Expression and regulation of B7-H3 immunoregulatory receptor, in human mesothelial and mesothelioma cells: Immunotherapeutic implications†
Disclosure: The authors declare no conflicts of interest.
Abstract
No treatment prolongs the survival of malignant mesothelioma (MM) patients. Since MM elicits anti-tumor host's immune responses, immunotherapy represents a promising strategy for its control. Immunomodulatory antibodies against components of the B7 family of immunomodulatory molecules that regulate T cell activation are being investigated in human malignancies including MM. The expression of B7-H3, a new component of the B7 family was investigated in primary cultures of human mesothelial cells (HMC) and in MM cell lines by flow cytometry and molecular analyses, and in MM tissues by immunohistochemistry. The role of DNA hypomethylating agents in modulating levels of B7-H3 expression in MM cells was also studied. Reverse transcriptase-polymerase chain reaction (RT-PCR) demonstrated that B7-H3 mRNA was consistently detectable in mesothelial and MM cells investigated; however, real-time quantitative RT-PCR analyses showed highly heterogeneous levels of B7-H3 mRNA among investigated MM cells. The analysis of B7-H3 protein expression indicated that comparable levels of B7-H3 were expressed on both cell types. Treatment with the DNA hypomethylating agent 5-aza-2′-deoxycytidine did not significantly affect the expression of B7-H3 mRNA in MM cells. In vivo, while B7-H3 was expressed in all 13 tumor biopsies of the epithelial variant, with high levels in 54% of cases, it was rarely detectable in spindle type MM in which 1/5 biopsies weakly expressed B7-H3. These findings suggest that B7-H3 is a promising target for new immunotherapeutic strategies in MM, with particular emphasis in the epithelial variant. J. Cell. Physiol. 226: 2595–2600, 2011. © 2010 Wiley-Liss, Inc.
Malignant mesothelioma (MM) is a rapidly progressive lethal tumor with a steadily increasing incidence (Peto et al., 1999; Zona and Bruno, 2009) that occurs in three histological variants, the epithelial with a relative more favorable course, the sarcomatoid and the mixed variants, characterized by a more aggressive behavior (Rosai, 2004). Despite the unquestionable improvement in its diagnosis and the availability of new treatment strategies, the prognosis of MM patients remains dramatically poor (Ak et al., 2009); thus, the search for novel therapeutic approaches remains a priority for the management of MM patients.
In light of evidences suggesting that MM patients can spontaneously mount a tumor-specific immune response (Mutti et al., 1998; Robinson et al., 1998; Ho et al., 2005), different immunotherapeutic approaches have been experimented (Castagneto et al., 2001; Powell et al., 2006; Hassan and Ho, 2008); however, the clinical results of these studies have been quite disappointing so far. Among novel immunotherapeutic strategies great interest is being focused on the selective targeting by monoclonal antibodies (mAb) of cell surface molecules expressed on immune cells capable to modulate the anti-tumor activity of T cells. Within the targets so far identified for these immunomodulating mAb, members of the B7 family such as CTLA-4 and PD-1, are being extensively investigated in different human malignancies (Berger et al., 2008; Ridolfi and Ridolfi, 2009). Along this line, the anti-CTLA-4 mAb Tremelimumab is currently being utilized in a phase II trial in chemoresistant MM patients (http://oss-sper-clin.agenziafarmaco.it/cgi-bin/ossc_index_pub?FILE=area_PUB_new).
In spite of their use as therapeutic targets on immune cells, very limited data is available on the expression of most molecules of the B7 family on neoplastic cells. This aspect seems particularly relevant since a direct anti-tumor activity of immunomodulating mAb can also be postulated. Indeed, recent studies have focused on the analysis of the expression of B7-H3, a newly identified immunomodulatory molecule of unknown ligand, that also belongs to the B7 family (Collins et al., 2005). B7-H3 has been found to be broadly expressed at transcriptional level in several normal human tissues (Chapoval et al., 2001), while B7-H3 protein expression was limited to liver, lung, bladder, testis, prostate, breast, placenta, and lymphoid organs (Greenwald et al., 2005). As far as immune cells, B7-H3 protein is present on activated T cells, natural killer (NK) cells, and antigen-presenting cells (APC), and it is up-regulated during the differentiation of monocytes to dendritic cells (DC), and following the interaction between DC and regulatory T cells (Tregs) (Chapoval et al., 2001; Suh et al., 2003; Steinberger et al., 2004; Zhang and Allison, 2007). The different expression pattern between mRNA and protein has suggested that B7-H3 has post-transcriptional regulation(s), although the molecular mechanisms are still unknown (Hofmeyer et al., 2008). However, the presence of a CpG island in the promoter of B7-H3 (Sigalotti L, unpublished data) suggests a potential role of epigenetic mechanisms in regulating its expression, as it has already been described for other immune-related molecules (Sigalotti et al., 2007; Lal and Bromberg, 2009).
The physiologic role of B7-H3 in immune processes remains controversial, because both stimulatory and inhibitory effects have been described (Chapoval et al., 2001; Suh et al., 2003; Castriconi et al., 2004; Prasad et al., 2004; Wang et al., 2005; Fukushima et al., 2007), possibly due to presence of two or more receptor isoforms (Hashiguchi et al., 2008; Hofmeyer et al., 2008).
As far as neoplastic cells, recent data have shown that B7-H3 is involved in their proliferation, adhesion, migration, and invasiveness (Chen et al., 2008) and its expression has been described in a wide spectrum of human tumors, including lung, prostate, renal, pancreas, colorectal, ovarian and gastric carcinomas as well as in gliomas, neuroblastomas, and osteosarcomas (Castriconi et al., 2004; Sun et al., 2006, 2010; Wu et al., 2006; Roth et al., 2007; Zang et al., 2007, 2010; Chen et al., 2008; Crispen et al., 2008; Gregorio et al., 2008; Loos et al., 2009).
In view of its potential use as target of novel immunomodulating mAb, in this study we investigated the expression of B7-H3 in human normal and transformed mesothelial cells. In addition, the role of the DNA hypomethylating agent 5-aza-2′-deoxycytidine (5-AZA-CdR) in modulating the constitutive levels of B7-H3 expression in MM was investigated.
Our results demonstrate that B7-H3 is differentially expressed in vitro and in vivo in MM, thus suggesting for its promising role in setting up innovative immunotherapeutic strategies in MM patients.
Materials and Methods
Cell lines, 5-AZA-CdR treatment
The epithelioid MM cell lines MES-1, MES-2, MMCA, MES-CM-98, MPP-89, REN, MMB, the sarcomatoid MM cell lines MES-MM-98, MES-OC-99, and primary human mesothelial cells (HMC) from pleural effusion of patients with heart failure, were grown as previously described (Orengo et al., 1999). Treatment with 5-AZA-CdR (Sigma Chemical Co., St. Louis, MO) at a concentration of 1 µM every 12 h for 2 days was performed as described (Sigalotti et al., 2002).
Peripheral blood mononuclear cell (PBMC) from healthy subjects and the SKNBE neuroblastoma cell line (kindly provided by Dr. Chiara Castelli, Istituto Nazionale dei Tumori, Milan, Italy) were utilized as B7-H3-negative or B7-H3-positive controls, respectively.
RNA extraction, qualitative and quantitative RT-PCR analysis
Total RNA was extracted from cultures of MM or HMC cells using TRIzol reagent (Invitrogen, Milan, Italy), according to the manufacturer's instructions, and digested with RNAse free DNAse (Roche Diagnostics, Milan, Italy) to remove contaminating genomic DNA. Synthesis of cDNA was performed on 1 µg total RNA using MMLV reverse transcriptase (Invitrogen) and random hexamer primers (Promega, Milan, Italy), as previously described (Calabrò et al., 2005). PCR analyses of B7-H3 expression were performed on 5 µl complementary DNA (cDNA) by 35 cycles of 94°C for 30 sec, 58°C for 30 sec, and 72°C for 1 min, using 50 pmol of sense (CGTGTGCTGGAGAAAGATCA) and antisense (AGAAGAGGGTGGTGATGTGG) primer under procedures previously described (Coral et al., 1999). The integrity of RNA and cDNA was confirmed by amplification of all cDNA samples with β-actin-specific primers as previously described (Coral et al., 1999). Ten microliters of each RT-PCR sample were run on a 2% agarose gel and observed by ethidium bromide staining.
Real-time quantitative RT-PCR analyses were performed essentially as described (Calabrò et al., 2005). Briefly, TaqMan quantitative PCR reactions were performed on 20 ng retrotranscribed total RNA in a final volume of 25 µl 1× TaqMan Universal Master Mix (Applied Biosystems, Milan, Italy). TaqMan primers/probe sets were as follows: β-actin forward, CGAGCGCGGCTACAGCTT; β-actin reverse, CCTTAATGTCACGCACGATT; β-actin probe, FAM-ACCACCACGGCCGAGCGG-BHQ1; B7-H3 forward, AGCTGTGAGGAGGAGAATGC, B7H3 reverse, TGCTGTCAGAGTGTTTCAGAGG; B7-H3 probe was the #7 of the Roche's Universal ProbeLibrary (Roche Diagnostics). Real-time measurement of fluorescent signals was performed utilizing the ABI PRISM 7000 Sequence Detection System (Applied Biosystems) and the copy number of cDNA molecules of B7-H3, and of the reference gene β-actin, was established in each sample by extrapolation of a standard curve containing serial dilutions of the B7-H3 or β-actin DNA amplicon, respectively. The number of B7-H3 cDNA molecules in each sample was then normalized to the number of cDNA molecules of β-actin.
Flow cytometry analysis
Cell surface expression of B7-H3 was determined by indirect immunofluorescence staining and flow cytometric analysis utilizing the FACscan flow cytometry equipped with the CELLQuest analysis software (Becton Dickinson, BD Bioscience, San Jose, CA), as previously described (Coral et al., 1999). Briefly, cells were incubated with 5 µg/ml anti-B7-H3 mAb (R&D System, Abingdom, UK and Europe) for 30 min at 4°C. After washing thoroughly with PBS, cells were stained with phycoerythrin (PE)-labeled anti-mouse antibodies (DAKO Corp., Carpinteria, CA) for 30 min at 4°C. Non specific staining was determined by using an isotype-matched mouse IgG. The results were expressed as values of mean fluorescence intensity (MFI) and percentage of stained cells on a logarithmic scale. A sample was classified as positive when more than 10% of cells were stained with the relevant mAb, and the value of MFI was higher than 15. Values of MFI obtained with isotype-matched mouse IgG were lower than 15 and percentage of positive cells was lower than 10% on all cell lines tested.
Immunohistochemistry
A tissue microarray of 23 MM (1.5 mm core in duplicate) of different histotype (13 epithelial, 5 mixed, 5 sarcomatoid), and three normal pleural tissues was purchased from US Biomax, Inc. (Rockville, MD). No data of disease stage were available. Four full sections of 2 epithelial and 2 sarcomatoid MM were kindly provided by the Pathology Department of the “Regina Elena” National Cancer Institute (RENCI), Rome. Tissues belonged to the archival bank of RENCI and were collected and used according to the RENCI Ethic Committee guidelines (latest guideline, no. 4, March 1st, 2006). Antigen retrieving was performed using 1 mmol/L EDTA (pH 8.0) at 96°C for 45 min. Array and sections were incubated with goat anti-B7-H3 antibody (R&D System) at a concentration of 10 µg/ml over-night at room temperature and further processed using the EnVision Peroxidase Detection System with biotinylated secondary antibody (Vector “Universal Reagent”, Burlingame, CA). Specimens were than counterstained with Mayer's hematoxylin solution for nuclear stain. Cores which were not stained by the anti-B7-H3 antibody served as negative controls. Immunohistochemical findings were scored as follows: negative, when the biopsy displayed no stain or dubious weak reactivity, 1+ when the stain was homogenous of moderate intensity, 2+ when the stain was homogenously intense. Scores were done by two independent readers.
Statistical analysis
Statistical significance was evaluated by the Student's t-test for paired data.
Results
Analysis of B7-H3 mRNA expression on HMC and in MM cell lines
Expression of B7-H3 on HMC and in MM cell lines was initially assayed at mRNA level by RT-PCR analyses. Results showed an homogeneous expression of B7-H3 mRNA among MM cell lines investigated (Fig. 1), which was also detected in HMC (Fig. 1). These information were further extended by quantitative real-time RT-PCR analysis in order to establish if levels of B7-H3 varied among the cells under analysis. Results showed highly heterogeneous levels of constitutive B7-H3 expression, ranging from 8.2 × 10−3 B7-H3/β-actin molecules in MPP-89 to 7.8 × 10−2 B7-H3/β-actin molecules in MES-2 cells (9.6-fold difference, Fig. 2). Noteworthy, HMC constitutively expressed B7-H3 mRNA and at levels comparable to those observed in the majority of MM cell lines investigated (Fig. 2).
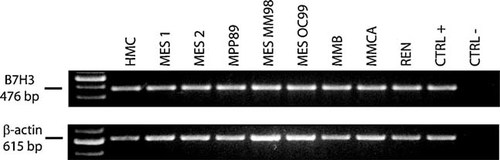
RT-PCR analysis of B7-H3 expression in HMC and in MM cell lines. Total RNA was extracted from HMC and MM cells, and from B7-H3-negative healthy donor PBMC (CTRL −) and B7-H3-positive SKNBE neuroblastoma cells (CTRL +). RT-PCR analysis was performed using B7-H3- or β-actin-specific primer pairs. PCR products were then separated on a 2% agarose gel and visualized by ethidium bromide staining.
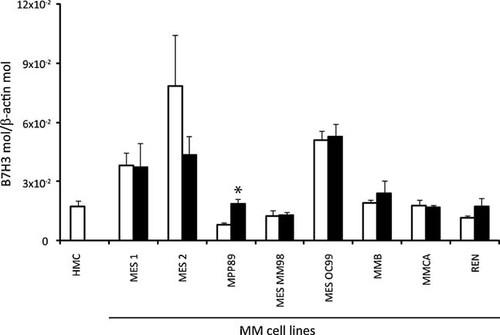
Quantitative RT-PCR analysis of B7-H3 expression on HMC and in MM cell lines. Total RNA was extracted from four independent cultures of normal mesothelial cells and 8 MM cell lines, either untreated (empty squares) or treated (black squares) with 5-AZA-CdR, and subjected to reverse transcription. The obtained cDNA was utilized in quantitative real-time PCR analysis using B7-H3- and β-actin-specific TaqMan primer/probe sets. B7-H3 expression was normalized to the expression of the housekeeping gene β-actin, and data are reported as mean value + SD of B7-H3 molecules/β-actin molecules obtained in three independent experiments. *Statistically significant (P < 0.05).
To evaluate the potential epigenetic regulation of B7-H3 in MM, suggested by the presence of a CpG island in its promoter, we investigated the effect of treatment with the DNA hypomethylating agent 5-AZA-CdR in MM cell lines. Except for a 2.3-fold up-regulation in MPP-89 cells, 5-AZA-CdR did not significantly modulate the constitutive expression of B7-H3 in MM cell lines investigated (Fig. 2).
Analysis of B7-H3 protein expression on HMC and in MM cell lines
Expression of B7-H3 on HMC and in MM cell lines was assessed in two independent experiments by indirect immunofluoresence and flow cytometry analysis. Representative results, expressed as percentage of positive cells (Table 1) and MFI (Table 1 and Fig. 3), demonstrated that B7-H3 was consistently expressed on normal and neoplastic mesothelial cells investigated. Levels of B7-H3 expression were rather comparable between mesohelial and MM cells, except for the cell line MES-2 (MFI = 1,353), deriving from a tumor of epithelial histotype, and MES-OC-99 (MFI = 658), originating from a sarcomatous malignancy, which showed the highest levels of B7-H3 expression.
| Cells | Histologic type | % positive cells | MFI |
|---|---|---|---|
| PBMC | 0.9 | 3 | |
| SKNBE | 100 | 1,059 | |
| Mesotelial | |||
| HMC | 99 | 272 | |
| MM | |||
| MES-MM-98 | Sarcomatoid | 99 | 149 |
| MES-1 | Epithelial | 99 | 312 |
| MES-2 | Epithelial | 99 | 1,353 |
| MES-OC-99 | Sarcomatoid | 99 | 658 |
| MMCA | Epithelial | 100 | 178 |
| MES-CM-98 | Epithelial | 100 | 268 |
| MPP-89 | Epithelial | 99 | 251 |
| REN | Epithelial | 98 | 202 |
| MMB | Epithelial | 99 | 191 |
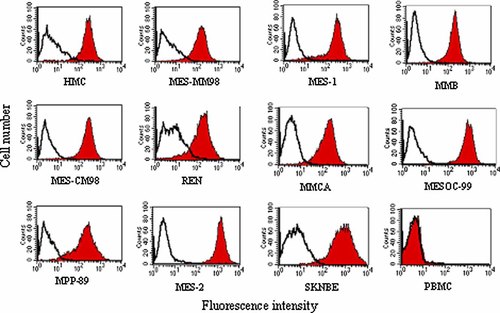
Indirect immunofluorescence analysis from one representative experiment of B7-H3 expression on HMC and in MM cell lines. B7-H3 expression on HMC and in MM cell lines was analyzed by flow cytometry using anti-B7-H3 mAb (red histograms) as well as isotype control mAb IgG (empty histograms). PBMC and SKNBE cell line were used as negative and positive controls, respectively.
Phenotypic analysis of B7-H3 expression in MM specimens
To investigate the expression of B7-H3 in vivo, we employed an indirect immunoperoxidase staining of a panel of 23 MM lesions of different histotype, arranged into a tissue microarray.
The results demonstrated that normal appearing pleural cells and those from two specimens classified as benign mesothelioma, displayed only a weak expression of B7-H3 (Fig. 4a) as compared to malignant mesothelial cells (Fig. 4b). Interestingly, B7-H3 although at different levels was expressed in all biopsies of the epithelial tumor variant (Fig. 4b), but not in the sarcomatoid histotype (Fig. 4c). In the mixed tumor variants the stain was restricted to the epithelial component (Fig. 4c inset. Asterisk: epithelial area. Triangle: sarcomatoid area). Furthermore, high scores (2+) of B7-H3 intensity expression were frequently (54%) observed only in tumors of epithelial morphology (Fig. 5). In contrast, only one out of five sarcomatoid tumors was B7-H3 positive and was characterized by a moderate staining intensity (1+) (Fig. 5). This findings were also observed in the tumor full sections analyzed. The staining pattern of the positive tumors which was very homogeneous appeared cytoplasmic, but in six out of seven lesions of epithelial variant that overexpressed B7-H3 a cell membrane staining was also observed in limited areas. No B7-H3 staining was found in the tumor vessels and stroma. B7-H3 expression was unrelated to age and sex and site of origin of investigated lesions. Notably, all cases tested lacked lymphatic cell infiltrates independently of their histotype.
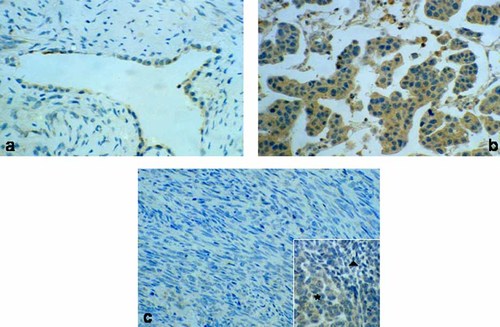
Tissue microarray analysis of B7-H3 expression in MM tissues. While low expression of B7-H3 is detected in mesothelial cells of a benign mesothelioma (a), high expression is present in a case of epithelioid MM (b). No B7-H3 expression is observed in a sarcomatoid type MM (c). Only the epithelial component of a mixed type MM displays B7-H3 expression (c, inset: asterisk) while the sarcomatoid area is negative (c, inset: triangle). Original magnification a, b and inset X250, c X160.
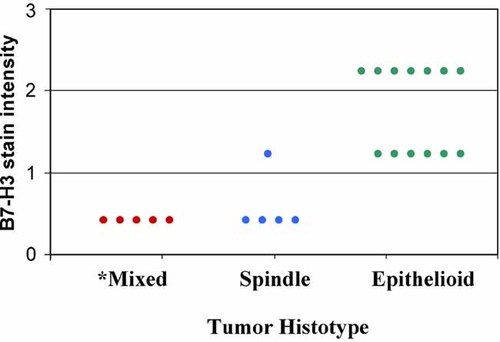
Tissue microarray analysis of B7-H3 expression among MM variants. B7-H3 was expressed in all 13 tumor specimens of epithelial morphology, most of them (7/13) with high scores (2+). In contrast, only one out of five sarcomatoid tumors was B7-H3 positive with a moderate staining intensity (1+). No B7-H3 expression was observed in 5 mixed tumors (sarcomatoid areas). *Referred to the spindle component.
Discussion
The identification of an increasing number of immunologic key checkpoints regulating T cell activation and suppression is stimulating a growing interest because of their potential therapeutic exploitation. Different cell membrane molecules expressed on immune cells regulate the activity of specific checkpoints and among those so far identified, members of B7 family including CTLA-4 and PD-1 are currently under active clinical investigation (Berger et al., 2008; Ridolfi and Ridolfi, 2009).
B7-H3 is a newly identified regulatory checkpoint of T cell signaling that is expressed on immune cells, thus representing an additional therapeutic target for immunomodulating mAb. However, selected tumor histotypes have also been showed to express B7-H3 (Castriconi et al., 2004; Sun et al., 2006, 2010; Wu et al., 2006; Roth et al., 2007; Zang et al., 2007, 2010; Chen et al., 2008; Crispen et al., 2008; Gregorio et al., 2008; Loos et al., 2009) suggesting for a potential dual role of anti-B7-H3 therapeutic mAb. Based on these comprehensive evidences, B7-H3 will likely represent an additional candidate for novel immunotherapeutic approaches with specific emphasis on B7-H3-positive human malignancies where a direct functional effect of the therapeutic agent can also be expected.
In the present study we have evaluated in vitro and in vivo the expression of B7-H3 in human mesothelial cells. Our in vitro findings provided sound evidences that B7-H3 is constitutively expressed by normal and neoplastic cells of the mesothelial lineage. These in vitro results were further corroborated by the in vivo data. In fact, B7-H3 expression was clearly identified both in mesothelial benign and malignant cells in the tissue arrays utilized. Altogether these evidences demonstrate that the B7-H3 phenotype observed in vitro is not the result of culture conditions, and that it does not represent a marker of neoplastic transformation of mesothelial cells.
The discordant patterns of B7-H3 expression described at mRNA and protein level (Hofmeyer et al., 2008), have led to the suggestion that a post-transcriptional regulation of B7-H3 may occur, even though the molecular mechanism(s) regulating B7-H3 expression are still unknown. Due to the presence of a CpG island in its promoter, in this study we explored the potential role of DNA hypomethylation in the transcriptional regulation of B7-H3 expression. Treatment with 5-AZA-CdR did not significantly affect the constitutive expression of B7-H3 by the investigated MM cells, suggesting that epigenetic events should not represent a major mechanism accounting for the expression of B7-H3 in MM. Noteworthy, as recently reported for human melanoma cells (Fonsatti et al., 2007), treatment with 5-AZA-CdR significantly upregulated the constitutive expression of HLA-class I antigens, intercellular adhesion molecule-1 (ICAM-1) and CD58 in MM cells investigated (Calabrò L, unpublished data). These findings are quite interesting, since these molecules are crucial for the immunogenicity and efficient recognition of neoplastic cells by the host's immune system.
As previously reported in prostate, renal and colorectal carcinoma (Roth et al., 2007; Crispen et al., 2008; Sun et al., 2010), the tissue arrays utilized in this study provided evidences that levels of B7-H3 expression are increased in MM cells as compared to mesothelial cells. This suggests that B7-H3 might play a role in the progression of MM. However, a high expression of B7-H3 in tumor specimens was found mostly restricted to the epithelial type of MM and to the epithelial component of the mixed tumors, a feature not fully appreciated by our in vitro testing. Although we cannot exclude that in sarcomatoid type tumors low concentrations of B7-H3 are present in vivo below the sensitivity of our testing method, the expression of B7-H3 in the two MM cell variants appears to be differently regulated. This different level of expression was also confirmed in a small but well defined number of mesothelioma analyzed as full sections. Whether this occurs constitutively or under the influence of tumor cell related and/or microenvironment-derived factor(s) (Chapoval et al., 2001), which may operate through autocrine and paracrine mechanisms, is at present unclear. Whatever the biological basis of this difference, B7-H3 is likely to play a role of undefined functional relevance predominantly in the epithelial MM tumors. Along this line the correlation between expression of B7-H3 and its functional role is far from being fully established; in fact, both immune stimulatory and inhibitory effects have been described for B7-H3 in gastric, pancreas, renal, lung, prostate, ovarian, colorectal carcinomas, and in neuroblastoma (Castriconi et al., 2004; Sun et al., 2006, 2010; Wu et al., 2006; Roth et al., 2007; Zang et al., 2007, 2010; Crispen et al., 2008; Gregorio et al., 2008; Chavin et al., 2009; Loos et al., 2009). Nevertheless, our findings suggest that B7-H3 expression may variably influence the biology of MM depending on its histotype. Moreover, the lack of lymphocytic infiltrates in all MM biopsies examined, regardless of their specific histotype, suggests that the functional role of B7-H3 in MM cells is possibly independent from their interaction(s) with immune cells.
A compelling evidence for abrogating inhibitory signaling by T cells to enhance anti-tumor immunity has been well-established with the experience of immunomodulating mAb directed against different immune regulatory molecules, such as CTLA-4 and PD-1. Concerning B7-H3, we are still in the infancy of its clinical exploitation; however, our data strongly suggest that it may represent an additional therapeutic target to develop immunotherapeutic protocols for MM patients.
Acknowledgements
This work was supported in part by grants from: Fondazione Buzzi Unicem Onlus, Associazione Italiana per la Ricerca sul Cancro (AIRC) (IG6038 to M.M.), (MFAG9195 to L.S.) and PGN, Istituto Toscano Tumori and Istituto Superiore di Sanità—Alleanza Contro il Cancro.




