Down-regulation of cyclin E1 expression by microrna-195 accounts for interferon-β-induced inhibition of hepatic stellate cell proliferation
Abstract
Recent studies have suggested that interferons (IFNs) have an antifibrotic effect in the liver independent of their antiviral effect although its detailed mechanism remains largely unknown. Some microRNAs have been reported to regulate pathophysiological activities of hepatic stellate cells (HSCs). We performed analyses of the antiproliferative effects of IFNs in HSCs with special regard to microRNA-195 (miR-195). We found that miR-195 was prominently down-regulated in the proliferative phase of primary-cultured mouse HSCs. Supporting this fact, IFN-β induced miR-195 expression and inhibited the cell proliferation by delaying their G1 to S phase cell cycle progression in human HSC line LX-2. IFN-β down-regulated cyclin E1 and up-regulated p21 mRNA levels in LX-2 cells. Luciferase reporter assay revealed the direct interaction of miR-195 with the cyclin E1 3′UTR. Overexpression of miR-195 lowered cyclin E1 mRNA and protein expression levels, increased p21 mRNA and protein expression levels, and inhibited cell proliferation in LX-2 cells. Moreover miR-195 inhibition restored cyclin E1 levels that were down-regulated by IFN-β. In conclusion, IFN-β inhibited the proliferation of LX-2 cells by delaying cell cycle progression in G1 to S phase, partially through the down-regulation of cyclin E1 and up-regulation of p21. IFN-induced miR-195 was involved in these processes. These observations reveal a new mechanistic aspect of the antifibrotic effect of IFNs in the liver. J. Cell. Physiol. 226: 2535–2542, 2011. © 2010 Wiley-Liss, Inc.
Hepatic fibrosis is characterized by excessive accumulation of extracellular matrices (ECM) and is a common feature of chronic liver diseases. Hepatic stellate cells (HSCs) are considered to play multiple roles in the fibrotic process. HSCs maintain a quiescent phenotype and store vitamin A under physiological conditions. When liver injury occurs, they become activated and trans-differentiate into myofibroblastic cells, whose characteristics include the proliferation, loss of vitamin A droplets, expression of α-smooth muscle actin (α-SMA), secretion of profibrogenic mediators and ECM (Friedman, 2000; Bataller and Brenner, 2001). Therefore, controlling the population and activation of HSCs should be a potential therapeutic target against liver fibrosis.
Interferons (IFNs) are cytokines with antiviral, immunomodulatory, and cell growth inhibitory effects. IFN-α and -β are classified as type I IFNs (Pestka et al., 1987; Uze et al., 2007), which are generally applied for the therapy of eradication of hepatitis B and C viruses. Studies using rodent models and cultured HSCs have also suggested that IFNs have a direct antifibrotic potential independently of their antivirus activity (Mallat et al., 1995; Fort et al., 1998; Shen et al., 2002; Inagaki et al., 2003; Chang et al., 2005; Tanabe et al., 2007; Ogawa et al., 2009), although the detailed molecular mechanisms of these effects of IFNs remain to be clarified.
Recently, microRNAs (miRNAs), which are endogenous small non-coding RNA, have become a focus of interest as post-transcriptional regulators of gene expression through interaction with the 3′ untranslated region (3′UTR) of target mRNAs (Bartel, 2004). miRNAs are known to participate in cell proliferation, development, differentiation, and metabolism (Bartel, 2004). Moreover, it has been reported that expression of miRNAs could alter hepatic pathophysiology; microRNA-122 (miR-122) is involved in the IFN-β-related defense system against viral hepatitis C (Pedersen et al., 2007), and miR-26 is associated with survival and response to adjuvant IFN-α therapy in patients with hepatocellular carcinoma (HCC) (Ji et al., 2009a). Regarding HSCs, miR-15b and miR-16 are down-regulated upon HSC's activation, and their overexpression induces apoptosis and a delay in the cell cycle (Guo et al., 2009a,b). Knockdown of miR-27a and miR-27b in activated HSCs allowed a switch to a more quiescent phenotype and decreased cell proliferation (Ji et al., 2009b). miR-150 and miR-194 suppress proliferation, activation, and ECM production of HSCs (Venugopal et al., 2010). Recently, we showed that miR-29b was induced by IFN and suppressed type I collagen production in LX-2 cells (Ogawa et al., 2010).
In the present study, we measured the levels of miR-195 in primary-cultured mouse HSCs and found that its expression was markedly reduced in their activation phase, suggesting the regulatory role of miR-195 in the activation/deactivation process of HSCs. Because miR-195 is categorized into the same family as miR-15b and miR-16 and has been reported to regulate cell cycle by targeting E2F3, CDK6, and cyclin D1 (Xu et al., 2009), we suspect the involvement of miR-195 in the proliferation of HSC and in type I IFN, in particular IFN-β, -induced inhibition of their growth.
Materials and Methods
Materials
Human HSC line LX-2 was donated by Dr. Scott L. Friedman (Mount Sinai School of Medicine, New York, NY) (Xu et al., 2005). Necessary reagents and materials were obtained from the following sources: Dulbecco's modified Eagle's medium (DMEM) from Sigma Chemical Co. (St. Louis, MO); fetal bovine serum (FBS) from Invitrogen (Carlsbad, CA); human natural IFN-α and -β from Otsuka Pharmaceutical Co. (Tokushima, Japan) and Toray Industries, Inc. (Tokyo, Japan), respectively; precursor and inhibitor of miR-195, and the corresponding negative controls from Ambion (Austin, TX); mouse monoclonal antibody against cyclin E1, cyclin D1 and p21, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) from MBL (Nagoya, Japan), Cell Signaling Technology, Inc. (Beverly, MA), and Chemicon International, Inc. (Temecula, CA), respectively; rabbit polyclonal antibodies against cyclin-dependent kinase (CDK) 6 and E2F3 from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA); goat polyclonal antibody against CDK4 from Santa Cruz Biotechnology, Inc.; enhanced Chemiluminescence plus detection reagent from GE Healthcare (Buckinghamshire, UK); Immobilon P membranes from Millipore Corp. (Bedford, MA); reagents for cDNA synthesis and real-time PCR from Toyobo (Osaka, Japan); a cell counting kit from Dojindo Laboratories (Kumamoto, Japan); and all other reagents from Sigma Chemical Co. or Wako Pure Chemical Co. (Osaka, Japan).
Cells
LX-2 cells were maintained in DMEM supplemented with 10% FBS (DMEM/FBS) and were plated at a density of 0.7–1.5 × 104 cells/cm2 24 h prior to biological assay. Biological assays were done in DMEM/FBS unless stated otherwise. Mouse primary HSCs were isolated from male C57BL/6 mice by the pronase-collagenase digestion method as described previously (Uyama et al., 2006) and were cultured in DMEM/FBS.
Transient transfection of miRNA precursors and inhibitors
Precursor of miR-195, which was a double-strand RNA mimicking endogenous miR-195 precursor, and the negative control with a scrambled sequence were transfected into LX-2 cells using Lipofectamine 2000 (Invitrogen) at a final concentration of 50 nM in accordance with the manufacturer's instructions. Briefly, miRNA precursor and Lipofectamine 2000 were mixed at a ratio of 25 (pmol):1 (µl) in Opti-MEM I Reduced Medium (Invitrogen), incubated for 20 min at room temperature, and were then added to the cultures. After 24 h, the culture medium was replaced with fresh medium. Inhibitor of miR-195, which was designed to bind to endogenous miR-195 and inhibit its activity, and the negative control with a scrambled sequence were transfected similarly. After 6 h, the culture medium was changed and IFN-β was added successively.
Cell proliferation assay
LX-2 cells were plated at a density of 2 × 103 cells/well in 96-well plates 24 h prior to experiments. The culture medium was replaced by fresh medium containing different concentrations of IFNs at days 0 and 3. After 3, 5, and 7 days of treatment, cell proliferation was measured by WST-1 assay. In another experiment, the cells were plated at a density of 3 × 103 cells/well in 96-well plate for 24 h prior and were then transfected with the miR-195 precursor as described above. After 24 h, the medium was changed and the culture was continued for an additional 1–3 days before the measurement of cell proliferation.
Cell cycle analysis
Cells were serum starved for 24 h and then the medium was replaced with IFN-containing DMEM/FBS. At the indicated time points after treatment, the cells were harvested by trypsinization, washed in phosphate-buffered saline (PBS), and fixed in ice-cold 70% ethanol. The cells were washed in PBS and resuspended in PBS containing 500 µg/ml RNase A and incubated for 20 min. Cellular DNA was stained with propidium iodide at a final concentration of 25 µg/ml for 20 min. The cells were analyzed using a FACSCalibur HG flow cytometer (Becton Dickinson, Franklin Lakes, NJ). A total of 20,000 events were counted for each sample. Data were analyzed using ModFIT LT software (Verity Software House, Topsham, ME).
Quantitative real-time PCR
Quantitative real-time PCR was performed according to the method described elsewhere with use of a set of gene-specific oligonucleotide primers (Table 1) using an Applied Biosystems Prism 7500 (Applied Biosystems, Foster City, CA) (Ogawa et al., 2010). To detect miR-195 expression, the reverse transcription reaction was performed using a TaqMan microRNA Assay (Applied Biosystems) in accordance with the manufacturer's instructions. The expression level of GAPDH was used to normalize the relative abundance of mRNAs and miR-195.
| Gene | Accession no. | Sequence |
|---|---|---|
| Real-time PCR | ||
| CDK2 | NM_001798 | Forward: 5′-CTCCACCGAGACCTTAAACCTCAG-3′ |
| Reverse: 5'-TCGGTACCACAGGGTCACCA-3′ | ||
| CDK4 | NM_000075 | Forward: 5′-GATAGATGCTGACCCATACCTCAAG-3′ |
| Reverse: 5′-ATGCTGTGGTGCTTTGAGGTAG-3 | ||
| CDK6 | NM_001259 | Forward: 5′-ATATCTGCCTACAGTGCCCTGTCTC-3′ |
| Reverse: 5′-GTGGGAATCCAGGTTTTCTTTGCAC-3' | ||
| Cyclin E1 | NM_001238 | Forward: 5′-GCAGTATCCCCAGCAAATC-3′ |
| Reverse: 5′-TCAAGGCAGTCAACATCCA-3′ | ||
| Cyclin D1 | NM_053056 | Forward: 5′-GCTGTGCATCTACACCGACAACTC-3′ |
| Reverse: 5′-AGGTTCCACTTGAGCTTGTTCACC-3′ | ||
| E2F3 | NM_001949 | Forward: 5′-CCAACTCAGGACATAGCGATTGCTC-3′ |
| Reverse: 5′-AGGAATTTGGTCCTCAGTCTGCTGT-3′ | ||
| GAPDH | NM_002046 | Forward: 5′-GCACCGTCAAGGCTGAGAAC-3′ |
| Reverse: 5′-TGGTGAAGACGCCAGTGGA-3′ | ||
| p21 | NM_000389 | Forward: 5′-AGCAGAGGAAGACCATGTGGA-3′ |
| Reverse: 5′-GGAGTGGTAGAAATCTGTCATGCT-3′ | ||
| p27 | NM_004064 | Forward: 5′-AGCTTGCCCGAGTTCTACTACAG-3′ |
| Reverse: 5′-ACCAAATGCGTGTCCTCAGAGT-3′ | ||
| 3′UTR cloning | ||
| Cyclin E1 | NM_001238 | Forward: 5′-TTCTCGAGATCCTTCTCCACCAAAGACAGTT-3′ |
| Reverse: 5′-TTTCTAGAGAATGGATAGATATAGCAGCACTTACA-3′ | ||
- The forward and reverse primers for 3′UTR cloning carried the XhoI and XbaI sites at their 5′-ends, respectively.
Immunoblotting
Cells were lysed in RIPA buffer [50 mM Tris/HCl, pH 7.5, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS)] containing Protease Inhibitor Cocktail, Phosphatase Inhibitors Cocktail 1, and Phosphatase Inhibitor Cocktail 2 (Sigma). Proteins (20 µg) were electrophoresed in a 10% SDS–polyacrylamide gel and then transferred onto Immobilon P membranes (Ogawa et al., 2010). Immunoreactive bands were visualized by the enhanced chemiluminescence system using a Fujifilm Image Reader LAS-3000 (Fuji Medical Systems, Stamford, CT).
Luciferase reporter assay
Interaction of miR-195 to the 3′UTR of the cyclin E1 gene was tested according to the reported method (Ogawa et al., 2010). The 3′UTR of the cyclin E1 gene containing putative miR-195 target regions was obtained by PCR using cDNA derived from LX-2 and a primer set listed in Table 1. The obtained DNA fragments (497 bp) were inserted into a pmirGLO Vector (Promega, San Luis Obispo, CA). LX-2 cells, plated in 96-well plates at a density of 2 × 104 cells/well 24 h prior to experiment, were transfected with 200 ng of reporter plasmid and miRNA precursor using Lipofectamine 2000. After 24 h, the medium was changed to 20 µl of PBS. The Dual-Glo Luciferase Assay System (Promega) was used to analyze luciferase expression in accordance with the manufacturer's protocol. Firefly luciferase activity was normalized to Renilla luciferase activity to adjust for variations in transfection efficiency among experiments.
Statistical analysis
Data presented as graphs are the means ± SD of at least three independent experiments. Statistical analysis was performed using Student's t-test. P < 0.05 was considered significant.
Results
Reduction of miR-195 expression during activation of primary-cultured HSCs
It has been known that, when maintained in a plastic culture plate, freshly isolated primary-cultured HSCs undergo spontaneous activation and transformation into myofibroblastic cells that express α-SMA and produce fibrogenic mediators, such as type I collagen and transforming growth factor-β. In our preliminary experiments using primary-cultured mouse HSCs, we noticed that the cells drastically decreased the expression of miR-195 when they underwent spontaneous activation (unpublished observation). The present study confirmed this notion as shown in Figure 1A. miR-195 expression level certainly decreased in activation process of primary-cultured mouse HSCs. In contrast, the expression levels of α-SMA and cyclin E1 mRNA increased (Fig. 1B). Accordingly, we considered that miR-195 plays a role as an antiproliferative and antiactivating miRNA in HSCs. As a matter of fact, there was a study showing that miR-16 family including miR-195 inhibits proliferation of lung cancer cells by silencing cyclins D1 and E1, and CDK6 (Liu et al., 2008). The result indicated by Figure 1 and the cited study together drove us to explore the IFN's antiproliferative action on HSCs (Mallat et al., 1995; Shen et al., 2002), focusing on miR-195 and cell cycle-related genes.
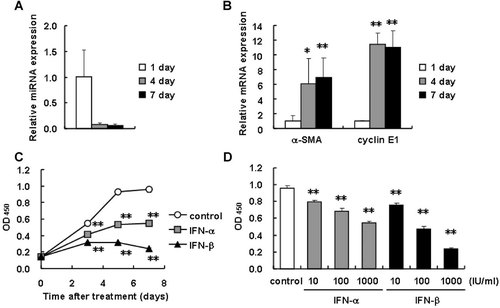
Expression of miR-195 in mouse HSCs during primary culture and growth inhibitory effect of IFN-α and -β on human stellate cells. A,B: Isolated mouse HSCs were cultured for the indicated periods. The expression levels of miR-195 (A), and α-SMA and cyclin E1 mRNA (B) were measured by real-time PCR. *P < 0.05, **P < 0.01 compared with 1 day. C,D: LX-2 cells were incubated with IFN-α or -β (1,000 IU/ml) for 3–7 days (C), or with IFN-α or -β at the concentration of 10–1,000 IU/ml for 7 days (D). Control indicates non-treated cells. The proportion of viable cells was determined using a WST-1 assay. **P < 0.01 compared with control.
Effects of IFN-α and -β on proliferation of HSCs
First, we investigated the effects of type I IFNs on the proliferation of LX-2 cells using a WST-1 assay. LX-2 cells in control culture continued to grow during the experimental period of 7 days (Fig. 1C). IFN-α and -β both, but the latter more actively, decreased cell proliferation time-dependently at a concentration of 1,000 IU/ml, supporting the previous studies (Mallat et al., 1995; Shen et al., 2002). Dose-dependency of the growth inhibition is shown in IFN concentrations from 10 to 1,000 IU/ml (Fig. 1D).
Effects of IFN-α and -β on cell cycle distribution
To elucidate the mechanism of the growth inhibitory effect of IFN, we next examined the change in cell cycle distribution in response to IFN-α and -β treatment by flow cytometry. LX-2 cells were synchronized in G0/G1 phase by serum starvation for 24 h. In non-treated cells (control), population in G0/G1 phase was reduced after serum exposure, which was accompanied by the increase of population in S phase. This cell cycle transition peaked at 24 h (Fig. 2, upper part). In cells treated with IFN-α or -β, the G0/G1 phase population was larger and the S phase population was smaller than in the control cells at 15 h and 24 h. In addition, the accumulation of cells in early S phase was observed at 32–48 h (Fig. 2, middle and lower parts). These delays in cell cycle shift were more potent in IFN-β-treated cells than in IFN-α-treated cells. It was concluded that type I IFN hampered HSC proliferation through a delay in the cell cycle at the transition from G1 to S phase and in the progression of S phase.
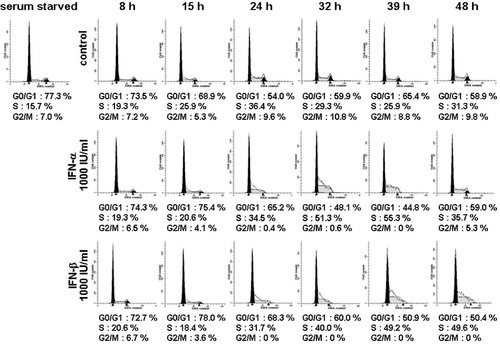
Effect of IFN-α and -β on cell cycle distribution in human stellate cells. LX-2 cells synchronized in G0/G1 phase were then incubated with IFN-α or -β (1,000 IU/ml) in DMEM/FBS for the indicated periods. Control indicates non-treated cells. The cell cycle was analyzed by flow cytometry. The white, black, and shaded region indicates the histogram measured by flow cytometry, G0/G1 phase (left) or G2/M phase (right), and S phase, respectively, as analyzed by ModFIT LT software.
Regulation of cyclin E1 and p21 expression by IFN-β
IFN-β was chosen in the following experiments because of its more potent inhibition of cell cycle progression than IFN-α as described above. The transition from G1 to S phase and the progression of S phase are known to be influenced by various regulators (Golias et al., 2004). Among them, we found that IFN-β significantly decreased cyclin E1 mRNA expression levels by 0.6- to 0.7-fold at 6 and 24 h and increased p21 mRNA expression levels by 1.4- to 2.3-fold at 6, 24, 48, and 72 h in LX-2 cells (Fig. 3). The expression levels of CDK4 and CDK6 were also reduced by IFN-β at early phase with less extent. The others showed negligible change within 24 h although variable dynamics were seen thereafter; changes of cyclin D1, CDK2, and p27 expression at late phase were toward cell cycle promotion with currently unknown reason.
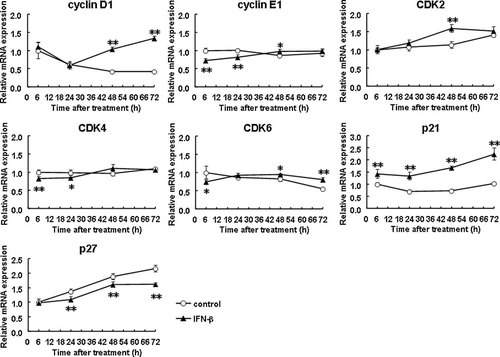
Expression of cell cycle-related genes in stellate cells. LX-2 cells were incubated with IFN-β (1,000 IU/ml) for up to 72 h for determining the expression levels of mRNAs of cyclin D1, cyclin E1, CDK2, CDK4, CDK6, p21, and p27. Control indicates non-treated cells. *P < 0.05, **P < 0.01 compared with control.
Regulation of miR-195 expression by IFN-β
The result indicated from Figure 1 strongly suggested the possibility that IFN-β increase the expression of miR-195 in LX-2 cells. To test this possibility, we examined the expression levels of miR-195 in IFN-β-treated LX-2 cells. As a result, the miR-195 expression level was significantly increased by IFN-β treatment at 24, 48, and 72 h (Fig. 4A).
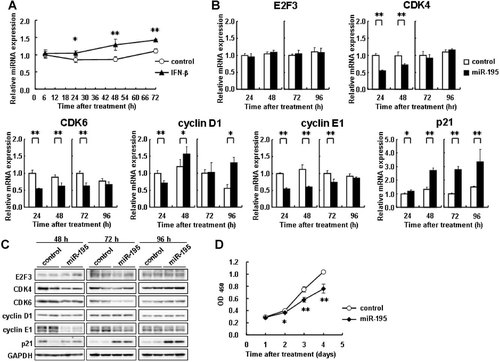
Regulation of expression of cell cycle regulators by miR-195. A: LX-2 cells were incubated with IFN-β (1,000 IU/ml) for up to 72 h for determining the expression levels of miR-195. Control indicates non-treated cells. *P < 0.05, **P < 0.01 compared with control. B–D: LX-2 cells were transfected with 50 nM miR-195 precursor or a negative control (control). B: mRNA expression levels of E2F3, CDK4, CDK6, cyclin D1, cyclin E1, and p21 measured at 24, 48, 72, and 96 h post-transfection. C: Protein expression of E2F3, CDK4, CDK6, cyclin D1, cyclin E1, and p21 examined at 48, 72, and 96 h post-transfection. D: Growth of LX-2 cells transfected with 50 nM miR-195 precursor or a negative control (control) was measured using a WST-1 assay. *P < 0.05, **P < 0.01 compared with control.
Regulation of cyclin E1 and p21 expression by miR-195
The results obtained from experiments shown in Figures 3 and 4A led us to hypothesize that IFN-β up-regulates the expression of miR-195, which then down-regulates the expression of cyclin E1 and up-regulates the expression of p21. In addition, there had been a study reporting that miR-195 targets E2F3, CDK6, and cyclin D1 in addition to cyclin E1 (Xu et al., 2009). Under these considerations, we examined the changes in the expression levels of the above-mentioned cell cycle-related molecules and CDK4 by introducing miR-195 precursor into LX-2 cells. Transfection of miR-195 precursor increased the miR-195 expression levels in LX-2 cells by up to 10,000–30,000 times compared with those in cells transfected with negative control (data not shown). Cyclin E1 mRNA and protein expression levels showed a remarkable reduction up to 72 h as result of miR-195 overexpression (Fig. 4B,C). On the other hand, p21 mRNA and protein expression levels showed a marked increase. CDK4, CDK6, and cyclin D1 expression levels were significantly changed at the mRNA level, but negligibly at the protein level. E2F3 mRNA and protein expression levels were unchanged (Fig. 4B,C). These results suggested that miR-195 mainly regulated cyclin E1 and p21 expression in LX-2 cells. Moreover, transfection of miR-195 precursor (50 nM) decreased the proliferation of LX-2 cells in the WST-1 assay (Fig. 4D). These results showed that miR-195 down-regulates endogenous cyclin E1 expression and up-regulates p21 expression, resulting in the attenuation of cell cycle progression and cell proliferation.
Interaction of miR-195 with cyclin E1 3′UTR in LX-2 cells
Next, we examined whether miR-195 interacted directly with cyclin E1 3′UTR in LX-2 cells. The predicted miRNA target sites for miR-195 in the cyclin E1 3′UTR were analyzed using TargetScan Human Release 5.1 (http://www.targetscan.org/). The cyclin E1 3′UTR contained two target sites for miR-195 (Fig. 5A,B). To investigate the direct interaction between them, the part of the cyclin E1 3′UTR containing the two miR-195 target sites (497 bp) was cloned from LX-2 cells, inserted the downstream of a firefly luciferase reporter gene in a pmirGLO vector (Fig. 5C), and cotransfected into LX-2 cells. As shown in Figure 5D, luciferase reporter activity decreased significantly in miR-195 precursor-transfected cells compared with cells transfected with a negative control of the precursor. These results suggested a direct interaction between miR-195 and cyclin E1 3′UTR in LX-2 cells. Binding site of miR-195 was not found in p21 3′UTR by TargetScan.
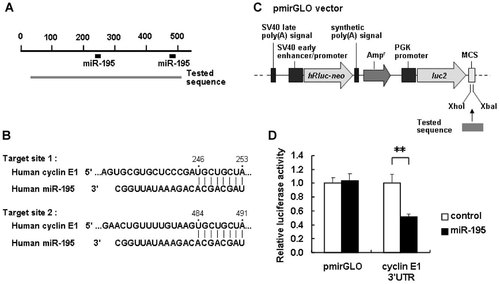
Interaction of miR-195 with the 3′UTR of cyclin E1 mRNA. A: Schematic indication of the putative miR-195 target sites in the 3′UTR of the cyclin E1 mRNA. Tested sequences indicate the regions that were inserted into the luciferase reporter vector. B: Predicted pairing of the target region and miRNAs. C: Structure of the luciferase reporter vector (Ogawa et al., 2010). The putative miR-195 target region in cyclin E1 3′UTR (tested sequence) was ligated into the MCS. Arrows indicate the gene directions. AmpR indicates an ampicillin resistance gene. D: Reporter gene assay of the interaction between the 3′UTR of cyclin E1 mRNA and miR-195 in LX-2 cells. Results are expressed as the relative activities against the activity in the presence of the control. *P < 0.05, **P < 0.01 compared with control.
Regulation of cyclin E1 expression by IFN-β and miR-195
To confirm the contribution of miR-195 to the inhibitory effect of IFN-β on cyclin E1 expression, LX-2 cells were first transfected with 50 nM miR-195 inhibitor and then treated with 1,000 IU/ml IFN-β. As shown in Figure 6A, miR-195 inhibitor blocked the inhibitory effect of IFN-β on cyclin E1 mRNA expression at 16 and 24 h. Although there was no difference in the cyclin E1 mRNA expression between IFN-β-treated cells and non-treated cells (control) at 48 h, the cyclin E1 mRNA expression level in miR-195 inhibitor plus IFN-β-treated cells was up-regulated compared with non-treated cells (Fig. 6A). Immunoblot analysis revealed that miR-195 inhibitor elevated the cyclin E1 expression level of IFN-β-treated cells at 24 and 48 h (Fig. 6B).
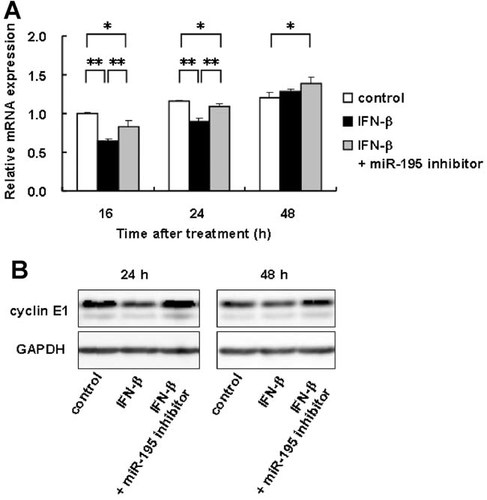
Regulation of cyclin E1 expression by IFN-β and miR-195. LX-2 cells were transfected with 50 nM miR-195 inhibitor or a negative control. After 6 h, the culture medium was changed and then IFN-β (1,000 IU/ml) was added. Cells were then incubated for the indicated time periods. A: mRNA expression levels of cyclin E1. B: Protein expression levels of cyclin E1. GAPDH are for loading adjustment. Control; cells were transfected with a negative control and incubated without IFN-β. *P < 0.05, **P < 0.01.
Discussion
In this study, we showed that IFN-β is more antiproliferative on LX-2 cells than IFN-α, which appears to be contradictory to their known mechanism of action: both IFN-α and -β exert their activities through the common signaling pathway, beginning with binding to the same type I IFN receptor (IFNAR) consisting of IFNAR1 and IFNAR2, which activate the common components of janus kinase/signal transducer and activator of transcription (STAT) pathway (Darnell et al., 1994). However, a similar activity difference between the IFNs has also been demonstrated in colon cancer cell lines (Katayama et al., 2007) and in rat HSCs (Shen et al., 2002). Some studies showed that IFN-β but not IFN-α formed a stable complex with IFNARs, suggesting that IFN-β may interact with IFNAR chains in a manner different from IFN-α (Croze et al., 1996; Russell-Harde et al., 1999).
We showed here that IFN-β down-regulated the expression of cyclin E1 and up-regulated the expression of p21, which caused the cells to be less active proceeding in the transition from G0 to G1 phase and in the progression of S phase. The cell cycle is regulated by various molecules, such as cyclins and CDKs. Cyclin E is essential in activating CDK2. The cyclin E-CDK2 complex phosphorylates pRb at G1 phase, leading to gene transcription activities that are needed in S phase, and also activates the factors involved in DNA replication at early S phase (Golias et al., 2004). It has been reported that cyclin E1 expression increased in non-parenchymal cells of human fibrotic liver and that cyclin E1-deficient mice developed milder liver fibrosis compared with wild-type mice after CCl4 administration (Nevzorova et al., 2010). These results imply that cyclin E1 regulates the progression of liver fibrosis by accelerating HSC proliferation.
The most frequent miRNAs that targets cyclin E1 are the miR-16 family, which consists of miR-15, -16, -195, -424, and -497 (Liu et al., 2008; Wang et al., 2009). We here observed the induction of miR-195 by IFN-β. miR-195 was reported to be down-regulated in human HCC tissues and to suppress HCC growth through the targeted interference of cyclin D1, CDK6, and E2F3 in a xenograft mouse model (Xu et al., 2009), while it was reported to target cyclin E1 in addition to the above-mentioned factors in A549 cells (Liu et al., 2008). miR-15b and miR-16 are down-regulated concomitantly with HSC activation and their overexpression induces apoptosis and a delay of cell cycle in HSCs by targeting Bcl-2 and cyclin D1 (Guo et al., 2009a,b). However, the role of miR-195 in HSCs remains unknown. We showed here that miR-195 expression was decreased during spontaneous activation of primary-cultured mouse HSCs and that miR-195 interacted with cyclin E1 3′UTR and lowered the expression levels of the cyclin E1 mRNA and protein in LX-2 cells. These results suggest that the down-regulation of miR-195 may associate with the proliferation of HSCs in fibrotic liver similarly to miR-15 and miR-16. In this study, the changes of the protein expression levels of E2F3, CDK6, and cyclin D1, which were reported to be regulated by miR-195 (Xu et al., 2009), were negligible by miR-195, although the exact reason for this phenomenon was not determined. However, because the total context scores obtained by TargetScan were −0.73 for cyclin E1, −0.33 for E2F3, −0.32 for cyclin D1, and −0.09 for CDK6, the result obtained here was thought to be reasonable. In addition, minimal or negligible effect of miR-195 on the expression of E2F3, CDK4, CDK6, and cyclin D1 was compatible with that of IFN-β on these factors. Furthermore, inhibition of miR-195 by miR-195 inhibitor attenuated the effect of IFN-β on cyclin E1 expression, though not so strong. Taken together, it is most likely that the down-regulation of cyclin E1 by IFN-β treatment in HSCs is mediated through miR-195 up-regulation. The mechanism through which IFN-β induces miR-195 in LX-2 cells need to be explored further.
It is well known that IFNs induce the expression of p21 in various cancer cells (Sangfelt et al., 1999; Katayama et al., 2007). We also observed the up-regulation of p21 in IFN-β-treated cells. Therefore, p21, in addition to cyclin E1, may play a role in IFN-induced growth inhibition of HSCs. Until now, it has been reported that IFNs induce p21 expression through the binding of STAT and IFN regulatory factor, which are critical signaling molecules after IFN-IFNAR interaction, to p21 gene promoter (Gartel and Tyner, 1999). Unexpectedly, we found the up-regulation of p21 by miR-195 (Fig. 4). The results obtained here raise a new possibility that the up-regulation of p21 by IFN-β in HSCs may be partially mediated through miR-195.
In conclusion, type I IFN, in particular IFN-β, inhibited the proliferation of human HSCs by delaying the cell cycle in G1 to early S phase through the down-regulation of cyclin E1 and up-regulation of p21. The cyclin E1 down-regulation and p21 up-regulation were partially mediated by miR-195 that was up-regulated by IFN-β. This study raises a new mechanistic aspect of the antifibrotic effect of IFN in liver fibrosis and the possibility of influencing miR-195 as a therapeutic strategy for liver fibrosis.
Acknowledgements
This work was supported by a grant from the Ministry of Health, Labour and Welfare of Japan to N. Kawada (2008–2010).




