
| 70 |
For HPV genotyping of CIN colonies, 10 colonies from each CIN2 and CIN3 primary culture were isolated with glass cylinder and subjected to DNA extraction, HPV-PCR and HPV genotyping as described above.
CIN and NUC primary cultures
Tissue fragments were kept at 4°C in a transporting medium containing DMEM-F12, 10% FBS and antibiotics (1,200 µg/ml amphotericin B, 200 IU/ml penicillin, and 200 µg/ml streptomycin), and transferred to the laboratory within 2 h. Tissue fragments were washed profusely in PBS 1× and digested over night (ON) with type II collagenase (Sigma–Aldrich, Milan, Italy) at 37°C, 5% CO2. Single cells were washed in PBS 1×, counted and seeded in T25 flasks (T1) with DMEM-F12 containing calcium standard concentration of 1.2 mM and 10% FBS and were left to attach for 3 days. Since epithelial cells have a slower-anchorage ability than fibroblasts, unattached cells were recovered and reseeded into new T25 flasks (T2) and left to attach for a further 3 days. To determine the number of attached cells, unattached cells were counted both in T1 and T2 flasks. Primary cultures were grown for 5 weeks which was considered an optimal culture period to allow the expansion and the growth of early and late colonies and to allow fibroblasts to expand without affecting the keratinocyte growth.
Cell clusters were considered keratinocyte colonies when they were visible to the naked eye and contained more than 50 cells (Chan et al., 2004).
Cloning efficiency (CE) was determined using the formula CE (%) = (number of colonies/number of keratinocytes and fibroblasts seeded) × 100 (Chan et al., 2004).
Colony staining with Rhodamine-B
Two additional specimens, one from CIN2 and one from the corresponding NUC tissue, were employed for colony staining with Rhodamine-B. Confluent primary cultures were fixed with 10% (v/v) formalin in phosphate-buffered saline. After 2 h, cultures were rinsed with water and stained with a 2% (w/v) Rhodamine-B aqueous solution for 1 h. After staining, cultures were exhaustively washed with water and left to dry at RT.
Cervical cell subcultures
CIN and NUC cultures were detached with 0.05–0.01% trypsin/EDTA. 1–2 × 105 cells were subcultured in T25 flasks (T1) with DMEM-F12 and 10% FBS. After 3 days, unattached cells were recovered and reseeded into new T25 flasks (T2) as reported above. Subcultures were grown for 4 weeks, which was considered an optimal culture period to allow the expansion and the growth of early and late colonies and to allow fibroblasts to expand without affecting the keratinocyte growth.
CIN and NUC primary colony expansion
To verify whether primary colonies could be independently expanded, the upper surface of the flasks was cut with a red-hot curved iron scissor and removed, then one primary colony from CIN3 and one from the corresponding NUC culture were isolated with cloning rings and cultured in a Petri dish (3 cm diameter) in DMEM-F12 and 10% FBS. After 24 h, the medium was replaced with a mixture of DMEM-F12 medium and defined keratinocyte serum-free medium (dKSFM), 1:1 ratio, in the presence of 5% FBS. At confluence, keratinocytes were detached with 0.05–0.01% trypsin–EDTA and expanded into T25 flasks.
Doubling population time (DPT) experiments were carried out in triplicate dishes when the keratinocytes were expanded into T25 flasks.
Immunofluorescence assays
CIN and NUC primary colonies were characterized by immunofluorescence. Colonies were fixed and assayed in culture flasks. Alternatively, in order to test the expression of different markers on the same colony, single colonies were isolated with cloning rings and keratinocytes were subdivided onto different coverslips. Keratinocytes were fixed and permeabilized with 4% paraformaldehyde and 0.5% Triton (1:1, v/v) in PBS 1× for 20 min. After blocking non-specific binding with goat serum, cells were incubated with different mouse anti-human monoclonal antibodies (mabs). To determine the epithelial and cervical markers, immunofluorescence staining with K5, K14, K17, and K19 keratins and with p63 (Dako SpA, Milan, Italy) was performed, as previously described (Quade et al., 2001; Radu et al., 2002; Martens et al., 2004; Harper et al., 2007; Tudor et al., 2007). The same markers were also investigated by immunofluorescence staining in individually expanded colonies. The substitution of primary antibodies with PBS 1× served as a negative control. Digital images from a Nikon TE2000E microscope were captured using the ACT-1 software for the DXM1200F digital camera (Nikon, Florence, Italy).
The percentage of cells expressing different keratin markers in the colonies was quantified by counting 1,000 cells in four randomly selected fields/colony.
Statistical analysis
Differences among CE were compared by confidence interval (CI 95%) according to Poisson distribution (Stata 8.0 software).
Results
HPV-DNA analysis of CIN and NUC specimens
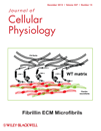
Cervical specimens were preliminarily investigated by PCR for HPV sequences. The nine NUC samples were all negative for HPV sequences (Fig. 1A, lanes 1n–9n). All nine CIN specimens tested positive for HPV high-risk types: 16, 18, 31, and 33 (Fig. 1A, lanes 1–9). Specifically, CIN1 specimens were positive for HPV-33, -31, and -16, respectively (Fig. 1B, lanes 1–3); CIN2 samples were positive for HPV-16 (Fig. 1B, lanes 4 and 6) and for HPV-18 (Fig. 1B, lane 5); CIN3 specimens were all positive for HPV-16 (Fig. 1B, lane 7).
HPV-PCR and HPV genotyping. Panel A: The agarose gel shows HPV-PCR results obtained from the CIN (lanes 1–9) and NUC-DNA samples (lanes 1n–9n). HPV-PCR products, 139–145 bp (Table 1) are only visible in the CIN samples (lanes 1–9). MW, molecular weight markers are 100 bp (left); HPV-16, PCR positive control; C−, negative control of the PCR reaction without DNA template. Panel B: The polyacrylamide gel shows HPV genotyping in seven representative PCR products from CIN samples (Table 1). CIN1 specimens (lanes 1–3) are positive for HPV-33, -31, and -16, respectively. CIN 2 specimens (lanes 4–6) are positive for HPV-16 (lanes 4 and 6), and HPV-18 (lane 5). The CIN3 specimen (lane 7) is positive for HPV-16. In lanes marked 1–6, DNAs have not been completely digested and bands with high molecular weight are also visible. Fragment lengths are reported in Table 1. MW, molecular weight markers are 100 bp (left) and 50 bp (right) ladder. HPV-6b, -11, -16, -18, -31, and -33: HPV controls.
CIN and NUC primary culture growth behavior
Cells isolated from CIN and NUC tissue fragments ranged from 2 × 104 to 1 × 105. Approximately, 50% of cells attached to the T1 flasks and 40% of the remaining cells in the T2 flasks. The cell density/cm2 of the attached cells, both fibroblasts and keratinocytes, varied from 180 to 2,400 cells/cm2, which are densities consistent with the clonal growth of keratinocytes (Hybbinette et al., 1999; Chan et al., 2004).
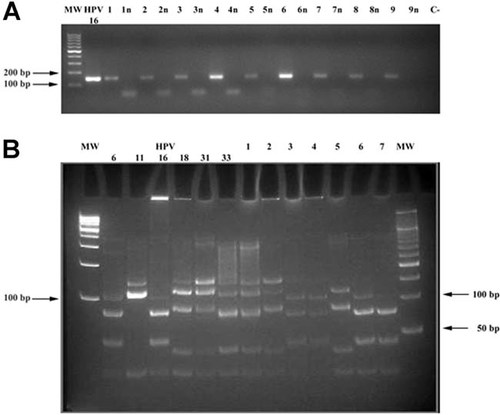
Daily cell monitoring with phase contrast microscopy indicated that a lag period of 3–5 days occurred in CIN and NUC cultures. During this time, cells appeared small and uniform in size and morphology (Fig. 2A). Fibroblasts displayed a spindly shape (Fig. 2B) at day 4–5, while keratinocytes were well distinguishable due to their rounded or polygonal morphology (Fig. 2B; Chan et al., 2004). Consistently, when the two cell types started to proliferate, they maintained their own growth and morphologic characteristics. Indeed, fibroblasts grew singly or in disordered clusters or parallel bundle groups (Fig. 2C,D), whereas keratinocytes formed colonies (Fig. 2E,F). Clusters of fibroblasts were visible at days 7–8 of culturing (Fig. 2C,D). Many of these clusters continued to expand over the 5-week culture period.
CIN cervical fibroblasts and keratinocytes cultured in DMEM-F12 medium and 10% FBS. Panel A: A culture at day 3. Fibroblasts and keratinocytes appear small, with uniform size and morphology. Panel B: A culture at day 5. Fibroblasts (arrows) and keratinocytes (head arrows) are well distinguishable. Panels C,D: A culture at day 8 displays clusters of disordered (C) or well parallel bundle organized fibroblasts (D). Panel E: An early CIN2 keratinocyte colony surrounded by fibroblasts, at day 8. Panel F: An early CIN1 keratinocyte colony without fibroblasts at day 15. Panel G: Two primary cultures, one from CIN2 (left) and one from the corresponding NUC tissue (right), stained with Rhodamine-B. Type II colonies (left-side arrow in the CIN2 flask and upper arrow in the NUC flask) stain more strongly than type I colonies (right-side arrow in the CIN2 flask and lower arrow in the NUC flask). [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/jcp]
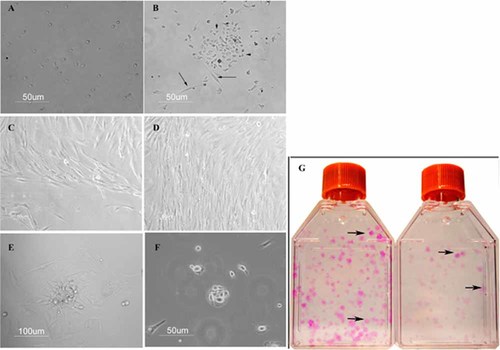
Keratinocytes started to proliferate at different times within the CIN cultures. Early colonies appeared at days 6–8 in CIN2 and CIN3 cultures (Fig. 2E), and at days 10–12 in CIN1 cultures (Fig. 2F). At this latter time, small epithelial colonies also arose in NUC cultures. Primary cultures were grown for a 5-week culture period. Then, individual primary keratinocyte colonies of T1 and T2 flasks were scored with phase contrast microscopy. The CIN and NUC cultures developed a similar pattern of morphologically differing keratinocyte colonies which were classified into three types, based on general morphology and cell content (Fig. 3A–E; Barrandon and Green, 1987; Chan et al., 2004; Tudor et al., 2007). Type I colonies contained cells with typical epithelial morphology; cells were irregularly sized, flattened or spindle-shaped, loosely spaced with a low nuclear:cytoplasmic ratio (Fig. 3A,D). Type II colonies consisted of cells which were smaller, more compact, more uniform in size with a higher nuclear:cytoplasmic ratio than cells of type I colonies (Fig. 3B,E). Type III colonies were visualized in CIN3 cultures only and contained cells which were similar to type II colonies with an additional central zone of yet smaller and more closely packed cells, grown in vertical layers (Fig. 3C). Keratinocyte colonies grew either surrounded by fibroblasts (Fig. 3F) or singly (Fig. 3A,B).
Keratinocyte primary colonies of CIN cultures. Panels A,D: A typical type I colony (A) and a magnified view of its center (D). Panels B,E: A typical type II colony (B) and a magnified view of its center (E). Panel C: A type III colony; the arrow indicates a group of cells grown vertically in the central area of the colony. Panel F: A type II colony surrounded by fibroblasts (arrow).
The two additional primary cultures, one from CIN2 tissue and one from the corresponding NUC tissue, employed for colony-staining assay, stained keratinocyte colonies positively with Rhodamine B (Fig. 3G).
Next, we counted types I, II, and III colonies in CIN and NUC cultures from T1 and T2 flasks and calculated the CE (Fig. 4).
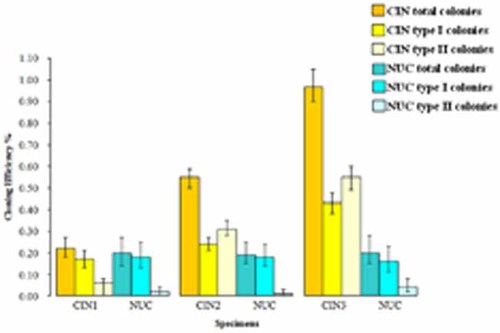
The cloning efficiency of CIN and NUC specimens. For every CIN group, CIN1, CIN2, and CIN3, in the graph, the total CEs, the CEs of type I and the CEs of type II colonies are compared to the total CEs, the CEs of type I and type II colonies of the corresponding NUC groups. Differences among CEs are compared by confidence interval (95% CI; Table 2). [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/jcp]
The total colony CE was significantly different among the CIN3, CIN2, and CIN1 groups: 0.97% (95% CI: 0.90–1.05%), 0.55% (95% CI: 0.50–0.59%), and 0.22% (95% CI: 0.18–0.27%), respectively (Fig. 4, Table 2). Significant data were also for the CE of the type I colonies between CIN3 group, 0.43% (95% CI: 0.38–0.48%), and CIN2 group, 0.24% (95% CI: 0.21–0.27%; Fig. 4, Table 2) as well as for the CE of the type II colonies among CIN3, CIN2, and CIN1 groups: 0.55% (95% CI: 0.49–0.60%), 0.31% (95% CI: 0.28–0.35%), and 0.06% (95% CI: 0.00–0.08%), respectively (Fig. 4; Table 2). The four type III colonies, obtained from two CIN3 cultures, were included in the type II colony CE of CIN3.
| N. | Specimens | Total colony CE (%) | 95% CI | CE Type I colonies (%) | 95% CI | CE Type II colonies (%) | 95% CI |
|---|---|---|---|---|---|---|---|
| 3 | CIN3 | 0.97 | 0.90–1.05 | 0.43 | 0.38–0.48 | 0.55 | 0.49–0.60 |
| 3 | NUC | 0.20 | 0.15–0.28 | 0.16 | 0.11–0.23 | 0.04 | 0.02–0.08 |
| 3 | CIN2 | 0.55 | 0.50–0.59 | 0.24 | 0.21–0.27 | 0.31 | 0.28–0.35 |
| 3 | NUC | 0.19 | 0.15–0.25 | 0.18 | 0.14–0.24 | 0.01 | 0.02–0.03 |
| 3 | CIN1 | 0.22 | 0.18–0.27 | 0.17 | 0.13–0.21 | 0.06 | 0.00–0.08 |
| 3 | NUC | 0.20 | 0.14–0.27 | 0.18 | 0.13–0.25 | 0.03 | 0.00–0.04 |
- Differences among cloning efficiencies (CE) are compared by confidence interval (95% CI).
The total colony CE, the CE of the type I and the CE of type II colonies were significantly different between CIN3 and the corresponding NUC group and between CIN and the corresponding NUC group (Fig. 4; Table 2).
Cervical cell subcultures
1–2 × 105 cells were sub-cultured in T25 flasks to verify how long they could support epithelial proliferation and colony forming.
A slight difference in cell growth behavior was observed in subcultures compared to primary cultures. Both CIN and NUC subcultures grew faster than primary cultures and were stopped at 4th week. Specifically, large clusters of fibroblasts were evident after 48 h in all cultures, whereas early CIN2, CIN3 colonies and CIN1, NUC colonies appeared after 48 h and 3–5 days, respectively. At each passage, CIN and NUC colonies were counted and classified both in T1 and T2 flasks, as reported above. Total colony CEs of CIN and NUC subcultures in each passage did not substantially differ from those obtained in primary cultures (Fig. 5A,B). However, the type I colonies progressively increased at each passage, whereas type III colonies were never detected. CIN and NUC cells were subcultured until only type I colonies and fibroblasts were present in the cell monolayer (Fig. 5A).
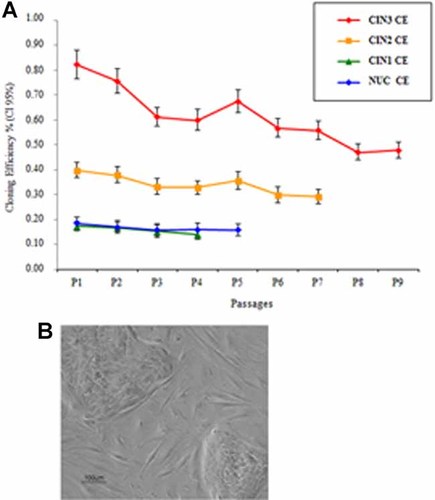
The cloning efficiency of CIN and NUC subcultures. Panel A: The graph indicates the CIN and NUC subculture CEs (y axis) obtained at each passage (x axis). Differences among CEs were compared by confidence interval (95% CI). Panel B: Two type II colonies surrounded by fibroblasts from a CIN3 subculture. [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/jcp]
CIN and NUC epithelial colony isolation and expansion
The aim of these experiments was to verify whether CIN and NUC colonies could be expanded with a high-proliferative rate and uniform size and morphology. One type II colony deriving from a CIN3 and one from the corresponding NUC primary culture were isolated with sterile glass cylinders and seeded into six-well tissue culture plates with DMEM-F12 and 10% FBS. After 24 h, 90% of the CIN and NUC cells were attached to the plastic dishes and the medium was replaced with a mixture of DMEM-F12/dKSFM (1:1 ratio) and 5% FBS. This choice was based on our preliminary experiments which showed that colonies seeded in DMEM-F12 and 10% FBS developed a heterogeneous population of small and large cells (Fig. 6A), whereas colonies cultured with dKSFM did not survive. In the presence of the new medium mixture, the keratinocytes assumed a morphologically homogeneous rounded or polygonal shape (Fig. 6B) and soon started to proliferate actively. The CIN3 and NUC cells took 4 and 7 days to reach confluence, respectively. Therefore, cells were expanded into T25 flasks and reached confluence at 7 and at 10 days in CIN3 and NUC cultures, respectively. Interestingly, during this culture period, the keratinocytes maintained their small-size, uniform morphology and very little differentiation occurred (Fig. 6C). PDT was 26 ± 3 for CIN3 and 39 ± 5 for NUC cells. Using this protocol, it was possible to passage CIN3 and NUC cells up to four times and twice, respectively, with a high growth rate and few signs of cell differentiation. After these passages, the cells were liable to excessive differentiation with a concomitant decrease in growth proliferation and a high level of cell loss.

Expansion of type II colonies. Panel A: A type II colony from CIN3 culture grown in DMEMF12-10% FBS. Keratinocytes are highly heterogeneous in size and morphology. Panel B: A type II colony from CIN3 culture grown in the new medium mixture at 3 days of culture. Keratinocytes display uniform size and morphology. Panel C: The same keratinocytes reported in panel B at confluence. Cells maintain small and uniform sizes with few signs of keratiocyte differentiation (arrows).
CIN and NUC primary colony characterization
In a preliminary assay, we assessed anti K5, K14, K17, and K19 antibody specificity in WI 38 fibroblasts used as negative controls (data not shown).
Immunofluorescence characterization was assessed in CIN and NUC type II primary colonies, only. Seventy-five colonies on flasks and 24 colonies on coverslips (seeded-colonies) were analyzed for keratin expression (Table 3), and one colony on flask and one seeded-colony from each CIN and NUC primary culture were analyzed for p63.
| Colony histotypes | Colonies analyzed | K14 staining and signal intensity | % Positive cells | K17 staining and signal intensity | % Positive cells | K19 staining and signal intensity | % Positive cells |
|---|---|---|---|---|---|---|---|
| CIN3a | 34 | 6/10+++ | 100 | 3/12+++ | 100 | 5/12+++ | 100 |
| 4/10++ | 100 | 2/12+++ | 50 | 2/12+++ | 75 | ||
| 2/12+ | 100 | 4/12+++ | 25 | ||||
| 1/12+ | 45 | 1/12− | 0 | ||||
| 4/12− | 0 | ||||||
| CIN2a | 25 | 3/7+++ | 100 | 2/8+++ | 100 | 2/10+++ | 100 |
| 3/7++ | 100 | 3/8+ | 100 | 5/10++ | 100 | ||
| 1/7+ | 100 | 3/8− | 0 | 2/10+ | 100 | ||
| 1/10− | 0 | ||||||
| CIN1a | 16 | 3/4+++ | 100 | 3/6+ | 100 | 4/6+++ | 100 |
| 1/4+ | 100 | 3/6− | 0 | 2/6− | 0 | ||
| NUCa | 44 | 6/9+++ | 100 | 4/10+ | 100 | 9/16+++ | 100 |
| 3/9+ | 100 | 6/10− | 0 | 4/16+ | 100 | ||
| 3/16− | 0 | ||||||
| CIN3b | 2 | 2/2+++ | 100 | 2/2+++ | 100 | 2/2+++ | 100 |
| 1 | 1/1+++ | 100 | 1/1+ | 100 | 1/1++ | 50 | |
| 6 | 6/6+++ | 100 | 6/6− | 0 | 6/6+++ | 100 | |
| CIN2b | 2 | 2/2++ | 100 | 2/2+ | 100 | 2/2++ | 100 |
| 5 | 5/5++ | 100 | 5/5− | 0 | 5/5++ | 100 | |
| CIN1b | 1 | 1/1+++ | 100 | 1/1+ | 100 | 1/1+++ | 100 |
| 1 | 1/1+++ | 100 | 1/1− | 0 | 1/1+++ | 50 | |
| 1 | 1/1++ | 100 | 1/1− | 0 | 1/1+++ | 50 | |
| NUCb | 3 | 3/3+++ | 100 | 3/3− | 0 | 3/3++ | 50 |
| 1 | 1/1+++ | 100 | 1/1− | 0 | 1/1+++ | 100 | |
| 1 | 1/1+ | 100 | 1/1+ | 100 | 1/1− | 0 |
- +++, ++, + mean high, moderate, and low-intensity signal, respectively.
- a Colonies were analyzed on flasks.
- b Colonies were analyzed on coverslips.
Most of the CIN3 colonies and seeded-colonies stained strongly for K14, K17, and K19 (Fig. 7A–C; Table 3) in all cells. In a few colonies and seeded-colonies K14 and K17 stained all cells with moderate and low signals, respectively (Table 3). Moreover, staining for K17 and K19 was also detected at differing strong, moderate, or weak signal intensities, at between 25% and 75% of cells (Table 3). P63 stained strongly in one seeded-colony (Fig. 7D,H,L). Most of the CIN2 colonies and seeded-colonies stained strongly for K14 (Fig. 7E; Table 3), for K17 (Fig. 7F; Table 3) and for K19 (Fig. 7G; Table 3), in all cells. In some CIN2 colonies and seeded-colonies, all cells stained moderately or weakly for K14 and for K19 and weakly for K17 (Table 3). P63 staining was negative. Most of the CIN1 colonies and seeded-colonies reacted strongly with K14 (Fig. 7M; Table 3) and with K19 (Fig. 7O; Table 3) and weakly with K17 (Fig. 7N; Table 3) in all cells. In some CIN1 colonies and seeded-colonies, all cells stained with a moderate or weak signal for K14 (Table 3) or stained strongly for K19 in only 50% of cells (Table 3). Staining for p63 was negative (Fig. 7P; Table 3). Keratin patterns for the NUC colonies and seeded-colonies resembled those of CIN1 being stained strongly for K14 (Fig. 7Q; Table 3) and for K19 (Fig. 7S; Table 3) and weakly for K17 (Fig. 7R; Table 3) in all cells. In some NUC colonies and seeded-colonies K14 stained weakly in all cells (Table 3) or K19 reacted with a moderate signal in only 50% of cells (Table 3). Staining for p63 was negative.
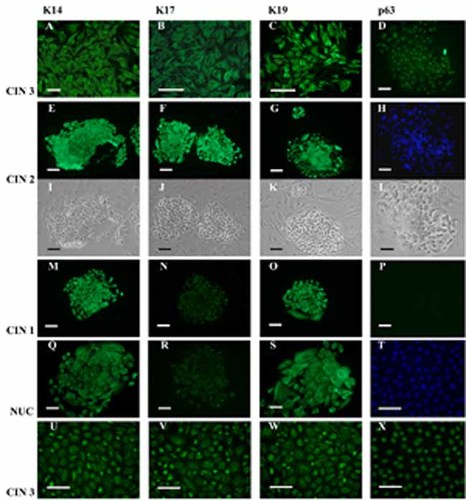
Characterization of NUC and CIN colonies. Panels A–D: Immunofluorescence staining of a CIN3 seeded-colony. All cells react strongly to K14 (A), K17 (B), and K19 (C) keratins; cells in phase contrast (L), and stained for DAPI (H), show a nuclear positive signal for p63 (D). Panels I–K and E–G: Phase contrast imagines (I–K) and immunofluorescence staining (E–G) of CIN2 colonies. All cells within the CIN2 colonies stain strongly with K14 (E), K17 (F), and K19 (G). Panels M–P: Immunofluorescence staining of a CIN1 colony. All cells within the CIN1 colonies react strongly to K14 and K19 (M and O) and weakly to K17 (N); no signal is visible for p63 (P). Panels Q–S: Immunofluorescence staining of a NUC seeded-colony. A strong signal for K14 and K19 (Q and S) and a weak staining for K17 (R) are shown in all cells. All keratinocytes expanded in the new medium mixture from a CIN3 colony show high expression of K14 (U), K17 (V), K19 (W); cells, stained for DAPI (T), show a nuclear positive signal for p63 (X). Bars: A, D, E–X = 100 µm; B and C = 50 µm. [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/jcp]
The CIN3 and NUC colonies expanded with the new media mixture stained strongly for K14, K17, K19, and p63 (Fig. 7U–X), and for K14 and K19, respectively.
All colonies and seeded colonies analyzed for K5 gave negative results.
HPV-DNA analysis of CIN colonies
Twenty colonies from CIN2 and CIN3 (10 each) primary cultures which were derived from HPV-16 positive tissue specimens, were HPV genotyped by PCR. HPV detected in 6/10 CIN2 colonies and in 9/10 CIN3 colonies were the genotypes HPV-16, -18, and -31 or -11 (DNA fragments of HPV-31 and -11 are very similar; Fig. 8, lanes 1–15). In 7/15 colonies, additional DNA fragments, that did not overlap with any of the control DNAs, were also detected (Fig. 8, lanes 2, 7 and lanes 10–14). All the 15 CIN colonies contained two or three HPV genotypes (Fig. 8). Specifically, the six CIN2 colonies were double infected with HPV-16 and -18 (n = 2), with HPV-16 and -31 or -11, (n = 2), with HPV-16 and undetermined genotype (n = 1) and triple infected with HPV-16, -18, and -31 or -11 (n = 3; Fig. 8). The nine CIN3 colonies were double infected with HPV-16 and undetermined genotype (n = 2), triple infected with HPV-16, -18, and -31 or -11 (n = 2) and triple infected with HPV-18, -31 or -11, and undetermined genotype (n = 5; Fig. 8).
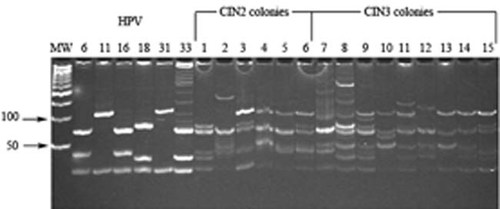
HPV genotyping of CIN colonies. The polyacrylamide gel shows HPV genotyping of 15 HPV-PCR products from six CIN2 (lanes 1–6) and 9 CIN3 (lanes 7–15) colonies. The six CIN2 colonies contain the HPV-16 genotype (lanes 1–6) together with the HPV-18 genotype (lane 1), with the HPV-18 and HPV11/31 genotypes (lanes 3 and 6), with the HPV-11/HPV-31 genotype (lanes 4 and 5), with DNA fragments that did not overlap with any of the control DNAs (lane 2). Four CIN3 colonies contain the HPV-16 genotype (lanes 7, 9, 12, and 15) together with the HPV-18 and HPV11/31 genotypes (lanes 9 and 15), with DNA fragments that did not overlap with any of the control DNAs (lanes 7 and 12). Five CIN3 colonies contain the HPV-18 genotype (lanes 8, 10, 11, 13, 14) together with the HPV11/31 genotype (lane 8), with additional DNA fragments that do not overlap with any of the control DNAs (lanes 10, 11, 13, 14). Fragment lengths are reported in Table 1. MW, molecular weight markers are 100 bp ladder. HPV-6b, -11, -16, -18, -31, and -33: HPV controls.
Discussion
In this study, we have demonstrated that CIN keratinocytes can be cultured from small pieces of neoplastic tissues. By completely digesting CIN tissues, it was possible to obtain an adequate number of keratinocytes which were able to grow in the presence of live cervical fibroblasts and medium-containing calcium and serum. We verified that the latent period (pre-growth) of the cultured keratinocytes was longer than that reported by others (Barrandon and Green, 1987; Stanley, 2002) probably because cells needed to exceed the stress of dissociation and adapt to the new culture environment before starting to proliferate. However, when primary keratinocytes started to proliferate, colonies arose with approximately the same growth rate as previously described (Stanley, 2002).
Although the presence of untreated fibroblasts is considered detrimental to keratinocyte cultures due to overgrowth being caused, this problem was avoided by co-seeding the cells at a very high dilution, that is, in T25 flasks. Thus, attached cells resulted at very low density and consequently keratinocyte colonies could be obtained before the fibroblasts colonized the flasks. On the other hand, the low cell density allowed the keratinocytes to reach the clonal density which is required to proliferate clonally (Locke et al., 2005; Harper et al., 2007). Moreover, reseeding the unattached cells from the first seeded flasks (T1) into new flasks (T2) was highly advantageous. Thus, the keratinocytes endowed with poor or slow anchorage capabilities attached later in T2 flasks, consequently increasing colony-forming efficiency in all cultures.
We have demonstrated that naturally HPV-infected CIN keratinocytes cultured in calcium and serum conditions resist terminal differentiation for 36, 28, and 16 weeks, respectively, and successfully grow-forming colonies. Interestingly, the CE of CIN specimens varied according to the grade of lesion from which they derived: CIN3 > CIN2 > CIN1. It is worth noting that the colony-forming capability of neoplastic keratinocytes is strictly related to the transformation grade of neoplastic cells (Locke et al., 2005; Mackenzie, 2005; Harper et al., 2007). The higher the neoplastic grade of keratinocytes, the higher the ability to attach and to proliferate clonally (Barrandon and Green, 1987; Chan et al., 2004; Locke et al., 2005; Mackenzie, 2005; Tudor et al., 2007). Three types of colonies (I, II, and III) were grown in the CIN primary cultures. As previously reported, the size and morphology of the keratinocytes which make up the colonies are the main parameter used in defining the proliferative potential of epithelial cells (Barrandon and Green, 1987; Chan et al., 2004; Locke et al., 2005; Mackenzie, 2005; Harper et al., 2007; Tudor et al., 2007). Therefore, the potential growth rate of the three type colonies was: type III > type II > Type I. Consistently, type III colonies were only detected in CIN3 cultures and the CE of CIN3 type II colonies was higher than CIN2 and the CE of CIN2 type II colonies was higher than CIN1. We want to point out that the CEs obtained in cultured cells is underestimated since CE has been calculated with the formula (colonies obtained/keratinocytes and fibroblasts seeded) × 100 instead of (colonies obtained/keratinocytes seeded) × 100 as previously reported (Chan et al., 2004).
Surprisingly, keratinocyte colonies, and even type II colonies, were obtained from HPV-negative NUC cultures. We speculated that the proliferative potential of NUC keratinocytes could be supported by cervical fibroblasts. To our knowledge, this is the first report which demonstrates that normal cervical keratinocytes can be cultured from small pieces of NUC tissues. Indeed, cultured normal cervical keratinocytes have been always obtained from large cervix pieces derived from hysterectomies (Woodworth et al., 1988; Stanley, 2002; Hougardy et al., 2008; Narisawa-Saito et al., 2008). Our finding is promising since normal tissues surrounding the neoplasia can be easily obtained from all CIN biopsies thus leading to opportunities for comparing normal and CIN keratinocytes from the same patient.
CIN and NUC subcultures maintained the capability to form type II colonies for a variable number of passages. A balance of CEs in each passage was observed. Indeed, keratinocytes endowed with a high proliferative rate, that is, from type III and type II colonies, are able to replace the exhausted type I colonies with new type II and type I colonies for some passages. Nevertheless, type II colonies decreased, whereas type I colonies and fibroblasts increased at each passage in all subcultures. These results indicate that CIN cultured keratinocytes are not-fully transformed, in agreement with the notion that CIN dysplasia are pre-neoplastic lesions (Reid and Coppleson, 1976; Mitchell et al., 1996; Baak et al., 2006; Woodman et al., 2007).
CIN and NUC type II colonies were successfully expanded and passaged at a high proliferative rate and uniform cell size using a new mixture of culturing medium (DMEM-F12:dKSFM, 1:1 ratio). This technical approach is useful for obtaining a sufficient number of keratinocytes to employ for different research purposes.
There are fewer reports on the in vitro keratin expression profile of CIN and normal keratinocytes than in vivo (Woodworth et al., 1988; Smedts et al., 1990, 1992a, b; Stanley, 2002; Martens et al., 2004). K5 and K19 expressions were previously associated with cultured normal cervical keratinocytes endowed with high proliferative capability (Stanley, 2002), whereas, K14 and K17 were associated with basal and parabasal layers of normal and CIN ectocervical epithelium (Smedts et al., 1990, 1992a, b; Martens et al., 2004). In our study, CIN and NUC primary colonies were tested for keratins 5, 14, 17, and 19 by immunofluorescence. Since type II colonies are of high interest for culturing purposes due to their high proliferative potential, immunofluorescence assays were only performed on these colonies. In most of the colonies and seeded-colonies keratin staining was uniformly present in all cells, suggesting that keratinocyte clonal proliferation occurred. However, keratin staining was also detected only in cell fractions of colonies and seeded-colonies indicating that some colonies did not originate clonally. Despite differing signal intensities, most of the CIN and NUC colonies, as well as the seeded colonies stained positive for K14, K17, and K19, indicating their common origin from basal and parabasal layers of the cervical epithelia (Smedts et al., 1990, 1992a, b; Martens et al., 2004; Akgül et al., 2007). However, CIN3 and CIN2 colonies stained more strongly for K14, K17, and K19 than CIN1 and NUC colonies. These results indicate that cultured CIN3 and CIN2 keratinocytes fully retained basal and parabasal markers, which, in turn, are predictive of a high proliferative potential. Similarly, CIN3 keratinocytes expanded from a colony stained strongly for K14, K17, and K19 in all cells. This result indicates that CIN keratinocytes did not change their original proliferative characteristics after expanding with the new medium mixture. Consistently, expanded NUC keratinocytes expressed K14 and K19 as shown in most NUC primary colonies. K5 negative results may account for the keratinocyte culturing method used. It is known that keratin expression pattern varies depending on keratinocyte culturing methods (Michelini et al., 2005; Roig et al., 2010). Indeed, K5 has previously been detected in cervical keratinocytes cultured on inactivated murine fibroblasts with serum medium supplemented with hydrocortisone, cholera toxin and EGF (Stanley, 2002). P63 has previously been detected in stem cells from normal and CIN ectocervical epithelia (Quade et al., 2001; Martens et al., 2004). Accordingly, p63 stained strongly in keratinocytes derived from CIN3 colonies (one seeded-colony and one expanded colony) suggesting that those keratinocytes are endowed with stemness characteristics.
In conclusion, we set up a rapid and easy method to obtain CIN and NUC keratinocyte colonies from small pieces of neoplastic and normal tissue fragments. Compared to previous CIN and NUC culture methods, our protocol is very simple. It allows primary NUC and CIN keratinocyte colonies to be obtained from small tissues in a single technical passage, that is, after tissue digestion, thus overcoming the necessity to isolate an adequate number of pure keratinocytes for successful cultures. Consequently, the time required and the risk of exogenous microbial contamination are highly reduced. Moreover, this culture method enables the HPV-CIN keratinocyte heterogeneity of neoplastic biopsies to be clonally reproduced in a single step, that is, in primary cultures. This is an important aspect because the clonal CIN keratinocytes enable the different genetic modifications occurring in each single HPV-CIN keratinocyte of tumor specimen to be characterized in vitro, both at cellular and molecular level, thus permitting the different phases of neoplastic progression to be reconstructed. To this purpose, our preliminary data on HPV genotyping of CIN colonies indicate that different HPV genotypes co-exist in single CIN2 and CIN3 colonies. These data suggest that the multistep process of the neoplastic onset/progression may occur because of different multiple HPV infections. However, due to the limited number of CIN colonies analyzed in this study final conclusions cannot be drawn. Further experiments with a large number of CIN colonies combined with in vitro functional reconstruction assays, that is, multiple HPV infections of cultured keratinocytes, may elucidate the mechanisms occurring during transition from normal to pathological keratinocytes and CIN progression toward higher grade. Moreover, comparative analyses of pathways in NUC and CIN cultured keratinocytes could led to identify dysregulated genes, as done before with other tumour models (Balatti et al., 2011; Varani et al., 2011). Altogether these findings may allow useful new biomarkers to be identified, to improve the diagnostic and prognostic accuracy of CIN lesions which, as is known, are far from perfect (Baak et al., 2006).
Acknowledgements
Supported in part by grants from Fondazione Guido Berlucchi, Borgonovo di Franciacorta; the Fondazione Cassa di Risparmio di Cento, Cento; FAR project, University of Ferrara, Ferrara; PRIN COFIN 2008, MIUR, Rome. Italy.