TGFBR3, a potential negative regulator of TGF-β signaling, protects cardiac fibroblasts from hypoxia-induced apoptosis†
Wenfeng Chu and Xiaoxue Li contributed equally to this work.
Abstract
A lot of evidence indicates that cardiac fibroblasts are essential for maintaining the structure and function of heart. The present study examined whether TGFBR3 (transforming growth factor type III receptor, also known as betaglycan) could prevent hypoxia-induced injury in neonatal mice cardiac fibroblasts, if so, its possible molecular targets. MTT, electron microscopy and TUNEL assay were used to identify cell viability and apoptosis in neonatal mice cardiac fibroblasts. Results showed that hypoxia for 24 h markedly reduce cell viability by 49.8 ± 8.9%, largely via apoptosis. However, hypoxia-induced apoptosis in cardiac fibroblasts were almost completely prevented by overexpression of TGFBR3. In the present study, hypoxia also induced TGF-β1, p-Smad2/3 expression, TGFBR1–TGFBR2 complex formation and collagen production in cardiac fibroblasts, which were attenuated substantially by TGFBR3 overexpression. TGFBR3 also reversed Bax up-regulation, Bcl-2 down-regulation and Caspase-3 activation induced by hypoxia in cardiac fibroblasts. Hypoxia or TGF-β1 itself triggered an increase of [Ca2+]i in cardiac fibroblasts, which were both inhibited by TGFBR3 overexpression. Taken together, our results indicate that TGFBR3 may act as a protective factor in apoptotic process of cardiac fibroblasts by negative regulation of TGF-β signaling and represent a potential therapeutic target for heart remodeling after hypoxia injury. J. Cell. Physiol. 226: 2586–2594, 2011. © 2010 Wiley-Liss, Inc.
Fibroblasts, which are the most numerous cell types in the heart, play very important roles in the myocardium. One of the main roles of cardiac fibroblasts is to give structural support to the heart by producing extracellular matrix such as collagens and fibronectin (Brown et al., 2005; Takeda et al., 2010). However, their excessive death accompanied by overproduction of extracellular matrix (ECM) contributes to heart remodeling even dysfunction of heart (Jensen and Host, 1997).
Hypoxia is a severe stress which induces physiological and molecular adaptations, and it is a known inducer of apoptosis in a variety of cell types including cardiac fibroblasts (Falanga et al., 1993; Mayorga et al., 2004). It has been reported that hypoxia induces both ECM over production and transforming growth factor-β1 (TGF-β1) activation in peritoneal, renal, and dermal fibroblasts (Steinbrech et al., 1999; Norman et al., 2000; Saed and Diamond, 2002). TGF-β signaling pathway is a multifunctional pathway which has a principal role in growth control through both its cytostatic effect on many different epithelial cell types and the ability to induce programmed cell death in a variety of other cell types (Schuster and Krieglstein, 2002). As a member of TGF-β family, TGF-β1 has diverse biological roles by interaction with two major types of cell surface receptors, TGFBR1 (transforming growth factor type I receptor) and TGFBR2 (transforming growth factor type II receptor) which form a complex and promote the activation of TGF-β signaling pathway (Attisano and Wrana, 1996).
TGFBR3 (transforming growth factor type III receptor, also known as betaglycan) is an accessory co-receptor of TGF-β. TGFBR3 is widely expressed in various cells, which with the cytoplasmic domain being able to bind TGF-β, to bind TGFBR2, and to enhance TGF-β signals or has no apparent intrinsic signaling activity (Lopez-Casillas et al., 1991; Wang et al., 1991; Blobe et al., 2001). However, in epithelial cells, some studies have suggested that TGFBR3 inhibits TGF-β signaling by preventing the formation of TGFBR1–TGFBR2 complex and it is also a potent TGF-β neutralizing agent (Vilchis-Landeros et al., 2001; Eickelberg et al., 2002). These results of the regulatory role of TGFBR3 in TGF-β signals raised a lot of controversy. Recently, TGFBR3 exhibits anti-fibrotic property in the heart and lung (Hermida et al., 2009; Ahn et al., 2010), suggesting it may be a potential inhibitor of TGF-β signaling. However, in cardiac fibroblasts exposed to hypoxia which TGF-β signaling accounts predominantly for the fibrogenesis, the physiological and pathophysiological role of TGFBR3 and its molecular regulatory targets and function remains to be determined.
The mechanism of TGF-β1 induced apoptosis involves regulating Bcl-2 and Bax proteins has been reported (Schuster and Krieglstein, 2002). These two proteins act as the hallmark of cell death and play a pathophysiological role in the protection or acceleration of apoptosis (Nishikawa et al., 2006). On the other hand, one of biochemical events in the apoptotic process is the elevation of intracellular Ca2+ ([Ca2+]i), as a consequence of deregulation of Ca2+ homeostasis has been suggested an injury of hypoxia (Kim et al., 2008). In addition to cytoplasmic signals, an increase in [Ca2+]i level can promote TGF-β1 secretion and activate TGF-β signaling pathway as well as an increase of TGF-β1 also can induce the enhanced calcium signals, thus, may facilitate apoptosis by triggering series of events in the apoptotic pathway. However, whether TGF-β signaling pathway and its regulated downstream events are involved in hypoxia induced cell apoptosis in cardiac fibroblasts have not been investigated.
In this study, firstly we demonstrated a TGF-β signaling pathway was involved in apoptosis induced by hypoxia in neonatal mice cardiac fibroblasts. Then we focused on TGFBR3, an accessory receptor member of TGF-β superfamily, acting as a protector which inhibited both TGFBR1–TGFBR2 complex formation and TGF-β1 production then subsequently initiated their downstream events targeting the apoptosis related genes in cardiac fibroblasts exposure to hypoxia. This study not only shed a light on a potentially novel regulator which can prevent cardiac fibroblasts from apoptosis via negative regulation of TGF-β signaling pathway, but also a new mechanism that may act as a piece of complementary evidence for a potential therapeutic target for heart remodeling after hypoxia injury.
Materials and Methods
Culture of neonatal mice cardiac fibroblasts and transfection of TGFBR3
Neonatal mice cardiac fibroblasts were prepared from hearts of 1- to 3-day-old B6 mice as described previously (Han et al., 2004; Callis et al., 2005). Fibroblasts were cultured in DMEM containing 10% fetal bovine serum (FBS) and used for all experiments. A total 2 × 103 of fibroblasts/cm2 were seeded in six-well plates and cultured for 72 h. Cells were transfected with pc-DNA3.1-mTGFBR3 plasmid (GeneChem Co., Ltd, Shanghai, China) by a DNA concentration of 10 ng/ml, 20 ng/ml and 40 ng/ml respectively. pc-DNA3.1-plasmid (Shanghai GeneChem Co., Ltd) was used as an empty vector, and Fugene6 (Roche Molecular Biochemicals, Mannheim, Germany) was used as transfection reagent.
Induction of hypoxia
Cardiac fibroblasts were placed in a hypoxic chamber which was kept at 37°C for 24 h, and a constant stream of water-saturated 93% N2, 5% CO2, and 2% O2 were maintained over it as previously reported (Hong et al., 2006).
Cell viability assay
Cells were seeded in 96-well plate with the same starting cell number per well (2.5 × 104 cells/well) and allowed to attach for 24 h. After treatment, the media in each well were removed and replaced with PBS solution with 5 mg/ml MTT (Sigma–Aldrich, St. Louis, MO) and then the plate was further incubated at 37°C for 3 h. All the remaining supernatant was then removed and 100 µl of DMSO was added to each well and mixed thoroughly to dissolve the formed crystal formazan. After 10 min of incubation to ensure all crystal formazan were dissolved, the cell viability was detected by measuring the absorbance of each well at 570 nm. Relative cell viability was calculated by the absorbance percentage of the treatment group to the control group.
TUNEL assay and DAPI staining
Apoptotic cells were detected in situ by a Cell Death Detection Kit, POD analysis which was performed with a commercially available kit for immunohistochemical detection and quantification of apoptosis (programmed cell death) at single cell level, based on labeling of DNA strand breaks according to the manufacturer's instructions (Roche Molecular Biochemicals) (Gavrieli et al., 1992; Gorczyca et al., 1993a,b). Cultured mice cardiac fibroblasts were prepared on cover slips in six-well plates. Briefly, cells were fixed in 4% paraformaldehyde and pretreatment of ethanol, proteinkinase K, TritonX-100, and pepsin. After washing in PBS for three times, the samples were then treated with the TUNEL reaction mixture terminal deoxynucleotidyl transferase (TdT) and TUNEL Dilution buffer at the ratio of 1:9, and incubated in a humidified chamber for 1 h at 37°C. After washing in PBS, anti-digoxigenin peroxidase conjugate was added, and incubation continued in a humidified chamber for 30 min at room temperature. The samples were washed with PBS, stained nuclei with DAPI (Roche Molecular Biochemicals) for 5 min at room temperature, and observed by confocal microscope (Olympus, FV-100). Further, the ratio of apoptotic (TUNEL-positive) cells to total (DAPI-stained nuclei) was calculated (n = 3). Measurements were performed using Scion Image software (Beta 4.03; Scion Corporation, MD, Frederick). All the measurements were performed in a double-blind manner by two independent researchers.
Electron microscopy
Cardiac fibroblasts grow in 60 mm plates and treated as indicated were fixed in 2.5% glutaraldehyde (Paesel-Lorei, Frankfurt, Germany) in Hank's modified salt solution (HMSS), postfixed in 1% OsO4 in 0.1 M cacodylate buffer, scraped off the plastic and dehydrated in ethanol. The 70% ethanol step was saturated with uranyl acetate for contrast enhancement. Dehydration was completed in propylene oxide. The specimens were embedded in Araldite (Serva, Heidelberg, Germany). Ultrathin sections were produced on an FCR Reichert Ultracut ultramicrotome (Leica, Bensheim, Germany), mounted on pioloform-coated copper grids and contrasted with lead citrate. Specimens were analyzed and documented with an EM 10A electron microscope (Zeiss, Oberkochen, Germany).
Western blotting and immunoprecipition
The protein samples were extracted from the whole cells with the procedures as previously described (Han et al., 2001, 2004). Cells were also lysed with standard sample buffer. After boiling the samples for 5 min, the protein samples were fractionated by SDS–PAGE (10–15% polyacrylamide gels). Primary antibodies for the detection of TGF-β1 and p-Smad2/3 were rabbit polyclonal and purchased from Cell Signaling (Beverly, MA); TGFBR1, TGFBR2, Bcl-2, and Bax antibodies were rabbit polyclonal and purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Western blot bands were quantified using Odyssey v1.2 software by measuring the band intensity (area × OD) for each group and normalizing to GAPDH (anti-GAPDH antibody from Kangcheng, Shanghai, China) as an internal control. Total protein (100–200 µg) was used to analyze TGFBR1 and TGFBR2 protein interaction. About 5 µl of antibody (rabbit polyclonal, Santa Cruz Biotechnology) specific to TGFBR1 or TGFBR2 protein was added to cell lysates then incubated for 12 h at 4°C. The antibody-protein complex was precipitated together with 20 µl of resuspended volume of Protein A/G PLUS-Agarose (rabbit polyclonal, Santa Cruz Biotechnology) which binds most antibodies, then incubated at 4°C on a rotating device for overnight. When TGFBR1 or TGFBR2 protein binds to one of them, TGFBR1 or TGFBR2 protein can be identified by Western blot analysis.
Measurement of collagen content
Total collagen content was quantitatively measured by Sircol Collagen Assay (Shan et al., 2009). Briefly, cells were lysed by 0.05 M Tris buffer, pH 7.5. The samples were stired at 4°C. A transparent solution was obtained, containing the salt soluble collagen. Lysates (100 µl) were stained with 1 ml of Sircol Dye reagent, then the contents were mixed by inverting 30 min. After spinned at >10,000g for 10 min, the unbound dye solution was carefully removed and 1 ml of the Alkali reagent was added to each tube. Usually the bound dye was dissolved by Alkali within 10 min, after totally dissolved 200 µl of each sample were transfered to a 96-well plate for measuring the absorbance at 540 nm. Total collagen (µg) was calculated by a linear calibration curve generated from standards (Vitrogen 100, Angiotech Biomaterials, Palo Alto, CA) and normalized to the total protein (µg) of each cell lysate.
Caspase-3 assay
To assess the activity of Caspase-3 (Zhao et al., 2010), the cardiac fibroblasts were scraped from the plates in ice-cold PBS. The cells from each 75-cm2 plate were then lysed in 160 µl of ice-cold cell lysis buffer for 30 min. The lysates were centrifuged at 13,000g for 30 min at 4°C. The supernatant was used for subsequent assay. The fluorogenic substrates for Caspase-3 were labeled with the fluorochrome 7-amino-methyl coumarin (AMC). AMC was released from these substrates upon cleavage by Caspase-3. The enzyme activity was determined by monitoring the fluorescence produced by free AMC using a spectrofluorophotometer (SHIMADZU Corporation, RF-5301PC, Kyoto, Japan) at 360/460 nm. Caspase-3 activity was expressed in picomoles AMC liberated as per minute per microgram of protein.
Intracellular Ca2+ measurements
Cytosolic free Ca2+ was measured by confocal microscopy and flow meter analysis (Chu et al., 2006; Kim et al., 2010). After cardiac fibroblasts adherent to the cover slips of the chamber, cells were rinsed once with the standard Tyrode's solution (in mM: 126 NaCl, 5.4 KCl, 10 HEPES, 0.33 NaH2PO4·2H2O, 1.0 MgCl2·6H2O, 1.8 CaCl2 and 10 Glucose; pH was adjusted to 7.40 ± 0.05 with NaOH) and then incubated with a working solution containing Fluo-3/AM (20 mM Molecular Probes, Eugene, OR) and Pluronic F-127 (0.03%) at 37°C for 45 min. After loading, cells were washed with the standard Tyrode's solution to remove the extracellular Fluo-3/AM. The images were captured by a confocal microscope (488 nm excitation, 530 nm emission). Scanning time lasts for 3 min. TGF-β1 (10 ng/ml) were added between the 3rd and 4th scan (10 sec apart) and the images were stored in hard disks. Fluorescent intensities before (FI0) and after (FI) the TGF-β1 administration were recorded. Fold change in [Ca2+]i was represented by the ratio of FI/FI0. For [Ca2+]i measured by cytometry, resuspended cells (1 × 106/ml) were loaded with 20 mM of Fluo-3 for 30 min. Fluorescence intensities were measured in the resuspended cells by FACSCalibur (Becton Dickinson Immunocytometry Systems, San Jose, CA) to obtain baseline readings. Mean channel fluorescence intensities were calculated using FACSDiva software.
Statistical analysis
Data are expressed as mean ± SD. Statistical comparisons of difference which were performed analysis of variance (ANOVA) followed by Dunnett's method were carried out using SPSS14.0 software. Otherwise, Student's t-test was used to compare the difference between means. A two-tailed P < 0.05 was taken to indicate a statistically significant difference.
Results
Effect of TGFBR3 on survival of cardiac fibroblasts exposure to hypoxia
In order to investigate the potentially protective effect of TGFBR3 against cell viability reduction when cardiac fibroblasts were exposed to hypoxia, the cell viability was detected by MTT assays. Figure 1A showed that cell viability of hypoxia group had dramatically decreased to 49.8 ± 8.9%, compared to non-hypoxia control group (P < 0.01). However, when cardiac fibroblasts were transfected by 10 ng/ml, 20 ng/ml, or 40 ng/ml of TGFBR3 24 h before exposing to hypoxia for 24 h, the inhibited cell viability by hypoxia was reversed by TGFBR3 in a transfect dose-dependent manner (Fig. 1A). TGFBR3 (40 ng/ml) significantly increased the viability to 98.4 ± 8.6% compared to 49.8 ± 8.9% (P < 0.01) of the hypoxia; 40 ng/ml of empty vector appeared no effect on the cell viability as expected (Fig. 1A). Western blotting showed that TGFBR3 expression was increased in a dose-dependent manner among 10 ng/ml, 20 ng/ml, and 40 ng/ml TGFBR3 plasmid (Fig. 1B), but was decreased when 60 ng/ml TGFBR3 plasmid was used (data not shown here). Thus, the results suggest that cardiac fibroblasts over-expressed TGFBR3 effectively resist to the hypoxia-induced cell viability reduction. Except for GAPDH, β-actin was also used as a reference protein in case that the housekeeping proteins would be changed by hypoxia. Western blotting (Fig. 1C) proved that fibroblasts under hypoxia or control situation showed equal amount expression of GAPDH or β-actin, so GAPDH was used as a reference protein in the study.
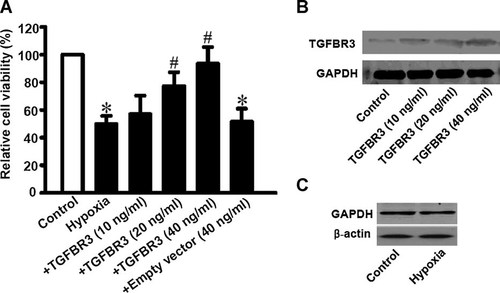
TGFBR3 promotes survival of cardiac fibroblasts exposed to hypoxia. Cardiac fibroblasts isolated from neonatal mice were transfected by TGFBR3 with a DNA concentration of 10, 20, or 40 ng/ml, respectively. After cells exposed to hypoxia for 24 h, protein lysates were submitted for Western blotting and MTT assays were done to estimate cell survival. A: TGFBR3 expression by Western blotting. B: Relative cell viability by MTT assay. Empty vector represents fibroblasts transfected with 40 ng/ml of pc-DNA3.1 plasmid. Data were obtained from six experiments for each group (n = 6). Values are mean ± SD. *P < 0.05 versus control; #P < 0.05 versus hypoxia. C: β-Actin as an alternative housekeeper protein was determined by Western blotting.
TGFBR3 prevents hypoxia-induced apoptosis in cardiac fibroblasts
To investigate whether TGFBR3 reversed hypoxia-induced cell viability reduction by preventing cardiac fibroblasts from apoptosis, TUNEL assay and electron microscopy were used to confirm the apoptosis changes. DAPI staining showed that cell number significantly decreased and nuclei condensation developed after cardiac fibroblasts exposed to hypoxia for 24 h, but it was reversed by TGFBR3 overexpression (Fig. 2A, upper part). TUNEL staining showed a dramatically increased amount of green stained cells which indicated that apoptosis occurred in cardiac fibroblasts exposed to hypoxia, but TGFBR3 significantly inhibited hypoxia-induced apoptosis (Fig. 2A, lower part). A statistical graph of the apoptosis abundance (Fig. 2B) showed that hypoxia caused an increased of apoptotic cells when compared to the normal oxygen condition (control, P < 0.05). But hypoxia induced apoptosis was prevented by TGFBR3 overexpression (P < 0.05). An empty vector did not affect the increased apoptotic cell abundance caused by hypoxia (P > 0.05).
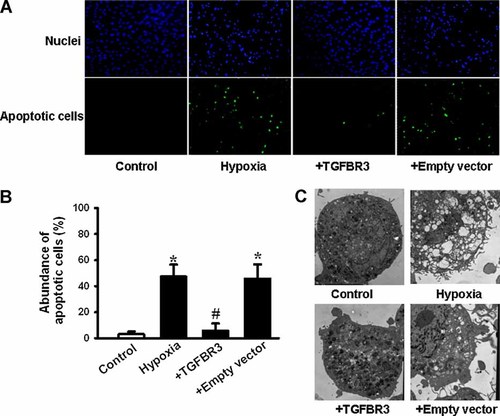
Hypoxia-induced apoptosis is prevented by over-expressed TGFBR3 in cardiac fibroblasts. Cardiac fibroblasts were transfected by an empty vector or TGFBR3 at an optimism DNA concentration of 40 ng/ml. After cells were exposed to hypoxia, TUNEL staining (A,B) and transmission electron microscope (C) were done to estimate cell apoptosis. Percentage of apoptotic cells (B) obtained from five independent experiments (n = 5). Values are mean ± SD. *P < 0.05 versus control; #P < 0.05 versus hypoxia. Magnification of transmission electron microscope was set at 8,000×. [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/jcp]
We further examined the micro-morphological changes using electron microscope at an original magnification of 8,000×, as an alternative indication of apoptosis. As shown in Figure 2C, the micro-structure of the cell under control condition appears to be normal. However, in the cells exposed to hypoxia there were considerable amount of cells that exhibited a robust change in microstructure including chromosomal DNA condensation, segmentation of the nucleus, sunken nucleus membrane, mitochondrial swelling and destruction of cristae. In contrast, changes in cellular microstructure, was at least in part prevented by transfected the cardiac fibroblasts with 40 ng/ml of TGFBR3. The results obtained from TUNEL assays and electron microscopy examinations are consistent with the notion that TGFBR3 prevents hypoxia-induced apoptosis in cardiac fibroblasts and it plays an important pre-protective role against hypoxia injury.
TGFBR3 inhibits the activation of TGF-β signaling pathway induced by hypoxia
TGF-β signaling pathway has been suggested to induce programmed cell death in a variety of cell types. In order to investigate whether TGF-β1 signaling pathway was involved in hypoxia-induced apoptosis in cultured neonatal mice cardiac fibroblasts and a regulatory effect of TGFBR3 on TGF-β signaling pathway under the hypoxia pathology situation, TGF-β1, and p-Smad2/3 expression and TGFBR1, TGFBR2 complex formation were determined. Western blotting and Co-ip analysis revealed up-regulation of TGF-β1 and p-Smad2/3 and increased complex of TGFBR1–TGFBR2 in cultured cardiac fibroblasts exposure to hypoxia compared to control cells. It indicates that hypoxia-induced apoptosis of cardiac fibroblasts might be due to activation of TGF-β signaling pathway. Interestingly, down-regulation of TGF-β1 (Fig. 3A), p-Smad2/3 (Fig. 3C) and complex of TGFBR1–TGFBR2 (Fig. 3B) were observed in mice cardiac fibroblasts when the cells were transfected with 40 ng/ml of TGFBR3, compared to hypoxia group and empty vector group.
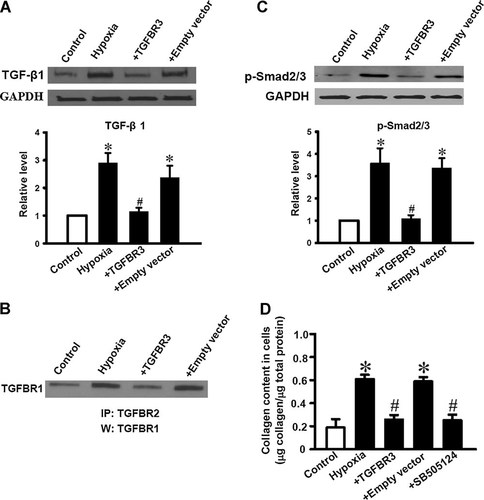
Activation of TGF-β signaling pathway and collagen production in hypoxia induced cardiac fibroblasts was reversed by TGFBR3. Control, TGFBR3 or empty vector transfected cardiac fibroblasts were exposed to hypoxia for 24 h, then TGFBR1–TGFBR2 complexes (B) were measured by Co-ip assay; TGF-β1 (A), p-Smad2/3 (C) were measured by Western blotting. TGF-β1 and p-Smad2/3 expression were normalized to GAPDH and the data are presented as relative fold (bar graphs below). Western blotting and co-ip assay shown are representative of three independent experiments (n = 3), *P < 0.05 versus control. #P < 0.05 versus hypoxia. Cardiac fibroblasts transfected with TGFBR3 or empty vector, or pretreated with a specific TGFBR1 blocker, SB505124 (10 µM for 1 h) then exposed to hypoxia for 24 h. Cells lysates were used to determine collagen production (D). Values are mean ± SD (n = 3). *P < 0.05 versus control; #P < 0.05 versus hypoxia.
Activation of TGF-β signaling not only promotes apoptosis, but also stimulates intracellular collagen production. As predicted, hypoxia resulted in collagen over secretion in neonatal mice cardiac fibroblasts. When the cells were pre-treated with a potentially small molecule inhibitor of TGF-β signaling pathway, SB-505124 (10 µM, Sigma–Aldrich) for 1 h, it blocked the overproduction of collagen induced by hypoxia in cardiac fibroblasts. TGFBR3 also abolished collagen over secretion induced by hypoxia. Taken together, TGFBR3 functions as a negative mediator of TGF-β signaling.
TGFBR3 overexpression inhibits Bcl-2, Bax proteins expression, and Caspase-3 activation in process of apoptosis induced by hypoxia
Previous studies demonstrated that Bcl-2 and Bax are not only critical molecules in the regulation of apoptosis in cardiac fibroblasts, but also downstream molecules of TGF-β1 mediated apoptotic signals (Falanga et al., 1993; Attisano and Wrana, 1996). So here we have to further investigate whether Bax and Bcl-2 are involved in the process of TGFBR3 preventing hypoxia induced apoptosis in cardiac fibroblasts. Figure 4 illustrated that hypoxia significantly increased Bax (Fig. 4A) and decreased Bcl-2 proteins expression (Fig. 4B) compared to control. However, TGFBR3 dramatically inhibited the hypoxia-induced up-regulation of Bax protein and down-regulation of Bcl-2 protein (P < 0.05). But empty vector did not affect both Bax (Fig. 4A) and Bcl-2 proteins expression under hypoxia condition (Fig. 4B).
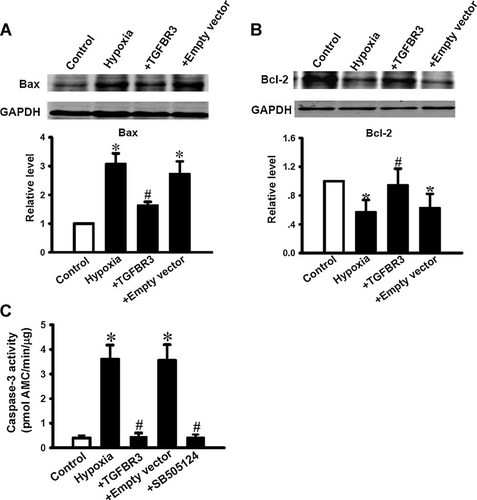
Bax Bcl-2 proteins expression changes and Caspase-3 activation in hypoxia-induced apoptotic cardiac fibroblasts were prevented by TGFBR3. Bax (A) and Bcl-2 (B) expression was normalized to GAPDH and the data are presented as relative fold (bar graphs below). Values are mean ± SD (n = 3). *P < 0.05 versus control; #P < 0.05 versus hypoxia. The activity of Caspase-3 (C) was detected by enzymatic assay. TGFBR3 over-expressed cardiac fibroblasts or fibroblasts pretreated with 10 µM SB505124 for 1 h prevented Caspase-3 activation by hypoxia. Data were obtained from five experiments for each group (n = 5) and shown as mean ± SD. *P < 0.05 versus control; #P < 0.05 versus hypoxia.
In addition, relative Caspase-3 activity was significantly increased to 3.56 ± 0.68-fold after cells exposed to hypoxia for 24 h, compared to control group (P < 0.05, Fig. 4C). Interestingly, hypoxia-induced Caspase-3 activity was dramatically attenuated by SB505124 or TGFBR3 overexpression, but not altered by empty vector. These results suggest downstream apoptotic molecules after TGF-β signaling activation by hypoxia are also regulated by TGFBR3.
TGFBR3 inhibits the elevation of [Ca2+]i induced by TGF-β1 or hypoxia
It is well known that increase of [Ca2+]i plays a key role in process of apoptosis in many cases (Deng et al., 2001). The present study showed that the expression of TGF-β1 increased in apoptotic cardiac fibroblasts induced by hypoxia (Fig. 3A). We stimulated the cardiac fibroblasts with TGF-β1 (10 ng/ml) to observe the change of [Ca2+]i by confocal microscopy. Figure 5A,B were original pictures and statistical graph from confocal microscopy. It showed that TGF-β1 triggered an elevated FI/FI0, reflecting an increase in [Ca2+]i in control cells; but TGFBR3 and SB502124 (10 µM), a specific blocker TGFBR1, dramatically abolished the effect of TGF-β1 on the elevation of [Ca2+]i. Hypoxia also induced an elevation of [Ca2+]i but was prevented by TGFBR3 overexpression (40 ng/ml) or SB502124 (10 µM) measured by flow cytometry (Fig. 5C). Together, these results suggest that the increase of TGF-β1 in apoptotic cardiac fibroblasts can induced the elevation of [Ca2+]i overload which is prevented by TGFBR3 though inhibiting the production of TGF-β1 which involved in TGF-β signaling pathway, albeit we do not have direct evidence.
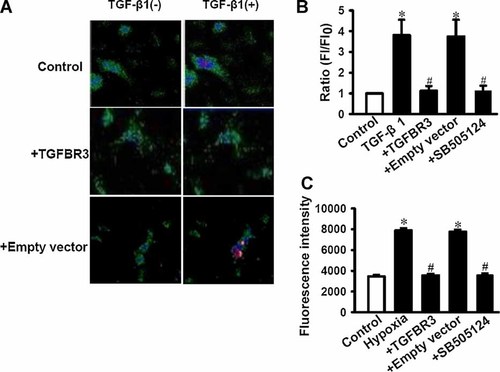
TGFBR3 inhibited [Ca2+]i induced by TGF-β1 or hypoxia in cardiac fibroblasts. Control, TGFBR3 or empty vector transfected cardiac fibroblasts in standard Tyrode's solution were treated with or without 10 ng/ml of TGF-β1. Intracellular Ca2+ was determined before (FI0) and after (FI) TGF-β1 was administered for 3 min. Confocal images (A, magnification: 200×) represent 1 min after TGF-β1 was given to the cells because TGF-β1 elicit an maximum [Ca2+]i at this time point. B: Summarized results of FI/FI0 obtained from experiments as shown in (A). Intracellular Ca2+ in cardiac fibroblasts measured by flow cytometry (C) under the condition of hypoxia for 24 h. Fluorescence intensity was calculated and showed in bar graph. Data were obtained from six experiments (n = 6) for each condition. Values are shown as mean ± SD. *P < 0.05 versus control; #P < 0.05 versus hypoxia. [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/jcp]
Discussion
Hypoxia-induced cell apoptosis in many other types of fibroblasts has been well established including embryonic fibroblasts and human peritoneal fibroblasts (Saed and Diamond, 2002). However, in heart, the vast majority of these studies have only focused on how to protect cardiomyocytes against apoptotic cell death whilst generally ignoring the other cells that make up the heart, namely the cardiac fibroblasts. In this study, we have observed that hypoxia directly induced apoptosis in cardiac fibroblasts. Our results have also revealed that TGFBR3 acting as a member of TGF-β family prevented hypoxia-induced apoptosis in cardiac fibroblasts. It is also predicted that TGFBR3 function as an inhibitor of TGF-β signaling pathway was involved in ECM production and inhibited TGF-β signals which was up-regulated in response to apoptotic stimuli. A model to account for the role of TGFBR3 as a protector of hypoxia-induced apoptotic process in cardiac fibroblasts was shown in Figure 6. Our finding provided the basis for further investigation of the protective mechanism and pathway through which hypoxia induces apoptosis in the myocardium, especially during cardiac remodeling.
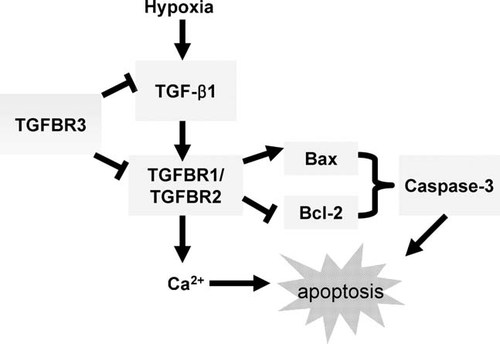
A model for the mechanism of TGFBR3 prevents hypoxia-induced apoptosis in cardiac fibroblasts. When cardiac fibroblasts are exposed to hypoxia, apoptosis occurs because of TGF-β signaling activation and subsequently apoptosis related gene expression changed (lightblue frame). TGFBR3 protects cardiac fibroblasts from apoptosis by inhibiting TGF-β1 production and TGFBR1–TGFBR2 complex formation, subsequently Bax, Bcl-2 expression changes and Caspase-3 activation are blocked and ultimately apoptosis is prevented. Besides, TGFBR3 also inhibits [Ca2+]i elevation induced by TGF-β1 or hypoxia, which makes another possible mechanism for the preventive role of TGFBR3 in hypoxia induced apoptotic cardiac fibroblasts.
In previous studies, severe hypoxia of 0.1% O2 was often used in cardiac fibroblasts (Tamamori et al., 1997; Mayorga et al., 2004), but 2% of O2 was also often used in cardiac myocytes (Baudino et al., 2006; Ronkainen et al., 2007). Indeed, cardiac fibroblasts coexist with cardiomyocytes and abnormality of cardiac fibroblasts is able to affect physiological and pathological function of cardiac myocytes (Rook et al., 1992; Baudino et al., 2006; LaFramboise et al., 2007). In this study, 2% of O2 causes apoptosis in cardiac fibroblasts, suggesting under same hypoxic condition and duration (2% of O2 for 24 h) excessive death of fibroblasts is possible to affect cardiomyocytes function, which probably mediated by increase of cytokine secretion (TGF-β1) or injury of structured couple (connexin) (Chen et al., 2000; Xie et al., 2009). The mechanisms of TGF-β1 mediated apoptosis in many other cell types has been elucidated in recent studies. In the present study, hypoxia also activated TGF-β signaling, including up-regulated TGF-β1 expression, promoted the formation of TGFBR1–TGFBR2 complex and increased p-Smad2/3 expression. It strongly indicates that TGF-β signaling is involved in hypoxia induced apoptosis in cardiac fibroblasts.
It has been demonstrated that Bax, Bcl-2, and Caspase-3 are downstream molecules of TGF-β signaling in some apoptosis cases. In cardiac fibroblasts, it is also reported that Bax, Bcl-2, and Caspase-3 are key molecules participating in apoptosis (Mockridge et al., 2000; Deveraux et al., 2001; Mayorga et al., 2004). For instance, in cardiac fibroblasts, the norepinephrine or osmotic-induced apoptosis involved the activation of Caspase-3 (Mockridge et al., 2000). Interleukin-1β was capable of increasing the Bax expression while the anti-apoptotic protein Bcl-2 remained unchanged in cultured cardiac fibroblasts (Deveraux et al., 2001). But Mayorga et al. (2004) proved that Bcl-2 is a key factor for cardiac fibroblast resistance to apoptosis induced by hypoxia. In consistent with these studies, our study showed that hypoxia induced apoptotic process involved the activation of Caspase-3, up-regulation of the pro-apoptotic gene (Bax) and down-regulation of the anti-apoptotic gene (Bcl-2) in cardiac fibroblasts. Previous studies indicate that Smad2/3 act as downstream molecules of TGF-β signaling which may regulate the expression of Bax and Bcl-2 (Schuster and Krieglstein, 2002). More interestingly, in this study, overexpression of TGFBR3 not only inhibits activation of TGF-β signals including expression of TGF-β1 and p-Smad2/3, formation of TGFBR1–TGFBR2 complex, but also recovers the balance of Bax and Bcl-2 expression and Caspase-3 activity suggesting a powerful negative regulation of TGFBR3 in TGF-β signaling involved apoptosis.
Ca2+ signals are essential for diverse cellular functions including gene expression, cell differentiation, proliferation, growth, and death. In fibroblasts, several lines of evidence suggest that Ca2+ entry is essential for cell functions induced by TGF-β1. Nesti et al. (2002, 2007) have demonstrated that TGF-β1 elicits a rapid, transient [Ca2+]i elevation which is necessary for enhancement of cell adhesion in human osteoblasts adhesion. Antagonizing TGF-β1 induced liver fibrosis by a retinoic acid derivative through regulation of ROS and Ca2+ influx (Yang et al., 2008). In human artrial fibroblasts, TGF-β1 stimulated fibrogenesis in arterial fibroblasts requires TRPM7-mediated Ca2+ signals (Burstein and Nattel, 2008). As far as cell apoptosis concerned, [Ca2+]i is also necessary for TGF-β1 mediated apoptosis in tumor cells. Andjelic et al. (1997) demonstrated [Ca2+]i elevation and cyclosporin A synergistically induced TGF-β1-mediated apoptosis in lymphocytes. All these studies suggest that [Ca2+]i elevation plays a key role in the TGF-β signaling regulated cell functions. Our study showed that TGF-β1 elicited an transient elevation of [Ca2+]i and hypoxia also induced [Ca2+]i increase. Both of these increased [Ca2+]i were abolished by a specific TGFBR1 blocker, SB505124 or TGFBR3 overexpression, which further proved a negative regulatory role of TGFBR3 in TGF-β1 induced [Ca2+]i elevation in cardiac fibroblasts.
Evidence exists from recent observations indicating that TGFBR3 exhibits antagonistic activities of TGF-β signals. For instance, Eickelberg et al. (2002) have demonstrated TGFBR3 inhibits TGF-β signaling by preventing TGFBR1–TGFBR2 complex formation. Vilchis-Landeros et al. (2001) also found recombinant soluble TGFBR3 is a potent and TGF-β neutralizing agent in vivo. More importantly, a most recent study demonstrated a synthetic peptide from TGFBR3 prevents myocardial fibrosis in spontaneously hypertensive rats (Hermida et al., 2009). However, the role of TGFBR3 in cardiac fibroblasts apoptosis through a TGF-β signaling pathway has not been fully elucidated. The present study showed that TGFBR3 regulated TGF-β signaling in the process of hypoxia induced apoptosis in cultured cardiac fibroblasts by inhibiting TGF-β1 production and TGFBR1–TGFBR2 complex formation. TGF-β1 mediated collagen secretion and multiple apoptotic molecules including Bax, Bcl-2 and Caspase-3 in cardiac fibroblasts were also reversed by TGFBR3 over-expression. More interestingly, TGF-β1 or hypoxia induced [Ca2+]i elevation was abolished by TGFBR3. Taken together, TGFBR3 prevents hypoxia induced apoptosis in cardiac fibroblasts by interacting with TGF-β1 signaling, regulating apoptotic related gene expression or through recovering intracellular second messenger, Ca2+.
In summary, our data demonstrate for the first time that TGFBR3 protects cardiac fibroblasts against hypoxia-induced apoptosis via the inhibition of TGF-β signaling pathway. TGFBR3 appears to inhibit TGF-β1 production and TGFBR1–TGFBR2 complex formation, subsequently reverse TGF-β1 induced downstream events, such as Smad2/3 phosphorylation, increased Bax/Bcl-2 ratio and Caspase-3 activation. In addition, TGFBR3 also prevents [Ca2+]i elevation induced by TGF-β1 or hypoxia in cardiac fibroblasts. In conclusion, modulation of TGFBR3 is emerging as a novel approach for the prevention or therapy of hypoxia related heart injury.




