Analysis of p53 and NF-κB signaling in modulating the cardiomyocyte fate during hypertrophy
Abstract
Cardiac hypertrophy leading to eventual heart failure is the most common cause of mortality throughout the world. The triggering mechanisms for cardiac hypertrophy are not clear but both apoptosis and cell proliferation have been reported in sections of failing hearts. In this study, we utilized both angiotensin II (AngII) treatment of cardiomyocytes and aortic ligation in rats (Rattus norvegicus, Wistar strain) for induction of hypertrophy to understand the cellular factors responsible for activation of apoptotic or anti-apoptotic pathway. Hypertrophy markers (ANF, β-MHC), apoptotic proteins (Bax, Bad, Fas, p53, caspase-3, PARP), and anti-apoptotic or cell proliferation marker proteins (Bcl2, NF-κB, Ki-67) were induced significantly during hypertrophy, both in vitro as well as in vivo. Co-localization of both active caspase-3 and Ki-67 was observed in hypertrophied myocytes. p53 and NF-κBp65 binding to co-activator p300 was also increased in AngII treated myocytes. Inhibition of p53 resulted in downregulation of apoptosis, NF-κB activation, and NF-κB–p300 binding; however, NF-κB inhibition did not inhibit apoptosis or p53–p300 binding. Blocking of either p53 or NF-κB by specific inhibitors resulted in decrease in cell proliferation and hypertrophy markers, suggesting that p53 initially binds to p300 and then this complex recruits NF-κB. Thus, these results indicate the crucial role of p53 in regulating both apoptotic and cell proliferation during hypertrophy. J. Cell. Physiol. 226: 2543–2554, 2011. © 2010 Wiley-Liss, Inc.
Cardiac hypertrophy leading to heart failure is one of the common causes of mortality since the past decade. The triggering mechanism for progression of cardiac hypertrophy to heart failure is still not clear, but both in vivo and in vitro studies have shown involvement of multiple gene networks and factors other than blood pressure during the initiation of cardiac hypertrophy (Sarkar et al., 2004; Schaub et al., 2006). Programmed cell death (PCD), that is, apoptosis has been suggested as a major contributor to heart failure, as myocyte apoptosis has been observed during acute cardiac dysfunction (Olivetti et al., 1997; Sarkar et al., 2004a). On the contrary, Beltrami et al. (2001) showed myocyte replication in failing human heart which might compensate for loss of myocyte during hypertrophy. Activation of cellular pro-survival marker such as NF-κB and apoptotic marker p53 during cardiac hypertrophy is also observed (Gupta et al., 2002; Haupt et al., 2003). Thus myocyte proliferation may be a compensatory mechanism which may be activated in heart upon demand and this might challenge the dogma of the heart being a post-mitotic organ. Several in vivo studies have suggested that AngII, an octa-peptide, acts as a growth-promoting factor in myocytes in an autocrine or paracrine manner and also activates a variety of signaling molecules to induce various genes that promote cardiac hypertrophy (Pathak et al., 2001). Tissue homeostasis depends on proper relationships between cell proliferation, differentiation, and cell death. Any imbalance between these processes might result in compromised cardiac function (Sarkar et al., 2004a).
In this study, AngII was used for analyzing induction of hypertrophy in both neonatal and adult cardiac myocytes in vitro. In addition, we have generated an in vivo hypertrophic model by aortic coarctation in rats to compare it with AngII induced hypertrophy. Differential expression pattern of apoptotic markers and cell cycle regulating proteins were analyzed to evaluate crosstalk and balance between cell death and regeneration during hypertrophy. Induction and interactions between diverse factors such as stress induced p53, a key regulator of cellular apoptosis and NF-κB, the cell survival factor, in response to AngII treatment in myocytes, has been shown in this study. This is the first report showing simultaneous activation and crosstalk between p53 and NF-κB which is mediated through p300 in cardiomyocytes. This might play a significant role in activating either cell death or regeneration process in the hypertrophied cardiomyocytes leading to severely compromised cardiac function.
Materials and Methods
Animal used
Wistar rats used in this study were obtained from National Institute of Nutrition, Hyderabad, AP, India. The investigation conforms with the Guidelines for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and was also approved by the Institutional Animal Ethics Committee, University of Calcutta (Registration # 885/ac/05/CPCSEA), registered under “Committee for the Purpose of Control and Supervision of Experiments on Laboratory Animals” (CPCSEA), Ministry of Environment and Forests, Government of India.
Isolation of neonatal and adult cardiomyocytes
Neonatal myocytes were isolated from hearts of 2- to 3-day-old rat pups and cultured on laminin-coated (Sigma–Aldrich, St. Louis, MO) plates and cover glasses placed in standard six-well plates following the procedure described previously (Sil and Sen, 1997). On the third day, myocytes were incubated in serum starved DMEM for 12 h, before experimentation. Approximately 90% pure isolated cardiomyocytes were confirmed by staining with α-actinin antibody. Adult myocytes were isolated from 24 weeks old rats on laminin-coated plates following the procedure of Sarkar et al. (2004a) by collagenase dispersion method. In brief, dissected hearts were digested with collagenase (2 mg/ml) for 28 min, with gradual enhancement of CaCl2. After digestion with collagenase, the ventricles were separated from the atria, triturated for 30 sec and subsequently filtered through cheesecloth. The filtrate was centrifuged at 400 rpm for 2 min, the supernatant was removed, and the pellet was resuspended in 4% bovine serum albumin solution and observed under a phase-contrast microscope. Preparations with ∼70% rod-shaped cells were used for experimental purposes. Myocyte dimensions were quantitated by image scanning using the Image Pro Plus software program.
Treatment of neonatal and adult rat cardiomyocytes
Neonatal and adult rat cardiomyocytes were incubated for 24 h at 37°C in the absence (control) or presence (treated) of 10−8 mol/L [Sar1]AngII (BACHEM, Torrance, CA). AngII was replenished every 6 h throughout the incubation period. Pifithrin-α [PFT-α, 50 µM] (Biomol, Plymouth Meeting, PA), a p53 blocker (Komarov et al., 1999) was added to serum starved cardiomyocytes before treating cells with AngII. Neonatal cardiomyocytes were transfected with either p53 specific siRNA (Ambion Silencer Select siRNA#S220498 and S128540) to block the expression of p53 or negative control siRNA (Ambion #4390844) as per manufacturer's protocol. To block NF-κB activity, IκBα dominant negative (IκBα DN) construct (BD Biosciences, San Diego, CA) was transfected in neonatal cardiomyocytes using lipofectamine 2000 (Invitrogen, Carlsbad, CA) followed by AngII treatment as described above. Adult cardiomyocytes were infected with adenoviral vectors containing IκBα DN construct (Kind gift from Dr. Mamta Chawla Sarkar) after 1 h of plating, in serum starved condition for 24 h following Das et al. (2006). The cells were routinely infected with the viruses at a concentration of 1 × 103 particles/cell. Cells were also pre-treated with SN50 (10 µM) (Calbiochem, San Diego, CA), a specific NF-κB inhibitor, prior to AngII treatment, to confirm results obtained by IκBα DN transfection.
Cell viability assay (MTS assay)
Cell viability in the presence or absence of Pifithrin-α and SN50 was determined by Cell titer 96® Aqueous One Solution Cell Proliferation assay kit (Promega, Madison, WI) as per manufacturer's protocol.
Aortic coarctation
Twenty-four weeks old male rats (n = 10), weighing 285 ± 20 g, were used to generate left ventricular hypertrophy, by ligating their right renal artery with slight modifications (Rojo-Ortega and Genest, 1968). In brief, animals were anesthetized with a mixture of ketamine (80 mg/kg) and xylazine (12 mg/kg) intraperitoneally. After opening the abdomen, a stainless wire (outer diameter, 0.34 mm) was placed along the right renal artery and then tied around the artery by silk suture. The wire was then taken out from under the knot, leaving the artery constricted to the external diameter of the stainless wire. The sham-operated controls underwent the similar procedures without actual ligation of the artery. The abdominal wall and the skin were closed with silk suture. Animals were maintained in a climate-controlled, light-regulated space with 12 h light and dark cycles in the departmental animal facility of the University of Calcutta. Animals were used on 14th day after surgery. Hypertrophy was measured by the heart weight (HW) to body weight (BW) ratio (Sen et al., 1974).
siRNA delivery in vivo model
siRNAs for NF-κB and p53 were obtained from Ambion (Austin, TX) (In Vivo Direct siRNAs; s159516/s159517 for rat v-rel/NFkB and s220498/s142460 for rat TP53) and the siRNAs in 1× PBS were injected in ventricles of ligated rats (n = 5) at a concentration of 10 nmol following manufacturer's protocol for last 10 days of experimentation, before sacrifice.
Determination of cardiac function
Two-dimensional echocardiography was performed to determine cardiac function in vivo. Lightly sedated aortic ligated and sham operated rats were evaluated using the M-mode views on a transthoracic study, measuring left ventricular systolic (LVSD) and diastolic dimensions (LVDD) and fractional shortening (% FS). Data were correlated with timing of the QRS complex. Digitized images were obtained using an ultrasound system (Vivid S5 system, GE Healthcare, Milwaukee, WI).
Reverse transcriptase-PCR (RT-PCR)
Total RNA was isolated from heart tissues and isolated cardiomyocytes (both neonatal and adult) using TRIzol reagent (Invitrogen). Reverse transcription was done using Cloned AMV First-Strand cDNA Synthesis Kit (Invitrogen). Expression of hypertrophy marker genes was studied using forward (F) and reverse (R) primers (IDT, San Diego, CA) [ANF (F5′CACCAAGGGCTTCTTCCTC-3′; R5′CGAGAGCACCTCCATCTCTC-3′), β-MHC (F5′CCATACAGAGGACGGAGGAG-3′; R5′GCCTCCTTCTGGGAAGACTC-3′)]. GAPDH gene was amplified as an internal control (F5′GGGGTGATGCTGGTGCTGAG-3′; R5′GATGCAGG GATGATG TTCTG-3′).
Dual luciferase NF-κB reporter assay
Neonatal rat cardiomyocytes were transfected with NF-κB-luc and pRL-TK (Promega) using Lipofectamine 2000 (Invitrogen). A calcium phosphate procedure was used to transfect adult cardiomyocytes with luciferase reporter plasmids (Beate et al., 2002). After 16 h, cells were treated with AngII alone and with inhibitors and luciferase activity (Promega) was measured after 16 h using a luminometer (Varioskan Multimode Reader, ThermoFisher, Waltham, MA). NF-κB-luciferase activity was normalized with Renilla luciferase (Elewaut et al., 1999).
Western blotting
Protein extracts were prepared from cells as well as ventricular tissue following the procedure described previously (Sarkar et al., 2004a). Total protein extract (20 µg) was fractionated by SDS–PAGE and transferred to PVDF+ membrane, followed by incubation with monoclonal antibodies to Bax, Bcl2 (BD Pharmingen, San Diego, CA), polyclonal antibodies to caspase-3 (BD Pharmingen), p21, p27, NF-κBp65, cyclin B1, cyclin D1, cyclin D3, Cdk2, Cdk4, Cdk6 (Cell Signaling, Danvers, MA), Fas, p53, PCNA, p300 (Santa Cruz, Santa Cruz, CA), PARP (Biomol, PA), and HRP-conjugated secondary antibodies (Pierce, IL). For NF-κB nuclear translocation studies, cytoplasmic and nuclear extracts were used. Nuclear and cytoplasmic protein extracts from cells and tissue were prepared as described previously (Dignam et al., 1983). Immunoreactive bands were visualized using enhanced chemiluminescence (Perkin Elmer Life Sciences, Waltham, MA). HDAC1 (Cell Signaling) was used to normalize the nuclear protein expressions (Fernández-Majada et al., 2007) and GAPDH was used as internal loading control for the cytoplasmic proteins (Novus Biologicals, Littleton, CO). The blots were scanned and quantitated using GelDoc XR system and Quantity One® software version 4.6.3 (Bio-Rad, Hercules, CA).
Co-immunoprecipitation
Co-immunoprecipitation was done following manufacturer's protocol (Pierce Co-Immunoprecipitation Kit, ThermoFisher). After immunoprecipitating with anti-p300 antibody (Santa Cruz), Western blotting was done using antibodies against p53 and NF-κBp65 as described in the previous section. Normalization was done with p300 antibody.
Caspase-3 protease activity assay
Control and treated cells were harvested in 1× lysis buffer and caspase-3 activity was determined as per manufacturer's protocol (ApoAlert caspase-3 Fluorescent Assay Kit, Clontech Laboratories, Mountain View, CA). Caspase-3 inhibitor DEVD-AFC was used as internal control for the experiment. Due to poor transfection efficiency of adult myocytes in vitro, adult myocytes were isolated from p53 siRNA treated rats, followed by treatment with AngII for determination of caspase-3 activity assay.
Immunocytochemistry and immunohistochemistry
Frozen ventricular tissue sections (4 µm) were prepared using Leica CM 1850 cryostat. Cells and tissue sections were stained with antibodies to Bax, Bcl2 (BD Pharmingen), cyclin B1, cyclin D1, Cdk2, Cdk4, Ki-67 (Santa Cruz) and α-actinin, active caspase-3, NF-κBp65 (Cell Signaling), followed by incubation with labeled secondary antibodies [Alexa fluor® 488, Alexa fluor® 594, Alexa fluor® 633 (Molecular Probes, Eugene, OR) as described earlier by Sarkar et al. (2004a). After mounting with Vectashield [with DAPI] (Vector Laboratories, Burlingame, CA), cells were visualized under fluorescent microscope (Olympus BX51, Progres® C5).
Statistical analysis
Results were expressed as mean ± standard error (SE) of >3 independent experiments. Data were analyzed by independent sample t-test by SPSS (11.0). Values of P < 0.05 were considered as significant.
Results
Expression of hypertrophy markers and apoptotic genes in response to hypertrophy in vitro and in vivo
Compared to the untreated controls, 10−8 M AngII treatment induced hypertrophy in neonatal myocytes with >20% increase in cardiomyocyte cross-sectional area (1,689 ± 89.9 µm2 in control vs. 2,134 ± 293.1 µm2 in AngII treated). Similar results were obtained with adult myocytes treated with AngII with >26% increase in cell size (1,890 ± 101.67 µm2 vs. 2,563 ± 325.39 µm2). In vivo pressure overload hypertension model also showed hypertrophy with significant increase in HW (mg) to BW (gm) ratio compared to sham operated control (5.24 ± 0.43 vs. 3.51 ± 0.54; Fig. 1A). Significant upregulation (P < 0.05) in the expression of hypertrophy marker genes—ANF, β-MHC—was observed in all the three models (>3.5-fold in neonatal myocytes; >4-fold in adult myocytes; >3-fold in ligated heart) compared to their respective controls (Fig. 1B). Echocardiographic analysis showed significant increase in LVDD (0.54 ± 0.04 vs. 0.45 ± 0.02) and decrease in % FS in ligated model compared to age matched sham animals (43 ± 2.5% vs. 61 ± 3.7%; P < 0.05). Assessment of a part of apoptotic markers by immunoblot analyses, in AngII treated neonatal myocytes, revealed increase in expression of FAS (4.0 ± 0.5-fold) and Bax (2.0 ± 0.5-fold, P < 0.05). These markers were also found to be significantly upregulated in vivo (>3.5-fold and 5.0 ± 0.5-fold) compared to sham operated control (Fig. 2A,B). Similarly, increased activation and cleavage of caspase-3 and PARP proteins were observed both in AngII treated neonatal cells and in vivo ligated hearts (Fig. 2A). To further assess whether in same condition, cells also show changes in expression of the stress induced cell cycle regulators, Western blots were done using the same protein extracts. Increase in cell cycle regulator proteins namely p53 (5.5 ± 0.7-fold; >4.7-fold), p21 (>3.5-fold; >5-fold) and p27 (>4-fold; >6-fold) were observed in both AngII treated neonatal rat cardiomyocytes and ligated heart tissue respectively (Fig. 2C). In addition, pronounced phosphorylation of p53 at ser-46 (8 ± 1.0-fold for in vitro and 5.5 ± 1.2-fold for in vivo) was also observed in hypertrophic cardiomyocytes, implying significance of p53 during hypertrophy (Fig. 2C).
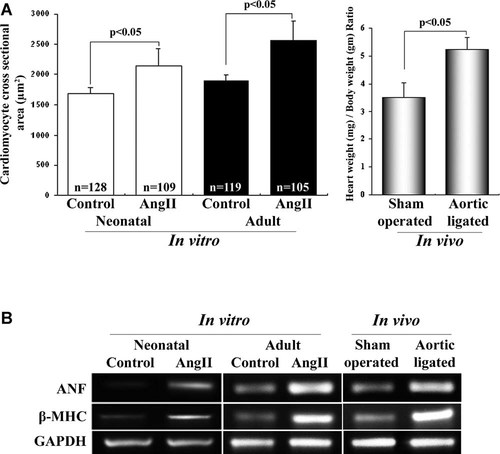
Initiation of cardiomyocyte hypertrophy in vitro and in vivo. A: Graph showing 10−8 M AngII treatment increased >20% and >26% myocyte cross-sectional area (µm2) in neonatal and adult rat cardiomyocytes, respectively, compared to control. Aortic coarctation model showed >1.5-fold increase in HW/BW ratio compared to sham operated control. B: Expression profile of transcripts of hypertrophy markers (ANF, β-MHC) by RT-PCR in AngII treated and untreated neonatal and adult cardiomyocytes. In vivo model also showed increased ANF and β-MHC expression in ligated heart compared to control. GAPDH gene was used as internal loading control.
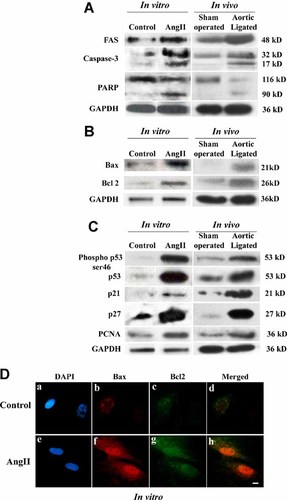
Activation of apoptotic and cell cycle regulatory genes in vitro and in vivo. Western blot analyses showed upregulation of Fas and cleavage of Caspase-3, PARP (A), induction of mitochondrial apoptotic protein Bax and anti-apoptotic protein Bcl2 (B) and cell cycle regulatory proteins phospho p53 ser46, p53, p21, p27, and PCNA (C) in AngII treated cardiomyocytes and in ligated heart. GAPDH was used as internal loading control. D: Immunofluorescence microscopy of neonatal myocytes (untreated or treated with AngII) with localization of Bax (red, parts b and f) and Bcl2 (green, parts c and g) in the same cardiomyocytes (parts d and h; overlay of b, c and f, g, respectively). Cells were counterstained with DAPI (blue, parts a and e). Bar = 10 µm. As shown in lower parts (f,g), increased expression of Bax and Bcl2 was observed in AngII treated cells compared to controls (b–c). [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/jcp]
Expression of cell proliferation markers in cardiomyocytes in response to hypertrophy
Since increased cell proliferation along with increased apoptosis during transition from hypertrophy to heart failure in a transgenic model has been reported (Sarkar et al., 2004a), we wanted to analyze the in vitro and in vivo effects of hypertrophy on factors implicated in either cell proliferation or in inhibition of apoptosis. Immunoblot analysis revealed, significant induction of anti-apoptotic mitochondrial protein Bcl2 (3.5 ± 0.5-fold; P < 0.05) and proliferating cell nuclear antigen (PCNA) (>3-fold) in AngII treated neonatal myocytes. It was also found that pressure overload induced hypertrophy also resulted in increased expression of both Bcl2 (5.5 ± 0.5-fold) and PCNA (>3.5-fold), compared to the control heart samples (Fig. 2B,C). This was further confirmed by immunofluorescence study where significant expression of both Bax and Bcl2 proteins within the same cardiomyocyte was observed (Fig. 2D).
As analyzed by immunofluorescence microscopy using antibodies against cyclin B1, cyclin D1, Cdk2, and Cdk4, expression of these markers were increased in AngII treated cardiomyocytes (Fig. 3A, parts f, l, r, x), compared to untreated controls (Fig. 3A, parts c, i, o, u). Like in vitro, significant (P < 0.05) increase in the expression of the cell cycle regulatory proteins like cyclins and Cdk proteins was observed in aortic ligated rat heart both by immunoblotting (Fig. 3B) and immunohistochemistry (Fig. 3C). AngII treated myocytes and adult heart sections were also stained with α-actinin, which is a cardiomyocyte specific marker (Fig. 3A,C). Increased expression of proliferation associated nuclear antigen Ki-67 positive nuclei in AngII treated hypertrophic cardiomyocytes was observed both in vitro as well as in vivo (Fig. 4A,B). Interestingly, apoptotic (active caspase-3) as well as cell proliferative marker (Ki-67) proteins co-localized in vitro as well as in the in vivo model in a single hypertrophied cardiomyocytes (Fig. 4A,B, part h). The rate of percent cell death (caspase-3 positive myocytes) versus rate of regenerating myocyte (Ki-67 positivity) was compared during hypertrophy, in vitro. Approximately 36.8 ± 5% active caspase-3 positive cells were found in AngII treated cells compared to the untreated controls (6.0 ± 2.0%) whereas 5.05 ± 1.2% Ki-67 positive nuclei could be traced in AngII treated cells compared to only 0.67 ± 0.2% Ki-67 positivity in untreated cells (Supplementary Fig. 1A). Similar observation was documented in vivo, in hypertrophied tissue sections, where number of active caspase-3 positive myocytes was much higher (39.8 ± 4.5%) than Ki-67 positivity (7.63 ± 2.2%) among hypertrophied cardiomyocytes (n = 6,124) compared to 9.5 ± 1.38% (caspase-3) and 1.25 ± 0.3% (Ki-67) positive cells in control (Supplementary Fig. 1B). Increased co-expression of active caspase-3 and Ki-67 was observed both in AngII treated myocytes (5.58 ± 1.35%) and aortic ligated hearts (6.85 ± 1.45%) compared to respective controls (<1.0%; Supplementary Fig. 1A,B).
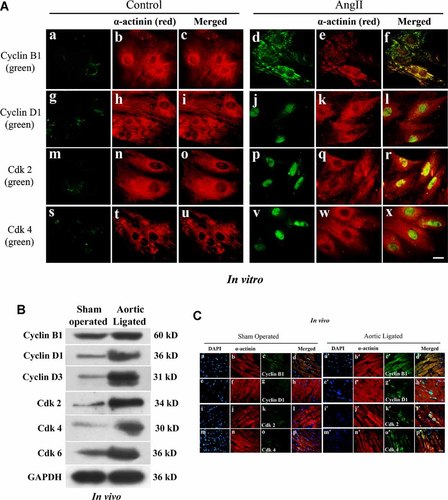
Activation of cell cycle regulatory and proliferation marker proteins in response to AngII and volume overload hypertrophy. A: Immunofluorescence microscopy of neonatal cells either treated with AngII or untreated controls with antibodies against cyclin B1 (parts a and d), cyclin D1 (parts g and j), Cdk2 (parts m and p), and Cdk4 (parts s and v) followed by Alexa fluor 488 (green) secondary antibody. All the cells were counter stained with α-actinin followed by Alexa fluor 633 secondary antibody (red, parts b, e, h, k, n, q, t, and w). As shown in right parts (d, j, p, and v) increased expression of cyclin B1, cyclin D1, Cdk2, and Cdk4 was observed in AngII treated cells. Parts c, i, o, u and f, l, r, x showed the merged images (Bar = 10 µm). B: Western blot analyses showing increased expression of cyclin B1, cyclin D1, cyclin D3, Cdk2, Cdk4, and Cdk6 in ligated hearts compared to control. GAPDH was used as internal loading control. C: Immunofluorescence study of cell cycle and cyclin dependent kinases in heart tissue sections showing increased expressions of cyclins and Cdks in aortic ligated model than the sham operated control. Tissue sections were incubated with primary antibodies against cyclin B1 (parts c and c′), cyclin D1 (parts g and g′), Cdk2 (parts k and k′), and Cdk4 (parts o and o′) followed by Alexa fluor 488 secondary antibody (green). All the sections were counter stained with α-actinin followed by Alexa fluor 633 secondary antibody (red, parts b, f, j, n and b′, f′, j′, n′) and nuclei with DAPI (parts a, e, i, m and a′, e′, i′, m′). Parts d, h, l, p and d′, h′, l′, p′ showed the merged images (bar = 10 µm).
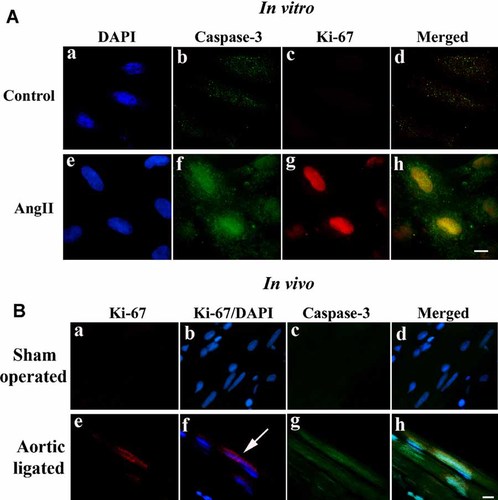
Immunofluorescence study showing apoptotic and regeneration markers in the single hypertrophied cell and tissue sections. A: Immunofluorescence study showing simultaneous activation of Caspase-3 (green, part f) and Ki-67 (red, part g) in the single neonatal cardiomyocytes treated with AngII that was absent in case of untreated cells (parts b and c). B: Immunohistochemistry showing expression of Ki-67 (red, parts e and f) within nucleus and active caspase-3 protein (green, parts g and h) in the same hypertrophied heart section. Sham operated control heart sections did not show expression of either Ki-67 or caspase-3 (parts a–d). Nuclei were counterstained with DAPI (bar = 10 µm).
Activation of Nuclear factor κB (NF-κB) in hypertrophic cardiomyocytes
Increased expression and translocation of NF-κBp65 subunit from cytoplasm to nucleus of cardiomyocytes was observed by immunoblotting in extracts of neonatal myocytes treated with AngII and hypertrophied heart (>3.0-fold; P < 0.05) compared to control (Fig. 5A). This was further corroborated by immunofluorescence study, where compared to untreated controls, increased p65 protein accumulation (green fluorescence) was observed in nuclei of majority of the AngII treated myocytes as well as in hypertrophic heart tissue (Fig. 5B).
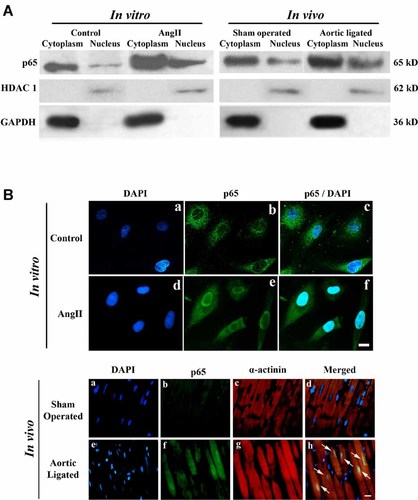
Activation and nuclear translocation of NF-κBp65 subunit during hypertrophy in vitro and in vivo. A: Immunoblot analyses of NF-κBp65 in cytoplasmic and nuclear extracts from neonatal cells and heart tissue showed increased activation and translocation in response to AngII treatment and aortic ligation. HDAC1 and GAPDH were used as internal loading control for nuclear and cytoplasmic proteins respectively. B: Immunofluorescence study showing expression of p65 in cytoplasm (green fluorescence) of control cells (part b) and increased translocation of p65 subunit to nucleus in AngII treated cells (part e) compared to control. In ligated heart section (part h), increased nuclear translocation of p65 was also observed compared to sham (part d). Tissue sections were counter stained with α-actinin antibody (red, parts c and g). Bar = 10 µm. [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/jcp]
Differential interaction of p53 and NF-κB with p300 during hypertrophy
To study the interaction between p53 and NF-κB with p300 during hypertrophy co-immunoprecipitation was done using p300 antibody. Differential binding of p53 and p65 with p300 was observed in control and AngII treated neonatal or adult cardiomyocytes. Binding between p65 and p300 was significantly (P < 0.01) increased in AngII treated neonatal and adult cardiomyocytes (Fig. 6A, part a) as well as in hypertrophic heart sections (Fig. 6A, part b). AngII treated neonatal and adult cells transfected with dominant negative construct of IκBα depressed this binding almost to the basal level compared to cells treated with AngII alone (Fig. 6A, part a).
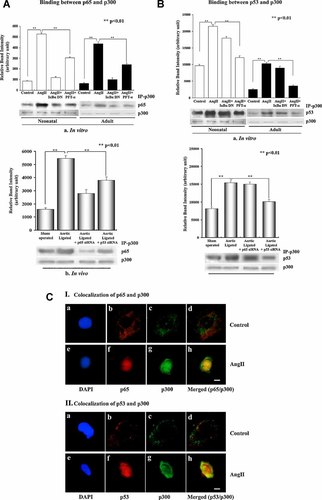
Interaction between p53 and p65 with p300 during hypertrophy. Cell extracts from untreated, treated with AngII, AngII plus IκBα DN, and AngII plus PFT-α were immunoprecipitated using antibody against p300 followed by immunoblotting using either p65 (A, part a) or p53 (B, part a) antibodies. Tissue extracts from sham operated control, aortic ligated heart as well as from p53 and p65 siRNA treated ligated hearts were also immunoprecipitated by p300 antibody followed by immunoblotting using p65 (A, part b) and p53 (B, part b) antibodies. Blots were reprobed with p300 specific antibody as internal loading control. Increased co-immunoprecipitation of p65 with p300 was observed in AngII treated cells (lanes 2 and 6, part a), which was significantly reduced in cells treated with IκBα DN (lanes 3 and 7, part a) or PFT-α (lanes 4 and 8, part a). Increased binding between p65 and p300 was also observed in vivo in hypertrophied heart compared to control, whereas p53siRNA inhibited this binding (part b). B: Significant increase in p53–p300 binding was observed in cells treated with AngII alone (lanes 2 and 6, part a) or in the presence of IκBα DN (lanes 3 and 7, part a). p53 inhibitor PFT-α inhibited p53–p300 binding (lanes 4 and 8, part a). p53–p300 binding was also found to be increased in ligated heart, compared to control, but inhibition of p65 by its specific siRNA did not affect this binding significantly (part b). C: Immunofluorescence study showing co-localization of p65 (red, part f) and p300 (green, part g) in AngII treated cells (part h, merged image), but it was absent in untreated control (C, I). p53 (red, part f) and p300 (green, part g) were also co-localized (yellow, part h) in AngII treated cells, but co-localization was absent in control (part d) (C, II). Bar = 5µm. [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/jcp]
Furthermore, significant increase in p53–p300 binding (P < 0.01) was also observed in AngII treated neonatal and adult cardiomyocytes as well as in aortic ligated hypertrophic hearts compared to untreated/sham controls (Fig. 6B, parts a and b). Since both NF-κB and p53 binding to p300 was increased in AngII treated myocytes, experiments were done in the presence of either p53 or NF-κB inhibitors to understand whether this binding is co-operative or independent of each other. Cells were either pretreated with DMSO control, PFT-α or transfected with IκBα DN followed by AngII treatment and assessed for p65–p300 and p53–p300 binding. In the presence of p53 inhibitor (PFT-α) both p53–p300 and p65–p300 binding was significantly decreased (P < 0.01) whereas in the presence of NF-κB inhibitor, no effect on binding of p53–p300 was observed in either neonatal or adult cells (Fig. 6A, part a; Fig. 6B, part a). Compared to controls, increased expression and co-localization of p65–p300 and p53–p300 was also observed within the cell nuclei in response to hypertrophic stimuli (Fig. 6C). p65–p300 binding was also reduced significantly (P < 0.01) in p53 siRNA treated hypertrophied heart in vivo (Fig. 6A, part b), but p53–p300 binding was not so pronounced in p65 siRNA treated hypertrophied rat (Fig. 6B, part b). Cell viability assay for SN50 (10 µM) and PFT-α (50 µM) showed 10 ± 5% cell mortality in cardiomyocytes, following 24 h treatment.
Role of p53 and NF-κB in regulating apoptosis and cellular proliferation pathways during hypertrophy
Caspase-3 activity was assayed to delineate role of p53 and NF-κB during apoptosis in AngII treated neonatal and adult myocytes under the influence of different inhibitors (Fig. 7A). Significant increase (>5-fold; P < 0.01) in caspase-3 activation and NF-κB promoter driven luciferase activity (>2.5-fold; P < 0.01) was observed in AngII treated neonatal and adult cells (Fig. 7A,B). PFT-α pretreatment followed by AngII showed significant decrease in caspase-3 activity in both neonatal (>60%) and adult myocytes (>2.6-fold) whereas, NF-κB inhibition (>3-fold) either by IκBα DN or SN50 had no effect in AngII induced caspase activation. To further confirm the role of p53, neonatal myocytes were transfected with p53 siRNA whereas adult myocytes were isolated from in vivo p53 siRNA treated rats. Similar to results from PFT-α treatment, significant reduction (>80% in neonatal and >66% in adult; P < 0.01) in AngII induced caspase-3 activity was observed, indicating positive role of p53 but not NF-κB in modulation of apoptosis during hypertrophy (Fig. 7A). Unlike caspase-3 activation, both PFT-α and NF-κB inhibitors resulted in significant reduction of AngII induced NF-κB promoter activity (Fig. 7B). Reduced expression of hypertrophic markers such as ANF and β-MHC was also observed in the presence of both NF-κB and p53 inhibitors, though the change in gene expression in cells treated with NF-κB inhibitor was not as pronounced as with PFT-α (Fig. 7C). These results imply that p53 is involved in modulation of apoptosis whereas NF-κB does not play a role in AngII induced apoptosis in cardiomyocytes during hypertrophy. Significant downregulation of both p65 and p53 protein expression with respective inhibitors was confirmed by immunoblot analyses (Fig. 7A, lower part).
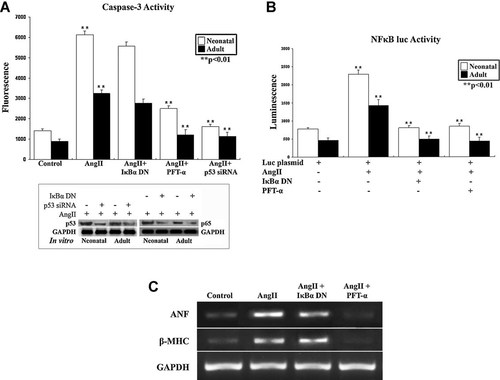
A: Caspase-3 enzymatic activity was measured using specific substrate in cellular extracts of neonatal and adult cardiomyocytes treated with AngII alone or in the presence of either IκBα DN, PFT-α or p53 siRNA. Increase in caspase-3 activity was observed in AngII treated neonatal and adult cardiomyocytes, which was significantly downregulated in the presence of PFT-α or p53 siRNA. IκBα DN did not inhibit AngII induced caspase-3 activity in either of the cell types. Immunoblot against p53 and p65 (A, lower part) shows significant downregulation of respective protein expression by p53 and NF-κB inhibitors in vitro. B: NF-κB-luciferase reporter assays were performed in both neonatal and adult cardiomyocytes treated with AngII alone or in the presence of either IκBα DN or PFT-α. Increase in NF-κB driven luciferase activity was observed in AngII treated cells (both neonatal and adult). AngII driven NF-κB activity was significantly reduced in the presence of either IκBα DN or PFT-α in both cell types. C: RT-PCR analyses showing reduced expression of hypertrophy markers (ANF and β-MHC) in hypertrophied cardiomyocytes treated with p65 and p53 inhibitors.
To analyze the effect of downregulation of p53 and NF-κB in vivo, expression of hypertrophy marker genes was assessed. As shown in Figure 8A, expression of ANF and β-MHC was significantly reduced (ANF >2.5-fold; P < 0.05 and β-MHC >3-fold; P < 0.05) in p53 siRNA treated hypertrophied rat heart compared to the respective controls, but no significant effect was observed in p65 siRNA treated rat heart (Fig. 8A). Successful downregulation of p53 (>3-fold) and p65 (>2.5-fold) proteins in vivo by respective siRNAs was shown in Figure 8A, respectively. Similarly, significant downregulation of apoptotic markers Fas, Bax, cleaved caspase-3, and PARP was observed in rat heart treated with p53 siRNA (Fig. 8B). In addition, p53 siRNA also downregulated the expression of anti-apoptotic protein Bcl2 and cell cycle regulatory proteins like p65, PCNA, cyclin B1, and Cdk2 proteins significantly (P < 0.05; Fig. 8C). However, effect of p65 siRNA on the expression of these apoptotic proteins was not so significant (Fig. 8B).
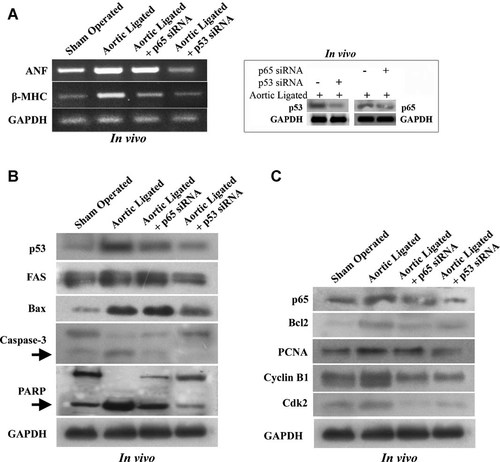
Effect of p53 or NF-κB inhibition in aortic ligated hypertrophy model. Significant downregulation of p65 and p53 proteins was observed in siRNA treated hypertrophied rat hearts (inset, part A). RT-PCR analyses showing reduced expression of hypertrophy markers (ANF and β-MHC) in pressure overload induced hypertrophy treated with p53siRNA and p65siRNA compared to sham operated control (A). Immunoblot analyses showing downregulation of p53, Fas, Bax as well as cleavage of caspase-3 and PARP in p53siRNA injected aortic ligated rat compared to in vivo hypertrophic model. p65siRNA injected hypertrophic rat show insignificant change of these apoptotic regulatory proteins. Arrow indicates the cleaved product of caspase-3 (17 kDa) and PARP (90 kDa) (B). Inhibition of p53 activity by p53siRNA also reduced the expression of cell cycle regulatory proteins like p65, PCNA, cyclin B1, Cdk2 as well as anti-apoptotic protein Bcl2 (C). Inhibition of p65 activity by p65 siRNA in vivo also reduced expression of these cell cycle regulatory proteins (C). GAPDH was used as internal loading control.
Discussion
In this study, we have shown the simultaneous occurrence of apoptosis and cell proliferation in cardiomyocytes during AngII induced hypertrophy in vitro as well as in vivo pressure overload hypertrophy model. Treatment of isolated neonatal and adult rat cardiomyocytes with AngII, a potent vasoconstrictor and a growth factor concurrently stimulated cell growth (Fig. 1A) and expression of the hypertrophy marker genes (Fig. 1B). AngII also led to increased expression of apoptotic protein Fas in myocytes in vitro and in ligated heart. It has been previously reported that Fas pathway is a critical mediator of cardiac myocyte death during ischemia-reperfusion in vivo (Lee et al., 2003). In addition to Fas, other apoptosis markers such as cleavage of caspase-8, caspase-3, and PARP reported previously in vivo (Black et al., 1998) were also observed in hypertrophic myocytes during this study. In concurrence to previous report on Bax mediated apoptosis in cardiomyocytes (Hou and Hsu, 2005), upregulation of both pro-apoptotic protein (Bax) and anti-apoptotic protein (Bcl2) was observed in both AngII treated hypertrophy and pressure overload hypertrophied rats (Fig. 2B). Nuclear degradation of PARP, accumulation of active caspase-3 protein and enhanced caspase-3 activity along with increased expression of p53 and cell cycle inhibitors—p27 and p21—in AngII treated cells also confirmed myocyte apoptosis during hypertrophy (Fig. 2A–C). Increased phosphorylation of p53 at ser46 and its nuclear accumulation in AngII treated myocytes indicated that activation of p53 may have a role in induction of apoptosis during hypertrophy. This is consistent with previous reports showing nuclear accumulation of p53 in different cell types during apoptosis. Elevated expression of p53 has also been documented in failing human heart and in animal models of cardiac hypertrophy (Sano et al., 2007; Das et al., 2010); but its significance was not clear. p53 has also been implicated in activation of both death-receptors and Apaf-1-dependent apoptosis (Haupt et al., 2003) which may also play a role in inducing apoptosis in hypertrophic cardiomyocytes. AngII, being a potent growth factor, also induces growth regulatory proteins in these cells.
Like apoptosis, cell division is also an essential and ubiquitous process in multicellular organisms. Cyclins and Cdks are the key regulators of cell cycle process and are well characterized (Norbury and Nurse, 1992; Morgan, 1995). Evidence exists to suggest that apoptosis and cell cycle may be interconnected (Meikrantz and Schlegel, 1995) as expression of the proto-oncogenes has been shown to stimulate both cell proliferation and apoptosis under certain conditions (Evan et al., 1992). Direct encroachment of apoptosis regulatory proteins on the cell cycle machinery is also documented (Brady et al., 1996; Mazel et al., 1996). In our study, we found significant upregulation of cell cycle regulatory proteins like cyclin B1, cyclin D1, Cdk2, and Cdk4 or cell proliferation marker Ki-67 in AngII treated cardiomyocytes (Figs. 3 and 4). Increase in mitotic index was reported during cardiac dysfunction but whether this was due to myocyte division or cardiac stem cells entering the area of stress, remained unclear (Beltrami et al., 2001; Jopling et al., 2010). Though myocytes are regarded as terminally differentiated cells, simultaneous upregulation of Bcl2, Ki-67, and PCNA (Figs. 2B,C and 4A) was observed in AngII treated cells, indicating that myocytes have capability to regenerate themselves during hypertrophic stimuli (Beltrami et al., 2001; Zorc et al., 2003).
Nuclear translocation of p65 subunit from the cytoplasm as well as increased expression of NF-κB in response to AngII was observed in our study (Fig. 5A,B), consistent with the previous reports regarding role of NF-κB in myotrophin induced cardiac hypertrophy (Gupta et al., 2005). Transcriptional activity of NF-κB is dependent on its phosphorylation (Wang et al., 1998) but some reports suggest that nuclear translocation of p65 does not always mean its transcriptional activation (Zhong et al., 1997; Wang and Baldwin, 1998; Campbell et al., 2001; Chen et al., 2001, 2002). To confirm transcriptional activation of NF-κB, luciferase reporter assay was performed which showed >2.5-fold increase in luciferase activity in response to AngII (Fig. 7B). Interestingly, inhibition of p53 by PFT-α inhibited both NF-κB induced luciferase activity and caspase-3 activation to almost basal level (Fig. 7A,B). In contrast, no significant change was observed in caspase-3 activity by blocking NF-κB in AngII treated myocytes both in vitro (Fig. 7A) and in vivo (Fig. 8B). This result leads us to speculate that activation of caspase-3 is not dependent on NF-κB activation directly. Though activation of AngII induced NF-κB is dependent on p53 activation which is implicated in cardiomyocyte apoptosis. It has also been previously shown that attenuation of NF-κB activation is not sufficient to block the deleterious effect of increased cardiomyocyte apoptosis in left ventricular remodeling (Zelarayan et al., 2009). Transcription co-activator p300 is essential for myocyte survival in the postnatal left ventricular myocardium (Nakagawa et al., 2009). p300/CBP is present in limited concentrations inside the nucleus and coordinates changes in the transcription of multiple genes in response to signals from the cytoplasm. p53 has been reported to be in a stable complex with p300 as it places the p53/p300 complex on DNA at p53-dependent promoters, consistent with the proposed role for p300/CBP as co-activators of p53 (Avantaggiati et al., 1997; Lill et al., 1997). Similar to a wide variety of other inducible regulatory transcription factors, NF-κB complexes with the co-activator protein p300 and CREB-binding protein (CBP) (Webster and Perkins, 1999). Immunoprecipitation experiments in cardiomyocytes treated with AngII confirmed stronger interaction of both p53 and NF-κB with p300 in AngII treated myocytes (Fig. 6A,B). Blocking of p53 by PFT-α or p53 siRNA reduced p65–p300 binding significantly compared to AngII treated cells (Fig. 6A, part a), however inhibition of NF-κB only altered NF-κB–p300 interaction but had no effect on p53–p300 binding (Fig. 6B, part a). Consistent with in vitro results, inhibition of p53 using specific siRNA blocked binding of both p53–p300 and p65–p300 in vivo (Fig. 6A, part b), but p65 siRNA had no effect on p53–p300 binding (Fig. 6B, part b). Based on these results it can be presumed that initially p53 binds to p300 and then the p53–p300 complex further recruits NF-κB during AngII induced hypertrophy. Reversal of hypertrophy as assessed by expression of ANF and β-MHC was observed both in vitro and in vivo following inhibition of p53 (Figs. 7C and 8A). In addition, if apoptotic signal p53 was inhibited, downregulation of both apoptotic marker proteins (Fig. 8B) and cell proliferation proteins, PCNA, cyclin B1, Cdk2, and NF-κB (Fig. 8C) was observed. Thus NF-κB activation and induction of other cell proliferation markers during AngII mediated hypertrophy are dependent on activation of p53 in cells. Thus, in spite of evidence of cell proliferation, apoptotic signals dictate terms to direct the cells towards apoptosis during hypertrophy and compromised cardiac function. This was confirmed when ratio of caspase-3 positive cells was found to be higher than Ki-67 positive cells in both neonatal and adult myocytes (Supplementary Fig. 1). Both active caspase-3 and Ki-67 positivity were also observed in same cardiomyocyte cells both in vitro (>5.5%) and in vivo (±7%) indicating induction of both apoptotic and regenerative signals during hypertrophic stress (Supplementary Fig. 1). This is consistent with recent report where direct evidence of proliferating cardiomyocytes during zebra fish heart regeneration, without involvement of stem cells has been shown (Jopling et al., 2010).
Based on these results, it can be concluded that during hypertrophy, myocytes undergo cell death via activation of p53 and other downstream apoptotic signals. Simultaneously p53 is also involved in induction of signaling pathways regulating cell proliferation markers such as NF-κB, to counter the cell loss in these terminally differentiated cells. The p53–p300–p65 complex plays an important role in balancing the two signaling mechanisms with antagonistic function. Inhibition of NF-κB activation balances the shift toward apoptosis. However blocking p53 results in inhibition of both apoptosis as well as cellular hypertrophy. Even with the cardiomyocyte's effort to reenter the cell cycle cell death signaling predominates, resulting in massive cell loss that might lead to heart failure. The crosstalk of these counteracting transcription factors may depend on access to cofactors such as p300 which appear to be highly important in fine-tuning of these diverse signals in cells and dictate the fate of a myocyte cell towards death or regeneration.
Acknowledgements
This work was financially supported by grants from Council of Scientific and Industrial Research [37(1393)/10/EMR-II] and Department of Science and Technology [SR/SO/HS-100/2009], Government of India. A. Chatterjee was supported by Research Fellowship [UGC/681/Jr.Fellow (Sc.) 05-06] from UGC, Government of India. We gratefully acknowledge Dr. Mamta Chawla-Sarkar, Department of Virology, National Institute of Cholera and Enteric Diseases, Kolkata for providing adenoviral vectors containing IκBα DN construct and her valuable inputs during manuscript preparation.




