Long-term in vitro expansion of osteoarthritic human articular chondrocytes do not alter genetic stability: A microsatellite instability analysis
Abstract
In this study, we investigated genetic damage acquisition during in vitro culture of human osteoarthritic (OA) chondrocytes to evaluate their safety for use in regenerative medicine clinical applications. In particular, we have addressed the impact of long-term in vitro culture on simple sequence repeat stability, to evaluate the involvement of the mismatch repair system (MMR) in the accumulation of genetic damage. MMR, the main post-replicative correction pathway, has a fundamental role in maintaining genomic stability and can be monitored by assessing microsatellite instability (MSI). MMR activity has been reported to decrease with age not only in vivo, but also in vitro in relationship to culture passages. OA chondrocytes from seven donors were cultured corresponding to 13–29 population doublings. Aliquots of the cells were collected and analyzed for MSI at five DNA loci (CD4, VWA, FES, TPOX, and P53) and for MMR gene expression at each subculture. Genetic stability was confirmed throughout the culture period. MMR genes demonstrated a strong coordination at the transcriptional level among the different components; expression levels were very low, in accordance with the observed genetic stability. The reduced expression of MMR genes might underline no need for increasing DNA repair control in the culture conditions tested, in which no genetic damage was evidenced. These data argue for the safety of chondrocytes for cellular therapies and are encouraging for the potential use of in vitro expanded OA chondrocytes, supporting the extension of autologous cell therapy procedures to degenerative articular diseases. J. Cell. Physiol. 226: 2579–2585, 2011. © 2010 Wiley-Liss, Inc.
Osteoarticular diseases are one of the principal fields for clinical applications of regenerative medicine due to the limited spontaneous repair capacity of articular cartilage (McNickle et al., 2008). Chondrocytes for cell-based therapies have been used long ago and autologous chondrocyte implantation (ACI; Brittberg, 2008, 2010) is a widely used approach for the repair of articular cartilage defects with good short- and long-term clinical results (Kon et al., 2010; Moseley et al., 2010; Vasiliadis et al., 2010). One of the major challenges for cartilage tissue engineering is to obtain sufficient cell numbers to fill a clinically relevant defect. Chondrocytes are limited in number, representing only 5–10% of cartilage tissue, therefore, after isolation from cartilage biopsies, they need to be expanded before their application.
The clinical use of in vitro expanded cells as therapeutic tools raises the question of their biosafety, particularly for long-term risks and for therapy efficacy. The theoretical risk of acquisition of genetic changes during in vitro growth is not completely addressed. Cells manipulated for these applications are considered by the rules of the European Union as drugs for advanced therapies and therefore subjected to very strict safety controls (Mayhew et al., 1998; Giordano et al., 2004). Cell factories usually assay for contamination by microorganisms and viability of the cell product, but with the exception of karyotyping, no screening is usually performed by specific molecular analyses of DNA damage prior to transplantation.
Regenerative potential diminishes with age and cells in culture undergo senescence possibly resembling in vivo aging. Indeed, long-term culture and aging seem to be regulated by similar mechanisms of gene expression modifications and methylation patterns (Bork et al., 2010). Replicative senescence induced by in vitro proliferation can lead to reduced telomere length, altered activity of the DNA repair systems, and altered fidelity of DNA polymerases, therefore favoring DNA damage accumulation. In addition, cells used for in vitro expansion retain their own in vivo replicative history that could interfere with in vitro replicative potential. This appears particularly relevant in osteo-cartilagineous regenerative therapy, because of the frequent advanced age of patients (Martin and Buckwalter, 2001).
Although thousands of patients have been treated with ACI, no data in the literature support the hypothesis of neoplastic transformation following culture of adult human chondrocytes. However, few data are available concerning acquisition of genetic damage during culture that could interfere with repopulation capacity and efficiency of cellular therapies. Ex vivo expanded human chondrocytes show telomere erosion equivalent to decades of aging in vivo (Parsch et al., 2002, 2004). They do not present chromosomal alterations during expansion with TGFβ and bFGF and the derived tissue-engineered cartilage shows 100% diploidy without cellular atypia (Kamil et al., 2002, 2003). No data are available concerning small molecular defects.
In this study, we investigated possible genetic damage acquisition during in vitro culture of osteoarthritic (OA) chondrocytes as surrogate biomarker to evaluate their safety for use in clinical applications. In particular, we have addressed the impact of long-term in vitro culturing on a specific compartment of genetic stability (guaranteed by several DNA repair enzymatic complexes), namely simple sequence repeat stability, in association with mismatch repair (MMR) gene expression, being the MMR system the cellular apparatus devoted to the correction of replication errors.
DNA MMR is the main post-replicative correction pathway playing a key role in maintaining genomic stability and is therefore crucial for proliferating cells. The system corrects mispairs generated during DNA replication and recombination. Defects in MMR are associated with genome-wide instability, particularly at microsatellites, defined as microsatellite instability (MSI). In humans, DNA MMR is mediated by several proteins combined in heterodimers: Msh2 bound to either Msh6 or Msh3 (MutSα and MutSβ, respectively); Mlh1 bound to either Pms2 or Pms1 (MutLα and MutLβ, respectively; Hsieh and Yamane, 2008; Li, 2008). There are several reasons to evaluate this repair system: firstly, its fundamental role in the maintenance of genome stability in actively replicating cells. Secondly, it appears that ageing leads to an accumulation of genetic instability manifesting as MSI both in vivo (Ben Yehuda et al., 2000; Coolbaugh-Murphy et al., 2005; Neri et al., 2005) and in vitro (Krichevsky et al., 2004; Neri et al., 2004; Annett et al., 2005), in association with altered MMR gene expression (Neri et al., 2007, 2008), arguing for an age-related alteration of this repair system.
In addition, the increased oxidative DNA damage observed in culture could alter MMR efficiency. Indeed, chondrocytes reside under low oxygen conditions in situ with glycolytic metabolism, but oxidative phosphorylation rises progressively during culture, with concomitant reactive oxygen species production (Heywood and Lee, 2010), thus limiting in vitro growth (Moussavi-Harami et al., 2004). Oxidative DNA damage can increase the frequency of MSI (Jackson et al., 1998) as occurs in chronic inflammatory pathologies (Brentnall et al., 1995, 1996; Ozaki et al., 2006) due to functional inactivation of MMR by non-cytotoxic levels of H2O2 (Chang et al., 2002; Lee et al., 2003).
To evaluate extreme condition of cell culture, our cellular model consist of osteoarthritic chondrocytes from aged patients. It was reported that oxidative stress may be involved in the pathogenesis of OA and OA cartilage exhibit more oxidative damage (Henrotin et al., 2005; Yudoh et al., 2005). Therefore, OA chondrocytes could already show, in vivo, an oxidative stress-mediated alteration of the MMR system further enhanced by in vitro growth.
Chondrocytes from seven OA donors were cultured and passaged corresponding to 13–29 population doublings (PD). MSI was analyzed at five microsatellite loci (CD4, VWA, FES, TPOX, and P53) at different PD. In addition, MLH1, MSH2, MSH3, MSH6, PMS1, and PMS2 MMR gene expression was analyzed by real-time RT-PCR. Our results point to the safety of adult human OA chondrocytes for therapeutic applications.
Materials and Methods
Isolation and long-term culture of osteoarthritic chondrocytes
Articular cartilage was harvested from femoral condiles and tibial plates of seven patients with OA (Kellgren–Lawrence grades 3–4) undergoing joint knee replacement surgery (3 men and 4 women; mean age: 66 ± 6.8 years, range 56–75 years). Informed consent was obtained from all patients. Chondrocytes were isolated by enzymatic procedure (Grigolo et al., 2002). Briefly, articular cartilage was minced into small pieces and submitted to sequential digestion: 30 min with 0.1% hyaluronidase (Sigma, St. Louis, MO), 1 h with 0.5% pronase (Sigma), and 45 min with 0.2% collagenase (Sigma) at 37°C in Dulbecco's modified Eagle's medium (DMEM; Sigma) with 25 mM HEPES (Sigma), 100 U/ml penicillin (Biological Industries, Beit-Haemek, Israel), 100 µg/ml streptomycin (Biological Industries, Israel), 50 µg/ml gentamicin (Flow Laboratories, Rockville, USA; complete DMEM). The isolated chondrocytes were filtered through 100 and 70 µm nylon meshes, washed and centrifuged. Cells were seeded at a density of 25,000 cells/cm2 and cultured in monolayer at 37°C and 5% CO2 in complete DMEM with 10% FCS. The flasks were monitored and the medium was changed twice a week until cells reached about 80% confluence. At this time point the cultures were designed as P0 and the cells were trypsinized using 0.25% trypsin/EDTA, seeded and propagated. At each split, aliquots of pelleted cells were stored at −80°C for DNA and RNA analysis.
Cell counting and viability were determined at every passage by eosin exclusion with a Neubauer counting chamber. PD level at each split was calculated from the cell count by using the equation NH/NI = 2PD, where NH = number of harvested cells and NI = number of seeded cells (Cristofalo et al., 1998). PD calculated at each passage was then added to the previous PD level, to yield the cumulative population doubling (CPD) level.
Microsatellite instability analysis
Total DNA was extracted from OA chondrocyte pellets (at least 100,000 cells) recovered at each split and from peripheral blood lymphocytes (PBL) by using the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) following manufacturer's instructions, then quantified by spectrophotometric determination. Five loci containing tetra- and pentanucleotide polymorphic tandem repeat sequences were genotyped by PCR with specific primers: CD4, VWA, FES, TPOX, and P53. Reaction conditions and amplification profiles were as previously described in detail (Neri et al., 2004).
A sample without template as negative control was included in all reactions. PCR products underwent electrophoresis on 10% non-denaturing polyacrylamide gels containing 5% glycerol, in TBE buffer, at 100–150 V for 16–18 h, together with allelic ladders.
Gels were stained using SYBR green dye (SYBR Green I Nucleic Acid Gel Stain, Roche, Germany) diluted 1:15,000 in TBE buffer, for 15 min at room temperature with continuous shaking. Images of the gels were acquired using the Kodak Image Station 4000MM.
Genotyping was made by side-by-side comparison with allelic ladders made up from a mixture of known alleles and genomic stability was assessed by comparing allele patterns of the different culture passages to the P0 typing (autologous control).
Mismatch repair gene expression analysis
Total cellular RNA was isolated from frozen sample pellets (200,000 cells) with Eurogold Trifast reagent (Euroclone, Milano, Italy). Total RNA (1 µg) from each sample was reverse-transcribed by using the reverse transcription-PCR Core kit (Applied Biosystems, Warrington, UK) with random examer priming and following manufacturer's instructions.
Complementary DNAs obtained from chondrocytes pelleted at different passages were analyzed by semi-quantitative real-time PCR with primers for MSH2, MSH6, MSH3, MLH1, PMS2, and PMS1 gene transcripts, as described (Neri et al., 2007). MMR gene transcripts were quantified in respect to GAPDH housekeeping gene. Real-time PCR was performed using the QuantiTect SYBR Green PCR kit (Qiagen). Specificity of RT-PCR reactions was checked with gel electrophoresis and confirmed at each run by melting curve analysis.
Amplification efficiencies were calculated according to the equation: E = 10[−1/slope] (Pfaffl, 2001) and corresponded to 1.98 for MSH2; 1.89 for MSH6; 1.92 for MSH3; 2.03 for MLH1; 2.05 for PMS2; 2.09 for PMS1; and 1.88 for GAPDH. Relative mRNA expression of MMR transcripts was obtained by normalization to GAPDH following the formula (1 + E)ΔCT, where E represents the reaction efficiency (approximated to 1 because >93% for all the transcripts) and ΔCT the difference between the GAPDH and the specific crossing point for each sample.
Statistical analysis
Results are reported as means ± standard deviation (SD) of mRNA relative expression, calculated as the ratio between the signal of the RNA of interest and the corresponding GAPDH signal.
Correlations among replication rates, days of culture, and MMR gene relative expression were determined using Spearman's correlation coefficients and/or by General linear model analysis. Statistical analysis was performed using the SPSS package for Windows (SPSS Inc., Chicago, IL).
Results
Long-term growth of osteoarthritic chondrocytes
Cells were grown as monolayers for up to 6 months (corresponding to a maximum of 18 passages), then the cultures were harvested to allow data analysis (except for samples OA2 and OA7 that were harvested earlier). Under the period of observation cells enlarged their morphology; none of the cell preparations reached the senescent phase. The seven samples (OA1–7) attained respectively 28.7, 13, 28.3, 25.9, 18.2, 29.3, and 13.6 PD, with a cell viability of 95.0 ± 3.7%, 94.8 ± 3.3%, 96.2 ± 2.9%, 95.8 ± 2.1%, 95.7 ± 2.8, 96.4 ± 2.8, 96.9 ± 1.1 (mean ± SD), respectively. The average number of CPD was 13 (range: 9.4–18.4) in about 100 days and 27 (range: 22.5–29.3) in about 255 days. Growth curves denote a moderate variability in the proliferative capacity among donors in terms of CPD accomplished in the same number of days of culture (Fig. 1). To examine long-term growth kinetics (proliferation rate) we measured PD in respect to days of culture in the seven donor samples. The proliferation rate varied over time although the cells were always splitted at the same density (about 80% confluence) and showed a decrease at advanced PD. Indeed, a negative correlation was found between cell passages and proliferation rate (PD/day) for those donor samples attaining the more advanced PD (Fig. 2).

Growth curves of osteoarthritic chondrocytes. Long-term growth curves are shown for cell preparations of seven OA donors (OA1–OA7). Cell numbers were determined at the end of every passage and cumulative population doublings (CPD) were calculated in respect to seeded cells and in relationship to the cell numbers at the first passage. Each symbol represents a single time point of trypsinization.
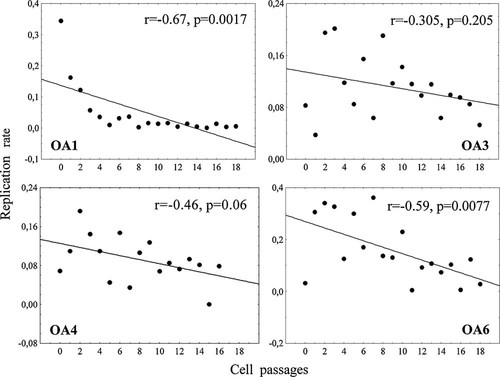
Replication rate. Scatterplot representation of replication rate (population doubling number/days of culture) and cell passage. Data relative to chondrocyte samples cultured for longer periods (OA1, OA3, OA4, and OA6) are represented. Replication rate decreases with cell passages in all donor samples even if at different degrees. Spearman's correlation coefficients and significances are indicated.
MSI analysis
Chondrocytes were characterized at different culture time points, from P0 to P18, by genetic typing at five microsatellite loci.
Allele typing for the seven donors is indicated in Table 1, as resulting from the analysis performed on DNA from cells in P0. Typing was then performed at each split until the end of in vitro propagation in order to highlight possible allele shifts due to uncorrected mispairs occurred during cell replications. As shown in Figure 3, samples were microsatellite stable and allele patterns were maintained overall the culture period for all analyzed donors, thus indicating that in this cellular model, repeated replications in vitro did not alter genetic stability at simple sequence repeats. In addition, the typing of constitutional DNA extracted from PBL from 3 out of 7 samples (OA3, OA6, and OA7) was identical to that of the corresponding OA chondrocytes (Table 1).
| Patient | CD4 | VWA | FES | TPOX | P53 |
|---|---|---|---|---|---|
| OA 1 | 4–9 | 16–17 | 10–10 | 8–11 | 8–8 |
| OA 2 | 4–5 | 16–18 | 9–10 | 8–10 | 7–9 |
| OA 3 | 5–9 | 15–18 | 10–13 | 8–9 | 7–8 |
| OA 4 | 4–4 | 15–18 | 10–12 | 8–10 | 7–8 |
| OA 5 | 5–5 | 16–16 | 11–12 | 8–11 | 6–8 |
| OA 6 | 9–9 | 15–15 | 10–11 | 11–11 | 8–9 |
| OA 7 | 4–4 | 17–17 | 10–11 | 9–11 | 7–7 |
- Typing of P0 chondrocytes from all the seven OA samples and of peripheral blood cells from OA3, OA6, and OA7 samples for the five analyzed microsatellite loci is shown. Allele nomenclature refers to the number of repeats: CD4 alleles: 4–11 repeats (88–123 bp amplicons); VWA alleles: 10–25 repeats (122–182 bp amplicons); FES alleles: 7–15 repeats (206–238 bp amplicons); TPOXalleles: 4–16 repeats (216–264 bp amplicons); and P53 alleles: 6–10 repeats (121–141 bp amplicons).
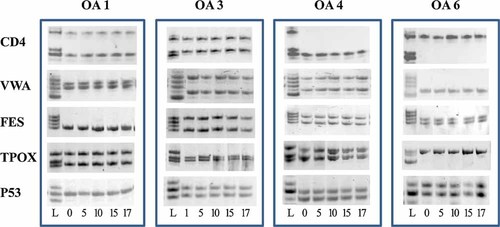
MSI analysis. Monitoring of allele patterns at CD4, VWA, FES, TPOX, and P53 microsatellite loci during in vitro expansion of osteoarthritic chondrocytes. Total DNA was extracted at different times of in vitro culture, PCR amplified, and run on acrylamide gels. Genetic stability is demonstrated by the maintainance of the same patterns overall culture period. Paradigmatic typing of 4 out of 7 OA donors (OA1, OA3, OA4, and OA6) is showed. In vitro passages are indicated under the images and allelic ladders (L) are shown on the left.
Mismatch repair gene expression
Transcript levels of the six principal MMR genes were quantified on total RNA extracted from chondrocytes at each split for all the seven OA donors by real-time semi-quantitative RT-PCR. In general, transcript levels of each gene were of the same magnitude in the different donor samples. In contrast, mean relative expression levels differed among the six genes, in accordance with previous observations (Neri et al., 2007): MLH1 and MSH6 appeared more highly expressed (mean relative expression ± SD: 0.008 ± 0.007 and 0.009 ± 0.01, respectively), while MSH2 was less expressed (0.000036 ± 0.000069), and MSH3, PMS2, and PMS1 showed slightly higher levels of expression than MSH2 (0.001 ± 0.001; 0.0002 ± 0.0005; 0.0002 ± 0.00008, respectively).
Furthermore, no gene expression modification was observed from the beginning to the end of the culture, except for the MLH1 gene (partial eta squared = 0.05, P = 0.046, by general linear model) that was found increased upon serial passaging.
Despite the very low expression observed, several positive correlations were found among the different MMR gene components. By considering separately donor samples, positive correlations were observed between heterodimer components: MLH1 versus PMS1/PMS2 and MSH2 versus MSH3/MSH6 mRNAs (the only exception being MLH1/PMS2 correlation in OA3 sample that was negative; Fig. 4).
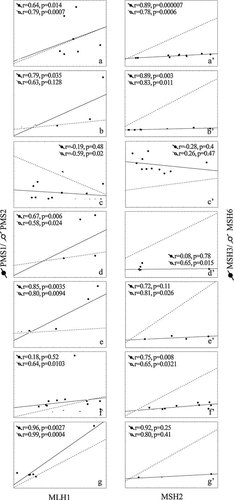
Correlations between MutSα/β and MutLα/β heterodimer components. Scatterplot representation of mRNA correlations in cultured OA chondrocytes. Correlations between MLH1 and PMS1/PMS2 mRNA (MutSα/β) and between MSH2 and MSH3/MSH6 mRNA (MutSα/β) are represented for each donor sample on the left (OA1–7: a–g parts) and on the right (OA1–7: a'–g' parts), respectively. Relative mRNA expression was determined by semi-quantitative RT-PCR at different culture passages and normalized to GAPDH. Spearman's correlation coefficients and significances are indicated.
By considering donor samples all together, almost all genes appeared positively correlated each other: MLH1, MSH3, PMS1, and PMS2 mRNAs were found positively correlated with all other mRNAs and only MSH2 and MSH6 mRNAs were not correlated each other (Table 2).
| MSH2 | MSH3 | MSH6 | PMS1 | PMS2 | |
|---|---|---|---|---|---|
| MLH1 | r = 0.365 | r = 0.307 | r = 0.492 | r = 0.441 | r = 0.214 |
| P = 0.002 | P = 0.004 | P < 0.001 | P < 0.001 | P = 0.053 | |
| MSH2 | r = 0.846 | r = 0.156 | r = 0.702 | r = 0.690 | |
| P < 0.001 | P = 0.196 | P < 0.001 | P < 0.001 | ||
| MSH3 | r = 0.208 | r = 0.692 | r = 0.487 | ||
| P = 0.052 | P < 0.001 | P < 0.001 | |||
| MSH6 | r = 0.376 | r = 0.436 | |||
| P < 0.001 | P < 0.001 | ||||
| PMS1 | r = 0.752 | ||||
| P < 0.001 |
- Correlations were calculated among the six mRNA components of the MMR system in all the seven donor samples at all analyzed PD. Spearman's coefficients and significances are indicated.
Discussion
Regenerative medicine offers great promise for osteocartilagineous medical applications and quality and safety are emerging as important issues due to in vitro processing of the cells used for these therapeutic approaches. ACI is the most widely used cell-based surgical procedure for the repair of articular cartilage defects (Brittberg, 2008, 2010). Cells used for these applications have to be expanded prior to reinfusion into the patient, but, at this time, no standardized genetic controls are usually performed although molecular alterations cannot be excluded. Indeed, non-transformed cells expanded in vitro become old during in vitro passages and their regenerative potential diminishes with age (Bonab et al., 2006). DNA damage accumulated by cultured cells could influence cell repopulation capabilities or represent a risk of failure or transformation. It is therefore really important, in our opinion, to deeply explore the kind of alterations possibly appearing.
Furthermore, it has to be taken into account that culture conditions could select some cells with occasional mutations already present in vivo. Actually, somatic mutations occurs during aging of normal cells and frequently patients with osteoarticular diseases are old persons, therefore, the amplification in vitro of a sporadic alteration acquired in vivo cannot be excluded. This highlights the need of molecular monitoring of these cells not only after culture, but also when harvested from donor tissues.
In this study, we have addressed the impact of long-term in vitro culturing of osteoarthritic human chondrocytes on genetic stability and MMR gene expression that would impact the quality control in cell therapies. We are not aware of any data concerning this particular genomic repair system in articular chondrocytes, but its relevance for actively replicating cells and its documented alteration during in vitro aging (Krichevsky et al., 2004; Neri et al., 2004; Annett et al., 2005) makes it very interesting to explore.
We performed the study on OA human articular chondrocytes as possible source for cellular therapies and we stressed the cells in extreme conditions of very long cell culture that are not usually used for tissue engineering.
Reported molecular analyses of DNA damage concerning this topic greatly differ in their sensitivity: most of the studies evaluated chromosomal alterations at the cytogenetic level and/or dose modifications (deletions or amplifications), but limited data are available on specific molecules or pathways. Overall, data available are negative for the presence of a transformation risk for cultured mature chondrocytes. No chromosomal alterations, aneuploidy or cellular atypia have been observed in cultured chondrocytes; mild atypia was only evidenced when cells were cultured in the presence of TGF-β, underlining the need of particular care when stimulating proliferation over the normal rate (Kamil et al., 2002, 2003). In accordance with these negative results, we also did not find molecular alterations in human articular OA chondrocytes cultured in vitro for up to 6 months. First, the time dependency of the growth speed up to the 18th passage was investigated. The actual age of a culture is normally recorded in PD: we expanded the cells at longest for about 29 PD (250–270 days). The proliferation rate of OA chondrocytes decreased progressively with time, as often observed in several types of normal cells during in vitro culture, but none of the samples reached the senescence phase of growth. There was a moderate variability in growth rates among donor samples. No marked changes in chondrocyte morphology were observed and the cells showed a stable microsatellite profile throughout the culture period. In addition, no modifications in respect to constitutional DNA were evidenced, pointing to the absence of genetic alterations acquired in vivo, before in vitro culture. These results greatly differ from those obtained in other cellular models of in vitro aging where a positive correlation between MSI phenotype and culture extension were observed (Krichevsky et al., 2004; Neri et al., 2004; Annett et al., 2005). In particular, an in vitro culture model of human T-cell clones indicated that MSI increased with increasing CPD in association with altered MMR gene expression (Neri et al., 2007, 2008).
As far as MMR gene mRNA expression in cultured OA chondrocytes is concerned, we found a very low expression of all the main genes of this pathway throughout the culture period. Expression levels were much lower than those observed in total RNA from normal peripheral blood mononuclear cells and T-cell clones (Neri et al., 2007). In addition, no modifications were evidenced during culture passages, except for MLH1 mRNA that increased at advanced PD. This could be a characteristic of this cellular type (indeed chondrocytes are in vivo low- or non-replicating cells) and could indicate a relatively weak involvement of these molecules in chondrocyte cellular metabolism. Our data indicate that this very low expression of MMR genes is maintained even ex vivo during in vitro culture, despite repeated expansion. The concomitant observation of genetic stability at microsatellite sequences is in accordance with these data, arguing for no need of MMR gene elevated expression due to the absence of DNA damage. No MSI was also observed in the synovium of OA patients undergoing joint replacement surgery for destructive arthritis after nine in vitro passages (Lee et al., 2003), but no data are available so far concerning OA chondrocytes.
Even if very low, MMR gene expression demonstrated a strong coordination at the transcriptional level among the different components, suggesting an expression reflecting an active system albeit at basal levels. Indeed, positive correlations were observed between heterodimer components of each donor sample: MLH1 versus PMS1/PMS2 and MSH2 versus MSH3/MSH6 mRNAs (the only exception being MLH1/PMS2 correlation in OA3 sample that was negative), in accordance with their binding in heterodimers, attending significance in several cases.
Conversely, observed basal expression levels could depend on the inactivation of the MMR system, possibly due to oxidative stress induced by in vitro culture. Conflicting results have been published concerning activity of the MMR system and oxidative stress. On one hand it seems that oxidative stress can inactivate this repair system as occurring in inflammatory chronic pathologies such as pancreatitis, ulcerative colitis, and rheumatoid arthritis (Brentnall et al., 1995, 1996; Lee et al., 2003; Ozaki et al., 2006), due to functional inactivation by non-cytotoxic levels of H2O2 (Chang et al., 2002). Further, MSI can result from reactive oxygen species-induced DNA damage (Jackson et al., 1998). On the other hand, hypoxia was shown to repress MMR gene expression and increase MSI in cancer cells (Mihaylova et al., 2003; Bindra and Glazer, 2007; Nakamura et al., 2008). Oxidative stress did not significantly alter the steady-state mRNA levels of MMR genes, but greatly reduces protein levels probably due to protein degradation and denaturation (Msh2, Mlh1, and Pms2; Lee et al., 2003). The ability of cells to proliferate when the MMR activity is inactivated by oxidative stress would presumably facilitate the introduction of additional mutations, which may explain why low-frequency MSI has been detected in chronically inflamed tissues. This seems not to be the case for OA chondrocytes, where, despite possible MMR inactivation or reduced activity, genetic damage do not appear even at the more advanced points of observation.
At present, used transplantation protocols are mainly indicated for traumatic cartilage defects in young patients and current International Cartilage Repair Society (ICRS) criteria do not recommend ACI as therapeutic option for patients with degenerative or inflammatory arthritis. Nevertheless, tissue engineering has an ongoing extension towards older individuals and degenerative defects. Preclinical studies suggest that chondrocytes from osteoarthritic patients may have the capacity to form cartilage repair tissue (Wenger et al., 2006; Stoop et al., 2007; Chung and Burdick, 2008) even if with decreased cell yields lower proliferation rates and diminished chondrogenic potential when compared to fetal and young chondrocytes. These limitations can be countered with the addition of growth factors (Barbero et al., 2004; Giannoni et al., 2005). Osteoarthritic chondrocytes ex vivo appear to be in a differentiation state similar to that of healthy chondrocytes, as indicated by ALK-1, type I collagen, type II collagen, and aggrecan gene expression. However, they contain significantly more IL-1β encoding mRNA that is downregulated upon primary culture, suggesting the importance to control the inflammatory stimuli in the articular environment to ensure the success of ACI in OA joints. This result could be obtained with anti-inflammatory or growth factor releasing scaffolds currently under study (Stoop et al., 2007). Chondrocytes from OA donors show comparable expression of cartilage markers and genes involved in matrix synthesis as healthy chondrocytes in 3D culture, thus suggesting that chondrogenic capacity is not significantly affected by OA (Dehne et al., 2009; Cavallo et al., 2010). All these data seem to indicate that osteoarthritic chondrocytes meet the prerequisites for their use in ACI and our data on their genetic stability during in vitro culture confer supplemental positive information.
Actually, applications of ACI to cartilage lesions in OA are under study. Clinical results have already been published by Hollander et al. (2006) indicating tissue regeneration after treatment with autologous chondrocytes seeded onto Hyalograft C in knee joints showing signs of OA. They found that OA did not inhibit tissue regeneration and may even enhance it. The treatment of mild degenerative and focal OA defects of the knee with second-generation autologous cartilage graft, Bioseed-C, showed good long-term clinical results (Kreuz et al., 2009). Promising results have also been observed in vivo in our laboratory in a rabbit model of OA using autologous chondrocytes (unpublished observations) and mesenchymal stem cells from the bone marrow (Grigolo et al., 2009).
Our results confirm the safety of chondrocytes for cellular therapies by evaluating specific molecular alterations and are encouraging for the potential use of in vitro expanded OA chondrocytes, supporting the extension of autologous cell therapy procedures to degenerative articular diseases.
Acknowledgements
This study was supported by Università di Bologna RFO (60% fund), Italy; Istituto Ortopedico Rizzoli (Ricerca Corrente), Bologna, Italy; and EU Project “ADIPOA” (FP7 grant no. 241719). We would like to thank Dr. Elettra Pignotti for support with statistical analysis.




