Group X secreted phospholipase A2 specifically decreases sperm motility in mice
Abstract
Different mammalian secreted phospholipases A2 (sPLA2s) are expressed in male reproductive organs and/or in sperm cells but their cellular functions are still not fully characterized. Because several reports indicate a link between cellular lipids and sperm motility, we have investigated the effect of mouse group IIA, IID, IIE, V, and X sPLA2s on sperm motility. Among these enzymes, only mouse group X sPLA2 (mGX sPLA2) acts as a potent inhibitor of sperm motility that decreases track speed (VCL) and lateral displacement of the head (ALH) of both noncapacitated and capacitated sperm. The inhibitory effect of mGX sPLA2 is dependent on its enzymatic activity because (i) both the proenzyme form of mGX sPLA2 (pro-mGX) and the H48Q mutant of mGX sPLA2 have very weak enzymatic activity and are unable to modulate sperm motility and (ii) LY329722, a specific inhibitor of sPLA2s, blocks the inhibitory effect of mGX sPLA2. Moreover, mGX sPLA2 exerts a gradual potency on sperm subpopulations with different velocities, an effect which may be linked to the heterogeneity of lipid composition in these sperm subpopulations. Finally, we found that endogenous mGX sPLA2 released during spontaneous acrosome reaction modulates sperm motility of capacitated sperm. Together, our results suggest a new role of sPLA2 in sperm physiology where the sPLA2 selects a sperm subpopulation for fertilization based on its effect on sperm motility. J. Cell. Physiol. 226: 2601–2609, 2011. © 2010 Wiley-Liss, Inc.
Asthenozoospermia (sperm with low motility) is one of the major causes of infertility or subfertility in humans. Deciphering the molecular mechanisms and pathways involved in sperm flagellum beat remains a challenging task. Although remarkable progresses have been made with respect to sperm flagellum beat regulations, issues are very complex and control pathways remain poorly characterized. Sperm flagellum beat is complex because the molecular structure of the axonema contains several hundreds of molecules (Escalier, 2006; Eddy, 2007), and even this number could be underestimated (Pazour et al., 2005). Control of flagellum beat is characterized by the importance of phosphorylation/dephosphorylation cycles via protein kinase A (PKA) or tyrosine kinases of numerous structural proteins like dynein chains (Luconi et al., 2004). Calcium and cAMP are key elements controlling PKA activation and play central roles (Esposito et al., 2004; Wang et al., 2007). Calcium is a complex actor since it controls soluble adenyl cyclase and thus cAMP production (Aoki et al., 1999; Carlson et al., 2007), phosphorylation/dephosphorylation cycles via calmodulin dependent pathways (Marin-Briggiler et al., 2005) and also binds directly to axonema structural component, leading to hyperactivation, a strong modulation of flagellum beat (Suarez, 2008). Interestingly, demembraned sperm or cilia present distinct sensitivity to Ca2+ or cAMP in comparison to control (Feng et al., 1988; Lansley et al., 1992) and these results suggest that the plasma membrane plays an important role in sperm flagellum beat regulation. Lipids are essential structural components of the plasma membrane which are likely to be important actors of sperm flagellum beat since they are involved in membrane fluidity, membrane potential and represent the precursors for many secondary messengers.
The role of lipid composition of the plasma membrane and of lipid metabolism in sperm motility has been poorly explored so far. A few studies have however suggested that lipid metabolism or sperm lipid content are important for sperm motility. First, human asthenozoospermia has been associated with a defective sperm plasma membrane composition characterized by an excess of cholesterol and desmosterol (Buffone et al., 2009; Zalata et al., 2009), a low concentration of polyunsaturated fatty acids such as docosahexaenoic acid (Gulaya et al., 2001; Tavilani et al., 2007) and an overall increase of fatty acid content (Ollero et al., 2001; Aksoy et al., 2006). These results suggest that lipid composition of the plasma membrane and/or its rearrangement during sperm maturation are important for flagellum beat. Membrane fluidity is directly linked to the lipid composition of the plasma membrane, and any change in lipid composition leads to a change of membrane fluidity which could in turn impact sperm motility. The link between sperm membrane composition and motility is reinforced by the fact that capacitation, the final maturation step of sperm, leads to both major reorganization of the plasma membrane and modification of sperm movement. Cholesterol efflux is the most well-known modification of the plasma membrane and plays a major role in sperm maturation both in vivo and in vitro (Visconti et al., 1999; Travis and Kopf, 2002). Other major lipid changes during capacitation include efflux of desmosterol, changes in sterol sulfates, phospholipids, sphingomyelins, and ceramides (Travis and Kopf, 2002). This remodeling of lipid distribution leads to the formation of membrane microdomains (Gadella and Harrison, 2000; Jones et al., 2007; Gadella et al., 2008). All these events likely contribute to increasing the membrane fluidity by changing lipid packing but also lead to the heterogeneity of the sperm population. Among the different families of lipolytic enzymes involved in these lipid rearrangements (Wang et al., 2004), phospholipases A2 (PLA2) are good candidates because of their large diversity of action in phospholipid remodeling in a large set of biological situations (Lambeau and Gelb, 2008). The involvement of PLA2 in sperm motility is reinforced by recent results obtained with knock-out mice where male deficient in group VIA Ca2+-independent phospholipase A2 (iPLA2β) or mouse group III secreted phospholipase A2 (mGIII-sPLA2) present asthenozoospermia (Bao et al., 2004; Sato et al., 2010).
The PLA2 superfamily comprises intracellular and secreted PLA2s (Schaloske and Dennis, 2006). Intracellular enzymes include Ca2+-dependent cytosolic PLA2s (group IV), Ca2+-independent iPLA2s (group VI) and Ca2+-independent PAF-acetylhydrolases (group VIII). Secreted PLA2s (sPLA2s) comprise up to 10 different members classified as group IB, IIA, IIC, IID, IIE, IIF, III, V, X, and XIIA.
Both intracellular and secreted phospholipases A2 have been described in mouse male reproductive organs (Koizumi et al., 2003; Yan et al., 2003; Bao et al., 2004; Masuda et al., 2004; Roldan and Shi, 2007). Spermatogenic cells, vas deferens and seminal vesicles express sPLA2s of group IIC, IID, IIE, IIF, V, and X; epididymis contains group IIC, IID, IIE, IIF, III, V, and X sPLA2s (Sato et al., 2010); prostate expresses group IIC, IID, IIE, and IIF sPLA2, and mature sperm specifically express mouse group X sPLA2 (mGX sPLA2) (Sato et al., 2010; Escoffier et al., 2010b). Deciphering the specific roles of each PLA2 present in male reproductive organs is challenging. Recent studies have revealed new roles for mGIII and mGX sPLA2s in sperm maturation. First, mGIII sPLA2 has been involved in sperm lipid homeostasis in the epididymis and lack of this sPLA2 leads to defective epididymal maturation, asthenozoospermia and infertility (Sato et al., 2010). Second, mGX sPLA2 is present in the acrosome of mature sperm and its paracrine secretion during sperm capacitation promotes a premature or spontaneous acrosome reaction (AR) that excludes a suboptimal sperm subpopulation from fertilization (Escoffier et al., 2010b). Third, venom sPLA2s from the Australian Taipan snake Oxyuranus scutellatus inhibit sperm motility (Escoffier et al., 2010a). These findings led us to evaluate the role of mammalian sPLA2 in sperm motility. We measured the impact on sperm motility of the mammalian sPLA2s which are known to be present in male reproductive organs (Masuda et al., 2004) and in the female tract (Valentin et al., 1999). These include mouse group IIA (mGIIA), mouse group IID (mGIID), mouse group IIE (mGIIE), mouse group V (mGV) and mGX sPLA2s. Using recombinant mature enzymes as well as catalytically inactive sPLA2s and specific inhibitors, we show for the first time that mouse sperm motility is decreased only by mGX sPLA2 in an enzymatically-dependent manner. Moreover, we demonstrate that mGX sPLA2 potency is dependent on sperm velocity and that mGX sPLA2 is less active on the high speed sperm subpopulation. Finally, we show that endogenous mGX sPLA2 released during capacitation decreases sperm velocity. Altogether, these results reveal a new physiological role for mGX sPLA2 in the process of sperm sorting.
Materials and Methods
Biological preparations
Sperm preparation
Sperm cells were obtained by manual trituration of caudae epididymides from OF1 male mice (2–6 months old—Charles River, France). All protocols were reviewed and have been approved by the local ethical committee of Grenoble Institute of Neurosciences. Sperm were allowed to swim in 1 ml of M2 medium for 10 min. Sperm were then centrifugated (600g, 10 min) and resuspended in M16 medium.
Capacitation and acrosome reaction assay
Sperm were capacitated in M16 medium with 2% fatty acid free BSA at 37°C in a 5% CO2 incubator for various times. For sPLA2 treatment, sperm were incubated with sPLA2 in M16 medium at 37°C for the last 10 min. Cells were transferred in PBS solution and then fixed with 4% PFA solution for 2 min. Sperm were washed (100 mM ammonium acetate, 2 min), wet-mounted on slides and air dried. Slides were then rinsed with water and stained with Coomassie blue (0.22%) for 2 min, and finally rinsed. Slides were counted and at least 150 sperm cells were scored.
Computer-assisted motility analysis
Noncapacitated or capacitated sperm suspension was immediately placed onto an analysis chamber (100 µm depth, Leja Products B.V., Nieuw-Vennep, the Netherlands) and kept at 37°C for microscopic quantitative study of sperm movement. Sperm motility parameters were measured at 37°C using a sperm analyzer (Hamilton Thorn Research Inc, Beverly MA). The settings employed for analysis were as follows: acquisition rate: 60 Hz; number of frames: 100; minimum contrast: 25; minimum cell size: 10; low static-size gate: 2.4; high static-size gate: 2.4; low static-intensity gate: 1.02; high static-intensity gate: 1.37; minimum elongation gate: 12; maximum elongation gate: 100; magnification factor: 0.70. The motility parameters measured were curvilinear velocity (VCL), average path velocity (VAP) and amplitude of lateral head displacement (ALH). At least, 100 motile sperm were analyzed for each assay. Progressive hyperactivated sperm were characterized by VCL > 250 µm/sec, VAP > 70 µm/sec, ALH > 18 µm, LIN < 45 and non-progressive hyperactivated sperm (star spin) by 120 < VCL < 250 µm/sec, VAP < 50 µm/sec and LIN < 30.
Method to trace the theoretical mGX-treated distribution: the sperm subpopulation belonging to the bin  will shift to the bin having a velocity of
will shift to the bin having a velocity of 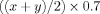 .
.
Production of recombinant sPLA2s
Recombinant mouse sPLA2s group IIA, IID, IIE, V, and X and the H48Q mutant of mGX-sPLA2 were produced as described previously (Singer et al., 2002; Rouault et al., 2007). Pro-mGX sPLA2 was produced as for mature mGX sPLA2 using the pAB3 vector in which the full-length cDNA coding for PromGX sPLA2 was inserted in frame with the ▵GST protein and the factor Xa cleavage site, which were removed from PromGX sPLA2 by using the factor Xa protease (Rouault et al., 2007).
Phospholipids staining/viability test
Control untreated sperm cells (noncapacitated or capacitated for 90 min) or sperm treated for 10 min with 200 nM mGX sPLA2 in M16 medium were washed twice with PBS, and then incubated in a staining buffer (from Apoptosis detection kit, Sigma–Aldrich, Saint Quentin Fallavier, France) containing either carboxyfluorescein diacetate (6-CFDA) or 1 µg/ml annexin V-Cy3 for 15 min in the dark at room temperature. After incubation with annexin V-Cy3, sperm was washed twice with the staining buffer and fixed in PBS with 4% paraformaldehyde. AnnexinV-Cy3 staining was analyzed using a confocal microscope (Leica Microsystems GmbH, Wetzlar, Germany).
Chemical compounds
M2 and M16 media, fatty acid free BSA (fraction V) and annexin V-Cy3 (from Apoptosis detection kit) were purchased from Sigma-Aldrich, Saint Quentin Fallavier, France. LY329722 was a generous gift from Pr. M. Gelb.
Statistical analyses
Statistical analyses were performed with SigmaPlot. Paired t-test were used to compare the effect of various compounds on AR. Data presented represent mean ± SEM. Statistical tests with a two-tailed P values ≤0.05 were considered as statistically significant. Linear regression was calculated with SigmaPlot.
Results
mGX sPLA2 specifically inhibits sperm motility among five different mouse sPLA2s
During its short life, sperm become exposed to several sPLA2s, including those present in the seminal plasma and originating from epididymis and prostate or present in the female genital tract (Valentin et al., 1999; Masuda et al., 2004; Lessig et al., 2008; Sato et al., 2010; Escoffier et al., 2010b). We thus evaluated the effect of five different mammalian sPLA2s known to be present in male or female genital tracts on sperm motility using the computer-assisted sperm analysis (CASA) system. These sPLA2s are mGIIA, mGIID, mGIIE, mGV, and mGX. Sperm tracks are characterized with the CASA system by four basic parameters evaluating sperm motility: the track speed (VCL), the progressive velocity (VSL), the path velocity (VAP) and the lateral displacement of the head (ALH). We first focused on noncapacitated sperm and because noncapacitated sperm have mostly linear progressive tracks, only the VCL and the ALH parameters were studied. Sperm were incubated with the 5 different recombinant sPLA2s at 200 nM during 10 min in M16 culture medium at 37°C. Figure 1A,B clearly shows that only mGX sPLA2 was a potent modulator of VCL and ALH: in the presence of 200 nM mGX sPLA2, the mean VCL was decreased by 23%, from 165.55 ± 5.44 (n = 19) to 124.73 ± 6.65 µm/sec (n = 16), P < 10−4 while the mean ALH was decreased by 14%, from 11.45 ± 0.35 (n = 19) to 9.81 ± 0.54 µm (n = 16), P = 0.01. The potency of mGX was variable between different males: no correlation between initial VCL values of the sample and the mGX sPLA2 potency was noticed (Fig. 1A), contrary to ALH values, which appeared to be negatively correlated with control levels (Fig. 1B, correlation coefficient 0.69). In order to better characterize the inhibitory effect of mGX sPLA2 on sperm motility, we determined the minimal concentration of mGX sPLA2 triggering the inhibitory effect in 10 min. Figure 1C shows mGX sPLA2 dose–response curves for VCL and ALH and indicates that concentrations of mGX sPLA2 as low as 20 nM affect significantly sperm motility, and more particularly VCL. sPLA2, as an enzyme hydrolyzing the lipids of the plasma membrane, may cause a premature cell death, affecting the sperm motility. To test this hypothesis, sperm were first treated with 200 nM mGX sPLA2 for 10 min and subsequently incubated with 6-carboxyfluorescein diacetate (6-CFDA) for 15 min. This compound is used as a marker of sperm viability: it enters freely into cells and produces green fluorescence after de-esterification by endogenous esterases only in living cells (Silva and Gadella, 2006). Both control (83.02% ± 1.2, n = 2) and treated sperm (85.42% ± 2.4, n = 2) showed a strong green fluorescence signal (Fig. 1D), indicating that the sperm motility decrease induced by the sPLA2 was not due to premature cell death during the duration of experiment.
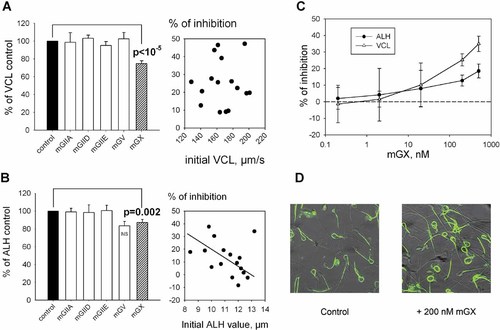
mGX sPLA2 is the most potent inhibitor of sperm motility among five different mouse sPLA2s. A,B: The effects of mGIIA, mGIID, mGIIE, mGV, and mGX sPLA2s at 200 nM on sperm motility were analyzed with the Computer Assisted Sperm Analysis system (CASA). Two different sperm motion parameters: “track speed” VCL (A) and “lateral displacement of the head” ALH (B) are shown. For each sPLA2, we compared the values obtained in the presence of the enzyme with those obtained in a control experiment with sperm cells from the same animal; n = 16 for mGX, n = 5 for mGV and n = 3 for mGIIA, mGIID, and mGIIE sPLA2s. The % of inhibition by mGX sPLA2 (right graphs) was quite variable between the different males, from ∼10% to ∼50% and independent of initial VCL value of the sample. Linear regression is traced for ALH (B, right part). C: Dose–response curves of mGX sPLA2 on two different sperm motion parameters ALH (●) and VCL (Δ). mGX sPLA2 concentrations tested were 0.2, 2, 20, 200, and 500 nM. D: Sperm viability is not reduced by sPLA2 treatment. Control or mGX sPLA2-treated sperm (200 nM), and subsequently incubated with 6-carboxyfluorescein diacetate (6-CFDA), showed similar strong green fluorescence signal.
The effect of mGX sPLA2 is dependent on its enzymatic activity
To determine whether the enzymatic activity of mGX sPLA2 is required for modulation of sperm motility, we first analyzed the effect of its H48Q mutant which has a very low enzymatic activity (Surrel et al., 2009). The mGX H48Q mutant was unable to affect sperm VCL (Fig. 2A) or sperm ALH (Fig. 2B). Second, since mGX sPLA2 is produced as a zymogen (pro-mGX sPLA2) which has a very low enzymatic activity (Morioka et al., 2000), we tested the effect of pro-mGX sPLA2 on sperm motility and found no change on VCL and ALH (Fig. 2A,B respectively). These results indicate that the enzymatic activity plays a crucial role in the effect of mGX sPLA2 on sperm motility. To further confirm these results, we tested the effect of the specific sPLA2 inhibitor LY329722. This compound is known to inhibit mGX enzymatic activity with an IC50 of 75 nM (compound A in Smart et al., 2006). Preincubation of mGX sPLA2 with LY329722 at 1 µM prevented its inhibitory effect on VCL (Fig. 2C).
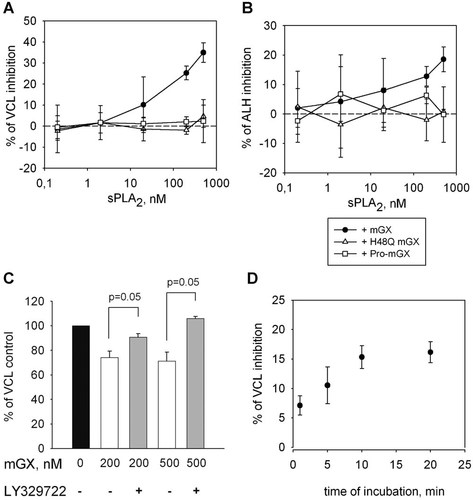
The effect of mGX sPLA2 on sperm motility is dependent on its enzymatic activity. A,B: The H48Q mutant of mGX sPLA2 and pro-mGX sPLA2, the inactive proenzyme form of mGX sPLA2 (200 nM) were unable to modulate VCL and ALH sperm motility parameters (n = 3). C: LY329722, a specific sPLA2 inhibitor, blocks the effects of mGX sPLA2 on sperm VCL (n = 5, for mGX sPLA2 at 200 nM and n = 3 for mGX sPLA2 at 500 nM, p as indicated). D: Kinetic of mGX sPLA2 effect on sperm VCL: at 200 nM mGX sPLA2, the maximal inhibition effect was reached in 10 min (n = 6).
Finally, we measured the kinetic of VCL inhibition by mGX sPLA2 at 200 nM. Sperm were incubated with mGX sPLA2 from 1 to 20 min: the VCL inhibition was proportional to the incubation period up to 10 min, then reached a plateau after 10 min (Fig. 2D). This result reinforces the importance of sPLA2 enzymatic activity in sperm velocity decrease and suggests that all substrates were hydrolyzed in 10 min at 200 nM.
Heterogeneous response of sperm to mGX sPLA2 treatment
The sperm population presents a high degree of heterogeneity in terms of motility, even in a cell population obtained from a single male. This heterogeneity is displayed by a wide range of VCL and ALH values, indicating different subpopulations of sperm. For instance, values for VCL ranged from 7.5 to 450 µm/sec in a control cell population (Fig. 3A, blue bars/curve). We noticed the same degree of heterogeneity for ALH, with values ranging between 0 and 40 µm (Fig. 1D). In this study, static sperm cells and slow cells were not counted and only sperm cells with a VAP > 7.5 µm/sec were analyzed. An important question was to determine whether mGX sPLA2 inhibits slow (VCL between 50 and 150 µm/sec) or fast sperm subpopulations (VCL between 200 and 300 µm/sec) with a similar potency (Fig. 3A). To address this question, we first compared the VCL distribution of control and mGX sPLA2-treated sperm populations, and then the VCL distribution of mGX sPLA2-treated sperm population versus a theoretical-treated distribution obtained after applying a identical reduction factor for the speed of each bin corresponding to the decrease of mean VCL calculated from the whole population: for this set of experiments we obtained a decrease of 30% (Fig. 3B, n = 3). As shown in Figure 3A, adding mGX sPLA2 modified the VCL distribution of sperm population, essentially by increasing the percentage of sperm with low speed (black bars/curve) and decreasing by a twofold factor the fast sperm subpopulation. We then traced a theoretical VCL distribution mimicking the effect of mGX sPLA2-treated sperm (Fig. 3C, red bars/line) by applying to all bins of control sperm an identical reduction factor (see Materials and Methods Section) corresponding to the reduction measured on the whole population, which was 30% (Fig. 3B). Comparing the distributions of measured and theoretical mGX sPLA2-treated sperm clearly shows that the theoretical distribution does not fit with that corresponding to mGX sPLA2-treated sperm (Fig. 3C, black vs. red curves). A similar discrepancy was observed when the size of the bins was reduced by a twofold factor (Size of 25, data not shown). This result suggests that some sperm subpopulations are differentially affected by the sPLA2 treatment. In particular, fast sperm were less inhibited by mGX sPLA2 than expected from the theoretical calculation. Because ALH and VCL amplitudes are correlated (correlation coefficient 0.6994 ± 0.025; Fig. 3D, n = 5), this result is consistent with our above result showing that mGX-inhibition of ALH appeared to be negatively correlated with its mean initial ALH amplitude (Fig. 1B). The heterogeneity of sperm population is illustrated by the tracks of sperm representing the position of the head of sperm over a period of 1.7 sec measured in control sperm and mGX sPLA2-treated sperm, respectively (Fig. 3E,F).
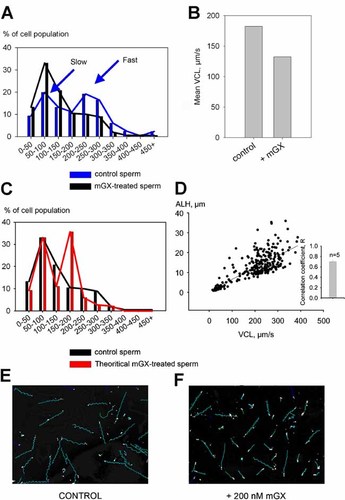
The inhibitory effect of mGX sPLA2 is stronger on low speed sperm than on high speed sperm. A: Sperm velocity is heterogeneous. Histogram of VCL values (in µm/sec) of control sperm (blue bars, n = 3; mean sperm counted by condition was 205) and mGX sPLA2-treated sperm (black bars, n = 3; mean sperm counted by condition was 310). For control sperm, VCL values ranged from 0 to 50 µm/sec to values above 450 µm/sec (blue bars/line) and allowed to identify slow and fast sperm subpopulations (blue arrows). In the presence of 200 nM mGX sPLA2, we observed a change of sperm VCL distribution (black bars/line), with a twofold decrease of the fast sperm subpopulation. B: Bar graph showing a 30% decrease of mean VCL induced by mGX sPLA2 treatment (n = 3). C: The VCL distribution of mGX sPLA2-treated sperm is different from the theoretical mGX sPLA2-treated distribution obtained by decreasing the control VCL values of 30% (red bars/line). Thirty percentage corresponds to the decrease of mean VCL in the presence of 200 nM mGX sPLA2 (part B). D: Positive correlation between ALH and VCL amplitudes. The scatter plot presents the result of the linear regression obtained in a control sperm population. Inset, mean value of correlation coefficient obtained with five independent sperm populations. E,F: Effects of mGX sPLA2 on sperm tracks (blue lines). Comparison of control sperm tracks (part E) and tracks of sperm incubated with 200 nM mGX sPLA2 during 10 min (part F) obtained from the same animal. Note that the length of tracks, which is proportional to the speed of the sperm cell, is more heterogeneous after application of mGX sPLA2. Static and slow cells were removed.
mGX sPLA2 is involved in phospholipid redistribution at the plasma membrane of the flagellum during capacitation
We have previously demonstrated that endogenous mGX sPLA2 is released via spontaneous AR during capacitation, and reaches after 45 min a concentration able to induce AR in a paracrine mode (Escoffier et al., 2010b). This process amplifies spontaneous AR by likely hydrolyzing phospholipids of the sperm plasma membrane right next to the acrosomal membrane. Because mGX sPLA2 is a potent modulator of sperm motility, we were wondering whether it may target phospholipids of the plasma membrane of the flagellum as well. To address this hypothesis, we first chose to measure the effect of exogenous mGX sPLA2 treatment on sperm phosphatidylserine distribution because (i) phosphatidylserine is readily hydrolyzed by mGX sPLA2 (Singer et al., 2002) and (ii) among phospholipids, phosphatidylserine is easily identified because it can be specifically stained by annexin V conjugated with the Cy3 fluorescent dye. Sperm incubated in a capacitating medium for 35 min present a strong annexin V-Cy3 staining in their midpiece. We thus compared the intensity of annexin V-Cy3 stainings of untreated capacitated sperm and capacitated sperm subsequently incubated with 200 nM mGX sPLA2 during 10 min (Fig. 4A). This experiment shows that recombinant mGX sPLA2 strongly decreased the annexin V-Cy3 staining of sperm capacitated during 35 min and demonstrates the ability of mGX sPLA2 to hydrolyze phospholipids of the sperm flagellum membrane.
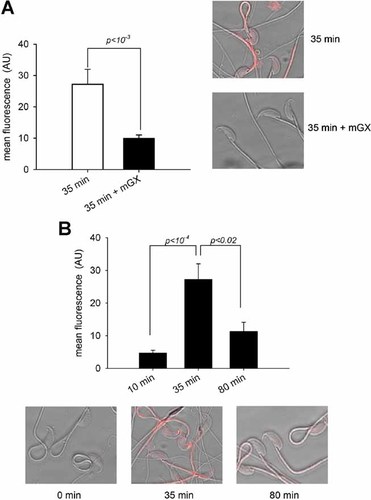
mGX sPLA2 and capacitation reorganize phosphatidylserine distribution in the midpiece of the flagellum. A: Annexin V-Cy3 staining vanished in the presence of 200 nM mGX sPLA2. Sperm were capacitated by incubating them for 35 min in 2% BSA in M16 medium in a 5% CO2 incubator at 37°C. They were then treated for 10 min in the presence of 200 nM sPLA2. Mean fluorescence density of the midpiece of sperm was measured (left). Confocal images of representative control and treated sperm are presented (right). B: Mean fluorescence density of the midpiece of sperm was measured at various durations of capacitation. Confocal images showing representative annexin V-Cy3 staining of sperm at various durations of capacitation (0, 35, and 80 min), illustrating changes in phosphatidylserine localization throughout capacitation. n = 3 separate experiments, the number of sperm measured by experiment and by condition was from 8 to 38.
Sperm capacitation leads to important changes in the lipid composition of the plasma membrane (Gadella and Harrison, 2000), including caspase-independent redistribution of phosphatidylserine in the external leaflet of the plasma membrane (de Vries et al., 2003). We confirmed that phosphatidylserine distribution changes during capacitation in our conditions. We observed that annexin V-Cy3 staining was absent or weak on noncapacitated sperm (Fig. 4B). After 35 min of capacitation, the staining was strong and localized mainly in the midpiece of flagella. On the other hand, sperm heads and principal pieces were weakly or not stained. At 80 min, a decrease in the annexinV-Cy3 staining was observed. This result confirms that capacitation, induced by cholesterol efflux and/or bicarbonate influx, leads to a modification of phospholipid asymmetry, promoting externalization of anionic phospholipids at the plasma membrane of the flagellum and specifies its variation throughout capacitation. The fact that the intensity of annexinV-Cy3 staining decreased between 35 and 80 min of capacitation (Fig. 4B) is in good accordance with both mGX sPLA2 enzymatic activity on sperm phospholipids (Fig. 4A) and the increase of mGX sPLA2 concentration in the capacitation medium after 45 min of capacitation (Escoffier et al., 2010b) and suggests that released mGX sPLA2 would be able to control sperm motility of capacitated sperm.
Endogenous mGX sPLA2 released during spontaneous AR decreases sperm velocity
We thus analyzed whether endogenous mGX sPLA2 was able to modulate sperm motility during the capacitation process. To test this hypothesis, we capacitated sperm in the presence of LY329722, the specific inhibitor of mGX sPLA2 tested in Figure 2C, during various times of capacitation (0, 55, and 90 min) and compared the VCL of LY329722-treated versus non-treated sperm (control). There was no statistically significant difference of the VCL between control and LY329722-treated sperm at 10 and 55 min of capacitation (Fig. 5A). In contrast, sperm treated during 90 min with LY329722 were faster than non-treated sperm: the mean VCL increased from 182.7 ± 5.1 to 191.1 ± 5.0 µm/sec (P = 0.036, n = 11) for LY329722-treated sperm and control sperm respectively (Fig. 5A). The fact that LY329722 treatment was effective only at 90 min is consistent with the kinetics of release of endogenous mGX sPLA2 during spontaneous AR, as demonstrated previously (Escoffier et al., 2010b).
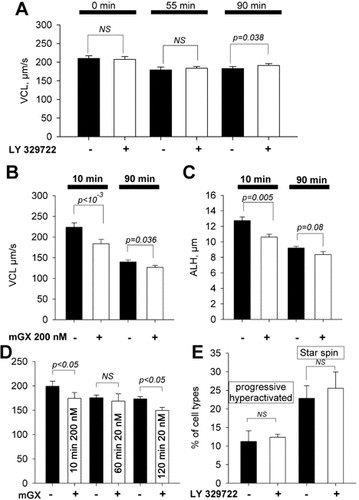
Endogenous mGX sPLA2 released during spontaneous AR decreases sperm motility, but does not modify hyperactivated sperm subpopulations. A: LY329722, a specific inhibitor of mGX sPLA2, increased the mean VCL of sperm only after 90 min of capacitation. Sperm were capacitated in the absence or presence of 1 µM LY329722. VCL of control (black bars) or LY329722-treated sperm (white bars) were compared at various times of capacitation (0, 55, and 90 min), n = 11. B,C: mGX sPLA2 potency decreased during capacitation. Mean VCL and ALH of control (black bars) or mGX-treated (200 nM) sperm populations (white bars), before capacitation (0 min, two left bars) or after 90 min of capacitation (90 min, two right bars), n = 6. D: Incubation of sperm with low concentration of mGX sPLA2 (20 nM) during 120 min produced a similar level of inhibition than the one obtained with 200 nM during a short incubation period of 10 min, n = 5. E: Progressive hyperactivated and star spin sperm subpopulations were not affected by LY329722 treatment (n = 4).
To confirm that mGX sPLA2 released during capacitation was able to reduce the sperm velocity, we compared the potency of recombinant mGX sPLA2 on the mobility of noncapacitated versus sperm capacitated for 90 min. Indeed, a 10 min application of 200 nM recombinant sPLA2 on capacitated sperm, a protocol sufficient to obtain a maximal sperm velocity inhibition (Fig. 2D), should produce a weaker inhibition than that obtained with uncapacitated sperm, since the capacitated cells are already partially inhibited by released mGX sPLA2. Noncapacitated and capacitated sperm were treated with mGX sPLA2 in the same conditions: the effect of mGX sPLA2 on sperm motility was measured after 10 min of incubation in M16 medium. Capacitated sperm were not treated in the capacitating medium (containing 2% fatty acid free BSA) in order to avoid an artefactual effect of BSA, which may chelate lipid metabolites produced by mGX sPLA2. Capacitated sperm were thus washed with M16 before mGX sPLA2 incubation in order to remove BSA. In this set of experiments, mGX sPLA2 inhibited the VCL of noncapacitated sperm by ∼18%; P < 10−3, n = 6 (Fig. 5B). On the other hand, the velocity of the same sperm, capacitated for 90 min and then treated for 10 min with mGX sPLA2, was inhibited by ∼9% (P = 0.036, n = 6). A similar result showing a decrease of mGX sPLA2 potency on ALH was observed at 90 min of capacitation (Fig. 5C). These results are in good accordance with the initial release of mGX sPLA2 and a subsequent phospholipid hydrolysis. Sperm were capacitated at a concentration of 200,000 sperm/ml. We have previously shown that after 90 min of capacitation, the amount of released mGX sPLA2 is around 1 ng/106 sperm (Escoffier et al., 2010b). Thus, during this experiment, sperm were swimming in a medium containing 0.02 nM of sPLA2. This concentration is very low when compared to those used in Figure 1, and thus the effect of LY329722 on sperm motility at 90 min of capacitation suggests that a long incubation (45 min) with a low concentration of mGX sPLA2 has an effect similar to the one observed after a short application of higher concentrations. This hypothesis was confirmed by comparing the effect of 200 nM mGX sPLA2 applied during 10 min versus the effect of 20 nM applied either during 60 or 120 min in a medium that does not promote capacitation (Fig. 5D): similar levels of inhibition were obtained at 200 nM during 10 min (12%) or at 20 nM during 120 min (13%), n = 5.
Finally, capacitation leads to a modification of sperm movements, and a small fraction of sperm switches from a linear movement to a more undulatory movement, and eventually to a circular movement. It is accepted that sperm presenting such movement changes are fully capacitated and are described as progressive and nonprogressive (or star spin) hyperactivated sperm, respectively (Mortimer, 2000). LY329722 treatment did not modify the hyperactivated sperm subpopulations (Fig. 5E) and this result suggests that released mGX sPLA2 does not interfere with the capacitation process leading to changes in sperm movements.
Discussion
The results presented in this article are important for two reasons. First, they evidence an unexpected role for sPLA2s as potent regulators of sperm motility. We found that among five different mouse sPLA2s present in male reproductive organs, recombinant mGX sPLA2 has the unique property of decreasing sperm motility. Furthermore, endogenous mGX sPLA2 released during in vitro capacitation takes part in the control of sperm motility, suggesting that sPLA2 control of sperm motility is physiologically relevant. Second, our results underscore the importance of phospholipid metabolism in sperm motility as we showed that the effect of mGX sPLA2 is dependent on its enzymatic activity, that is, the release of different types of fatty acids and lysophospholipids.
Sperm motility is controlled by mGX sPLA2
To our knowledge, this is the first report showing the modulation of sperm motility by an sPLA2 during capacitation. Platelet activating factor (PAF), a potent lipid mediator, has been shown to increase sperm chemotactism, but its effect on sperm motility parameters has not been investigated (Wu et al., 2001). Disruption of the gene for the intracellular PLA2 group VIA (iPLA2β) leads to a severe impairment of sperm motility, but the direct contribution of this PLA2 to sperm flagellum beat has not been demonstrated (Bao et al., 2004). Similarly, disruption of the Pla2g3 gene (coding for mGIII sPLA2) leads to a series of profound defects in sperm maturation, including asthenozoospermia (Sato et al., 2010). These effects appear to be due to maturation defects rather than to a defective control of sperm flagellum beat during capacitation.
We have previously shown that mGX sPLA2 is a strong inducer of AR (Escoffier et al., 2010b) and an obvious question concerns the relationship between the mGX-induced motility decrease and the modification of the acrosomal status of sperm. In Figure 6, we have plotted on the same graph the dose–response curves for mGX sPLA2-induced AR level and sperm motility decrease as a function of mGX sPLA2 concentrations. It clearly appears that AR did not induce a sperm motility decrease since there was not correlation between the two phenomena. The absence of correlation is obvious, especially at low and high concentrations of mGX sPLA2. For instance, at 2 nM, the motility was not affected whereas this concentration produced a 30% increase in the number of acrosome reacted sperm.
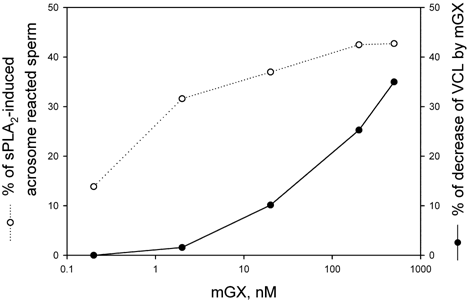
Absence of correlation between acrosome reaction and decrease of sperm motility induced by mGX sPLA2. Noncapacitated sperm were treated with different concentrations of recombinant mGX sPLA2 and both AR (○) and sperm motility (●) were measured. Spontaneous AR was subtracted and only sPLA2-induced AR is plotted.
We have not yet determined the molecular pathways explaining how mGX sPLA2 enzymatic activity reduces sperm motility. Since various fatty acids and lysophospholipids, the two products of sPLA2 enzymatic activity, can inhibit sperm motility (Hong et al., 1986; Siegel et al., 1986), the effect of mGX sPLA2 is likely due to the release of such metabolites. Reactive oxygen species (ROS) are produced when sperm are incubated with unsaturated fatty acids (Aitken et al., 2006) and sperm motility is known to be sensitive to oxidative damage (Agarwal et al., 2008). Although the production of ROS by sPLA2 on sperm remains to be determined, different sPLA2s have been involved in ROS production in several cell types (Adibhatla and Hatcher, 2008; Kim et al., 2009). An alternative hypothesis may involve changes of the biophysical properties of proteins embedded in the plasma membrane. Indeed, it has been recently shown that the functions of various membrane proteins, including ion channels, are specifically modulated by different types of lipids via protein–lipid interactions. For instance, the voltage sensor of Kv2.1 K+ channels is modulated by charged phospholipids and sphingomyelinase alters the biophysical properties of this channel (Xu et al., 2008). However the fact that LY329722 did not modify hyperactivated sperm subpopulations (Fig. 5D) suggests that mGX sPLA2 does not interfere with cell signaling involved in hyperactivation, at least in vitro. Hyperactivation is mostly controlled in vitro by calcium signaling (Suarez, 2008) and this result suggests that the mGX sPLA2 effect is not related to changes in calcium influx.
Physiological relevance of mGX sPLA2 action on sperm motility
By using CASA, we identified several subpopulations based on sperm velocity (Fig. 3). Because the CASA system takes a snapshot of the sperm population, the true existence of these subpopulations may still be debated. However, the existence of highly motile sperm subpopulation, sorted by swim-up techniques is well accepted for human sperm (Mortimer and Mortimer, 1992) and we obtained similar results by swim-up techniques with mouse sperm (not shown). This notion of sperm population heterogeneity is clearly illustrated by the histograms of sperm velocity showing the presence of different subpopulations in a single sperm sample. Moreover, we have demonstrated that the different subpopulations are not equally inhibited by mGX sPLA2, the slow speed sperm being more sensitive to mGX sPLA2 treatment. The stronger effect of mGX sPLA2 on slow sperm may be due to different lipid compositions of the plasma membrane since it has been shown that the heterogeneity of sperm population velocity is associated with a difference in lipid composition of slow versus fast sperm subpopulations (Buffone et al., 2009; Zalata et al., 2009). Although the effect of mGX sPLA2 on ALH was dependent of its initial amplitude (Fig. 1B), confirming therefore that less motile cells are more affected by mGX sPLA2, the absence of correlation between the mGX sPLA2 potency and the initial VCL amplitude of the sample (Fig. 1A) may appear controversial. However, the reasons for differences of sperm velocity between the different males are likely not exclusively related to lipid composition differences since the flagellum beat is controlled by numerous factors as described at the beginning of this article. Another facet of sperm heterogeneity has been recently described, showing that a fraction of the sperm population is resistant to AR induced by mGX sPLA2 (Escoffier et al., 2010b). This mGX resistant subpopulation of sperm gives higher fertility outcome than control sperm in in vitro fertilization experiments, and we have proposed that mGX sPLA2 may sort different sperm subpopulations presenting different levels of fertility. The fact that high-speed sperm (the likely most competent sperm subpopulation for fertilization) are less sensitive than low speed sperm to sPLA2 treatment, suggests that mGX sPLA2 may further contribute to sort competent sperm cells by slowing-down the slow, likely suboptimal sperm, and thus decreasing the chance for these latter to reach oocytes on time for fertilization.
From these results, we can propose that mGX sPLA2 released by sperm during spontaneous AR may sort competent sperm cells by two ways: first by promoting AR of a specific sperm subpopulation with a low fertility potency and second by decreasing the sperm motility of slow and likely suboptimal sperm.
Acknowledgements
This work was supported in part by the Région Rhône-Alpes (to C.A.), CNRS (to C.A. and G.L.), INSERM (M.D.W.), and the Association pour la Recherche sur le Cancer (to G.L.). J.E. was supported by a fellowship from Région Rhône-Alpes. V.P., L.M., and Z.B. are supported by a grant from the French ANR (Genopat 2009, ICG2I). We are grateful to Pr. Michael Gelb for the generous gift of LY329722.




