ClC-3 is a main component of background chloride channels activated under isotonic conditions by autocrine ATP in nasopharyngeal carcinoma cells
Abstract
In this study, the activation mechanisms of the background chloride current and the role of the current in maintaining of basal cell volume were investigated in human nasopharyngeal carcinoma CNE-2Z cells. Under isotonic conditions, a background chloride current was recorded by the patch clamp technique. The current presented the properties similar to those of the volume-activated chloride current in the same cell line and was inhibited by chloride channel blockers or by cell shrinkage induced by hypertonic challenges. Extracellular applications of reactive blue 2, a purinergic receptor antagonist, suppressed the background chloride current in a concentration-dependent manner under isotonic conditions. Depletion of extracellular ATP with apyrase or inhibition of ATP release from cells by gadolinium chloride decreased the background current. Extracellular applications of micromolar concentrations of ATP activated a chloride current which was inhibited by chloride channel blockers and hypertonic solutions. Extracellular ATP could also reverse the action of gadolinium chloride. Transfection of CNE-2Z cells with ClC-3 siRNA knocked down expression of ClC-3 proteins, attenuated the background chloride current and prevented activation of the ATP-induced current. Furthermore, knockdown of ClC-3 expression or exposures of cells to ATP (10 mM), the chloride channel blockers 5-nitro-2-(3-phenylpropylamino) benzoic acid (NPPB) and tamoxifen, or reactive blue 2 increased cell volume under isotonic conditions. The results suggest that ClC-3 protein may be a main component of background chloride channels which can be activated under isotonic conditions by autocrine/paracrine ATP through purinergic receptor pathways; the background current is involved in maintenance of basal cell volume. J. Cell. Physiol. 226: 2516–2526, 2011. © 2010 Wiley-Liss, Inc.
The regulation of cell volume is a fundamental function of cells for maintaining constant size and is involved in the process of cell proliferation, migration, and apoptosis. Regulation of cell volume requires the participation of ion transports across the cell membrane, including appropriate activities of chloride channels among others (Lang et al., 2007). Volume-activated Cl− channels play a crucial role in the process of regulatory volume decrease (RVD) induced by hypotonic stresses (Wang et al., 2000). Our previous experiments demonstrated that volume-activated Cl− channels and cell volume regulation mechanisms were involved in cell cycle progression (Chen et al., 2002, 2007; Wang et al., 2002) and migration (Mao et al., 2007, 2008) in human nasopharyngeal carcinoma CNE-2Z cells. It was reported by us and others that a background Cl− current was detectable under isotonic conditions (Duan et al., 1997a; Ransom et al., 2001; Borg et al., 2002; Chen et al., 2002; Do et al., 2005; Yoshise et al., 2009; Rinke et al., 2010). However, the properties, activation mechanisms and roles of this background chloride current have not been clarified.
By far, much attention has been paid to the chloride current activated by hypotonicity-induced cell swelling. As for the activation of the background current, it is far from clarified. Purinergic receptor pathways have been proposed to be involved in the process of RVD under hypotonic conditions (Okada et al., 2001). We demonstrated previously that micromolar concentrations of extracellular ATP activated a chloride current and shrank human nasopharyngeal carcinoma cells (He et al., 2004). The ATP-activated chloride current possesses the characteristics similar to those of the volume-activated chloride current, implying that purinergic receptor signaling pathways may be involved in the activation of volume-sensitive chloride channels under hypotonic conditions. However, it is not clear whether purinergic receptor signaling pathways are activated and contribute to the activation of the background current under normal isotonic conditions.
As for the molecular identification of the background chloride channel, it is not clear at present. Our previous work and the data obtained by others support the theory that ClC-3, a member of the voltage-gated ClC Cl− channel family, is the molecular component involved in activation of volume-sensitive Cl− currents under hypotonic conditions although it is still being debated (Duan et al., 1997b; Wang et al., 2000, 2003; Hermoso et al., 2002; Jin et al., 2003; Mao et al., 2008; Yoshise et al., 2009; Xiong et al., 2010). The ClC-3 protein may also be a molecule associated with the activation of the background current. In this study, the involvement of ClC-3 in the formation of the background chloride current, the activation mechanisms of the current and its role in basal cell volume maintenance were investigated in human nasopharyngeal carcinoma CNE-2Z cells.
Materials and Methods
Cell culture
The poorly differentiated human nasopharyngeal carcinoma cells (CNE-2Z cells) were routinely grown in 25 cm2 culture flasks with the RPMI 1640 medium containing 10% newborn calf serum, 100 IU/ml penicillin, and 100 µg/ml streptomycin and incubated in a humidified atmosphere of 5% CO2 at 37°C. The cells were subcultured every 2 days.
Preparation of cells for current recordings and volume measurements
Cells cultured in the flasks were trypsinized, centrifuged, and re-suspended in RPMI 1640 medium with 10% newborn calf serum, 100 IU/ml penicillin, and 100 µg/ml streptomycin. Cell suspension was plated on 22 mm round coverslips (150 µl/coverslip) located in 35 mm culture dishes and incubated at 37°C for 2–3 h before volume measurements and current recordings (Chen et al., 2007).
Whole cell current recording
Whole cell currents of single CNE-2Z cells were recorded using the patch clamp technique described previously (Wang et al., 2000), with a List EPC-7 patch-clamp amplifier (List Electronic, Darmstadt, Germany). The patch clamp pipettes were made from standard wall borosilicate glass capillaries with an inner filament on a two-stage vertical puller and gave a resistance of 5–10 MΩ when filled with the pipette solution. Electrode and whole cell capacitance was determined using the amplifier functions. Once the whole cell configuration was established, cells were held at the chloride equilibrium potential (0 mV) and were stepped repeatedly to 0, ±40, and ±80 mV using 200 msec pulses with 4 sec interval between steps. Command voltages and whole cell currents were recorded simultaneously by a computer via a laboratory interface (CED 1401, Cambridge, UK) with a sampling rate of 3 kHz. Voltage pulse generation, data collection, and current analysis were performed by the computer using the EPC software package (CED). Whole-cell background Cl− currents were measured under isotonic conditions. Experiments were carried out at room temperature (20–24°C). All current measurements were made at 10 msec after onset of each voltage step when the collected data were analyzed. The inhibition of background Cl− currents was calculated using the equation: Inhibition (%) = (CurrentCTRL − CurrentTEST)/CurrentCTRL × 100, where CurrentCTRL is the background current under isotonic conditions, CurrentTEST is the current recorded after inhibitory treatments.
Measurements of cell volume
Cell volume were measured using the methods described by us previously (Wang et al., 2002; Mao et al., 2005). Cell images were captured by a charge-coupled device digital camera (Mono CCD625, Leica, Wetzlar, Germany) that was connected to the microscope (Leitz DMIL; Leica Mikroskopie und Systeme, Wetzlar, Germany). The acquisition of the cell images was controlled by the Quantimet Q500MC image processor and analysis software (Leica). The cell volume (V) was calculated from cell diameters (d) with the equation V = 4/3π(d/2)3. The changes of cell volumes induced by different treatments were calculated using the formula: (VTEST − VCTRL)/VCTRL × 100%, where VCTRL is the basal cell volume under isotonic conditions, VTEST is the cell volume after various treatments.
Solutions and chemicals
The pipette solution contained (in mM): 70 N-methyl-D-glucamine chloride (NMDG-Cl), 1.2 MgCl2, 10 HEPES, 1 EGTA, 140 D-mannitol and 2 ATP. The isotonic bath solution contained (in mM): 70 NaCl, 0.5 MgCl2, 2 CaCl2, 10 HEPES, and 140 D-mannitol. Twenty-three percent and 47% hypertonic solutions were obtained by adding 70 and 140 mM D-mannitol into the isotonic bath solutions, respectively. The osmolarity of solutions was measured with a freezing-point osmometer (OSMOMAT 030; Gonotec, Berlin, Germany). The osmolarity of pipette and isotonic bath solutions was adjusted to 300 mOsmol/L with D-mannitol. The pH of the pipette and bath solutions was adjusted to 7.25 and 7.4, respectively, with 1 M Tris-base. ATP and the chloride channel blockers, 5-nitro-2-(3-phenylpropylamino)benzoic acid (NPPB) and tamoxifen were dissolved in distilled water, dimethyl sulfoxide (DMSO) and methanol, at the concentrations of 100, 50, and 20 mM, respectively, and diluted to the indicated final concentrations with the isotonic bath solution. Gadolinium chloride (GdCl3), apyrase, and the P2Y purinergic receptor antagonist reactive blue 2 (RB2) were dissolved in distilled water at the concentrations of 50 mM, 100 mM, and 120 U/ml, respectively, and diluted to the indicated final concentrations with the isotonic bath solution. The pH of the final solutions was adjusted to 7.4. All chemicals were purchased from Sigma-Aldrich, St. Louis, MO.
ClC-3 siRNA treatments
The sequences of ClC-3 siRNA duplex were as follows: sense, 5′-CAA UGG AUU UCC UGU CAU ATT-3′; antisense, 5′-UAU GAC AGG AAA UCC AUU GTA-3′. Sequences of the negative control siRNA duplex were: sense, 5′-UUC UCC GAA CGU GUC ACG UTT-3′; antisense, 5′-ACG UGA CAC GUU CGG AGA ATT-3′. siRNAs were synthesized and labeled with FAM carboxyfluorescein by GenePharma, Shanghai, China in China and stored at −20°C. CNE-2Z cells were incubated in RPMI 1640 medium without antibiotics in 24-well culture plates for 24 h and reached 30–50% confluence. The cells were transfected with ClC-3 siRNA (100 nM) or negative control siRNA plus lipofectamine 2000 (1 µl in 500 µl medium, Invitrogen, Carlsbad, CA) in serum and antibiotics-free culture medium for 6 h and incubated in normal RPMI 1640 medium containing serum at 37°C in a CO2 incubator for 48 h. Western blotting and electrophysiological recordings were carried out 48 h after transfection.
Western blotting
Cells were lysed using the buffer containing Tris–Cl (50 mM), NaCl (150 mM), NaN3 (0.02%), Nonidet P-40 (1%), SDS (0.1%), sodium deoxycholate (0.5%), leupeptin (5 µg/ml), and aprotinin (1 µg/ml). The protein content of cell lysates was quantified with Coomassie brilliant blue. Proteins were separated by SDS–PAGE and transferred to nitrocellulose membranes (Schleicher & Schuell, Keene, NH). The membranes were blocked at room temperature (24–26°C) for 1 h in a solution containing 130 mM NaCl, 2.5 mM KCl, 10 mM Na2HPO4, 1.5 mM KH2PO4, 0.1% Tween-20, and 5% BSA (pH 7.4). The membranes were treated with the primary antibodies (rabbit anti-ClC-3 antibody, 1:300, Alomone Laboratories, Jerusalem, Israel; mouse anti-actin antibody, 1:500, Boster Bio-technology, Wuhan, China) at 4°C overnight and then with the HRP-linked secondary antibodies (goat anti-rabbit IgG, 1:1,500; goat anti-mouse IgG, 1:1,000, ProteinTech Group, Inc, Wuhan, China) for 1 h at room temperature. Final detection was accomplished with Western blot luminol reagents (Santa Cruz Biotechnology, Inc, California).
Statistical analysis
Values are expressed as means ± standard error (number of observations) and, where appropriate, have been analyzed using ANOVA. Values of P < 0.05 were considered to be significant. All experiments were repeated at least three times.
Results
Background currents recorded under isotonic conditions
CNE-2Z cells were clamped at 0 mV in the whole cell configuration and stepped to 0, ±40, and ±80 mV repeatedly. Under isotonic conditions, a relatively stable background current was recorded. The current exhibited negligible time-dependent inactivation (Fig. 1A) and almost linear current–voltage (I–V) relationship, with the current density of 9.6 ± 0.6 pA/pF at +80 mV and −8.9 ± 0.6 pA/pF at −80 mV (n = 58, Fig. 1B). The current–voltage relationship demonstrated that the background current reversed at a voltage close to the calculated equilibrium potential for Cl− (about 0 mV). The current properties were not changed significantly by increasing the voltage pulses to ±120 mV (data not shown). In these experiments, there was no K+ present either in the electrode or bath solutions. The Cl− concentrations inside and outside the cells were almost equal, giving a value of −0.9 mV for ECl, which was very close to the experimental reversal potential. The equilibrium potentials for Na+ and Ca2+ were both predicted to be greater than +200 mV. Thus, the data strongly support the hypothesis that the background currents recorded in this study were carried primarily by Cl−.
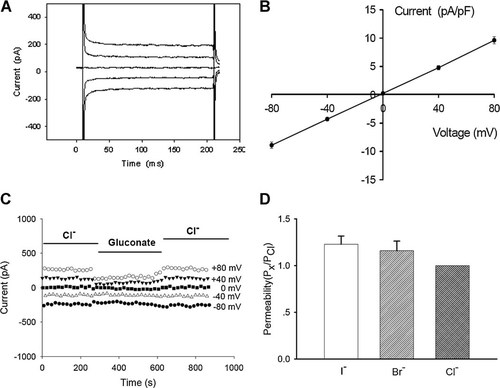
Whole cell background Cl− currents and anion permeability of background chloride channels in CNE-2Z cells. The cell was held at 0 mV and then stepped to 0, ±40, and ±80 mV using 200 msec pulses with an interval of 4 sec between voltage pulses. A typical sample of the background chloride current under the isotonic condition is shown in (A). The I–V relationship of the current is presented in (B) (mean ± standard error of 58 cells). C: Effect of replacing sodium chloride with sodium gluconate on the background current. D: Permeability ratios (PX/PCl) of various anions (X−) relative to Cl−, calculated using the modified Goldman–Hodgkin–Katz equation (mean ± standard error of six cells).
The effect of a higher extracellular osmolarity (320 mOsmol/L) on the background current was also tested. The background current was decreased from 9.8 ± 0.8 to 7.8 ± 0.7 pA/pF (n = 5) at +80 mV when the extracellular osmolarity was increased from 300 to 320 mOsmol/L. The remaining current could be inhibited further by 100 µM NPPB or 47% hypertonic solution.
Anion permeability of background channels
When the recorded background current was stable in the isotonic solution, the isotonic solution containing 70 mM of NaCl was replaced with the solution containing equimolar NaI, NaBr, or sodium gluconate. The anion substitution shifted the reversal potential. Figure 1C shows the typical time course of gluconate replacement. The replacement of sodium chloride with sodium gluconate inhibited the background current, especially the outward component of the current. The permeability ratios of PI/PCl and PBr/PCl, calculated from the shifts in reversal potential using the modified Goldman–Hodgkin–Katz equation, were 1.23 ± 0.09 and 1.16 ± 0.10, respectively (n = 6, Fig. 1D). The results indicate that the permeability of the channel to anions is I− > Br− > Cl−.
In the gluconate replacement experiments, apart from the shift in reversal potential, both the inward and outward currents were reduced when the isotonic solution containing 70 mM NaCl was replaced with the solution containing equimolar sodium gluconate (Fig. 1C). The phenomenon may imply a partial blockage of the chloride channels by sodium gluconate.
Inhibitions of background currents by chloride channel blockers and ATP
The above data indicate that the recorded background current was mainly carried by Cl−. To study further the involvement of chloride channels in the formation of the background current, the effects of ATP and the Cl− channel blockers NPPB and tamoxifen on the current were investigated.
As shown in Figure 2A,B, extracellular applications of 10 mM ATP inhibited significantly the background current. The effects of ATP on the current were voltage-dependent. The inhibition (60.4 ± 1.9%, n = 9) of the outward current at +80 mV was stronger than that (51.5 ± 1.5%, n = 9) of inward current at −80 mV (+80 mV vs. −80 mV, P < 0.01). The inhibitory effects were reversible. The background current recovered gradually once the isotonic solution containing ATP was washed out and exchanged with the isotonic solution without additives.
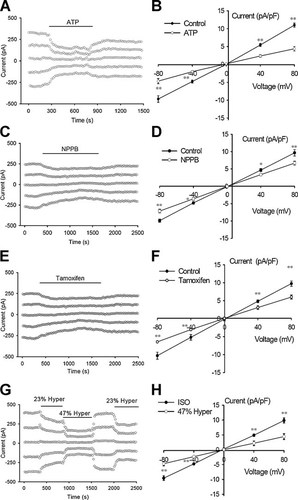
Inhibitions of the background current by ATP, chloride channel blockers NPPB and tamoxifen and hypertonic challenges. Extracellular applications of 10 mM ATP (A,B), 50 µM NPPB (C,D), 20 µM tamoxifen (E,F), or 23% and 47% hypertonic solutions (G,H) inhibited the background current recorded under isotonic conditions. Cells were held at 0 mV and then stepped repeatedly to 0, ±40, and ±80 mV using 200 msec pulses with an interval of 4 sec between voltage pulses. Typical time courses of the recordings are shown in (A,C,E,G). I–V relationships are presented in (B,D,F,H) (mean ± standard error of 9, 8, 8, and 8 cells, respectively). *P < 0.05, **P < 0.01 (vs. control).
Similar to the effects of ATP, extracellular applications of NPPB also inhibited the background current reversibly (Fig. 2C,D). However, the inhibition induced by NPPB was not voltage-dependent. NPPB (50 µM) decreased significantly the outward current at +80 mV and the inward current at −80 mV by 30.2 ± 2.6% and 28.5 ± 1.3%, respectively (n = 8; P < 0.01, vs. control). The difference between the inhibitions of the outward and inward currents was not significant (P > 0.05).
The characteristics of tamoxifen effects on the background current were similar to those of NPPB. Tamoxifen (20 µM) suppressed reversibly the current in a voltage-independent manner (Fig. 2E,F). The outward current at +80 mV and inward current at −80 mV were inhibited by 38.3 ± 2.6% and 36.9 ± 2.5%, respectively (n = 8; P < 0.01, vs. control).
Sensitivity of background currents to cell volume changes
To observe the sensitivity of the background current to cell volume changes, the effects of hypertonic challenges on the current were detected. As shown in Fig. 2G,H, exposures of the cells to hypertonic bath solutions inhibited the background current. The outward (at +80 mV) and inward currents (at −80 mV) were decreased, respectively, by 30.4 ± 1.5% and 24.2 ± 1.7% by 23% hypertonic solution (n = 8; P < 0.01, vs. control) and by 55.1 ± 1.9% and 51.1 ± 1.8% by 47% hypertonic solution (n = 8; P < 0.01, vs. control). These data indicate that the background current is volume-sensitive and may play important roles in maintenance of normal cell volume under isotonic conditions.
Cell volume changes under the hypertonic conditions were confirmed by the cell image analysis (data not shown). Exposures to 23% and 47% hypotonic solutions shrank the cells by 11.2 ± 0.7% (n = 18, P < 0.01) and 15.1 ± 1.0% (n = 20, P < 0.01), respectively.
Cell swelling induced by chloride channel blockers under isotonic conditions
To investigate whether or not the background current is involved in maintenance of normal cell size under isotonic conditions in CNE-2Z cells, the effects of extracellular applications of the chloride channel blockers, tamoxifen, and NPPB, on basic cell volume were tested. Cells were bathed in isotonic solution for 5 min and then perfused with isotonic solutions containing 20 µM tamoxifen or 50 µM NPPB for 20 min. The results demonstrated that extracellular applications of 20 µM tamoxifen or 50 µM NPPB swelled the cells by 11.3 ± 1.6% (n = 20, P < 0.01) or 6.5 ± 1.1% (n = 18, P < 0.01), respectively (Fig. 3). The results indicate that some volume-sensitive chloride channels are activated under the isotonic condition.
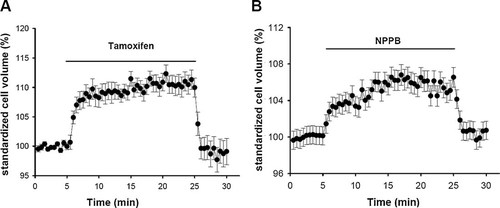
Cell swelling induced by chloride channel blockers under isotonic conditions. Perfusing the cells with the isotonic solutions containing 20 µM tamoxifen or 50 µM NPPB swelled the cells, suggesting that some volume-sensitive channels are activated under isotonic conditions. These data in (A,B) are mean ± standard error of 20 and 18 cells, respectively.
Inhibitions of the background Cl− current by purinergic receptor antagonist
To investigate the roles of P2Y purinergic receptors in the activation of the background Cl− current, the effects of reactive blue 2 (RB2), a P2Y purinergic receptor antagonist that inhibits virtually all P2Y receptors (Jacobson et al., 2009), was observed. Typical background Cl− current traces under the normal isotonic condition and after the treatment with 50 µM RB2 are shown in Figure 4A,B. As shown in Figure 4C, a stable background Cl− current was recorded in the CNE-2Z cell with continuous perfusion of the isotonic bath solution, and the extracellular application of 50 µM RB2 inhibited reversibly the current. Analysis of the data indicated that the inhibitory effects of RB2 on the background Cl− current were concentration-dependent (Fig. 4D,E). Fifty, 100, and 200 µM of RB2 inhibited, respectively, the outward current at +80 mV by 45.5 ± 4.7% (n = 6, P < 0.05), 49.2 ± 4.5% (n = 9, P < 0.01), and 55.8 ± 3.2% (n = 5, P < 0.05) and the inward current at −80 mV by 22.4 ± 4.2% (P < 0.05), 23.2 ± 6.0% (P < 0.05), and 36.2 ± 4.0% (P < 0.01).
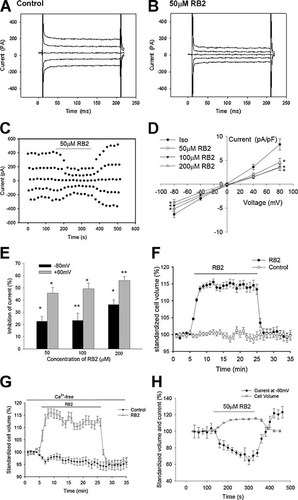
Inhibitions of background Cl− currents and increases of cell volume by the P2Y purinergic receptor antagonist reactive blue 2 (RB2) under isotonic conditions. The typical samples of the background Cl− current (at 0, ±40, and ±80 mV) in the normal isotonic bath solution and after the treatment with 50 µM RB2 are shown in (A,B), respectively. The typical time course of inhibition of the background current by 50 µM antagonist (RB2) is shown in (C). I–V relationships and inhibition rates of the current by different concentrations of RB2 are shown in (D,E). F,G: Changes of cell volume induced by 100 µM RB2 in the normal and Ca2+-free isotonic solutions. H: Relationship between the change of the background Cl− current recorded at −80 mV and that of cell volume induced by RB2 treatments. *P < 0.05, **P < 0.01 (vs. control). These data in (D,E,F,G,H) are mean ± standard error of 5–10.
Cell swelling induced by purinergic receptor antagonists
To further explore the roles of P2Y purinergic receptor signal transduction pathway in regulation of cell volume under isotonic conditions in CNE-2Z cells, RB2 was applied to block P2Y purinergic receptors. As shown in Figure 4F, cell sizes were relatively stable in the isotonic solution. However, exposures to the isotonic bath solution containing 100 µM RB2 swelled the cells. Cell swelling appeared in 30–60 sec and reached a peak in 3–4 min. Cell volume increased by 15.6 ± 0.9% (n = 10, P < 0.01) at peak. The cells regained gradually their initial sizes when the normal isotonic solution was returned to the bath.
Similar cell swelling induced by RB2 was also observed under Ca2+-free conditions. Cells were bath in the Ca2+-free isotonic solution (containing 2 mM EGTA) and exposed to the Ca2+-free solution containing 100 µM RB2. Cell volume was increased by the RB2 treatment by 16.2 ± 1.6% (n = 11, P < 0.01; Fig. 4G), suggesting that RB2-induced cell swelling is not dependent on extracellular calcium in the period observed.
As shown above, RB2 treatments inhibited the background current and swelled the cells. The relationship between the inhibition of the background Cl− current and the change in cell volume induced by RB2 treatments was shown in Figure 4H. Analysis of the data indicated that the change of the background current was negatively correlated with that of cell volume.
Effects of apyrase on the background Cl− currents
As shown above, P2Y purinergic receptor antagonists inhibited the background Cl− current in CNE-2Z cells. To explore the nature of extracellular messengers acting on the P2Y purinergic receptors, apyrase was applied to catalyze the hydrolysis of extracellular ATP.
Apyrase (20 U/ml) depressed the background Cl− current under isotonic conditions. Typical background Cl− current traces under the normal isotonic condition and in the isotonic solution with 20 U/ml apyrase are shown in Figure 5A,B. The inhibition of the current was reversible (Fig. 5C). The outward (at +80 mV) and inward (at −80 mV) background chloride currents were decreased by 72.2 ± 5.5% and 62.35 ± 5.1%, respectively, by 20 U/ml apyrase (n = 6; P < 0.05, vs. control; Fig. 5D). The inhibitory effect of apyrase was further verified by applications of more diluted apyrase. Apyrase at the final concentration of 2 U/ml suppressed the background current by 44.3 ± 7.6% at +80 mV and 36.6 ± 7.9% at −80 mV, respectively (n = 4; P < 0.05, vs. control; Fig. 5D).
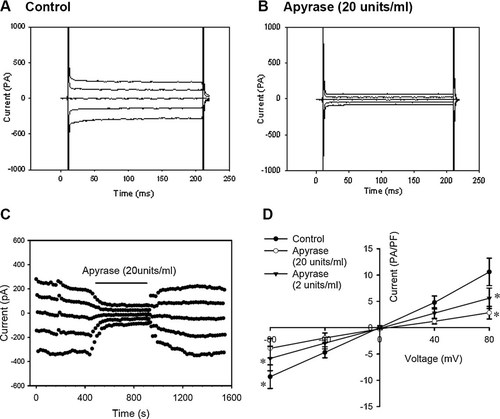
Inhibition of the background Cl− current by apyrase under isotonic conditions. Typical samples of the background Cl− current in the normal isotonic bath solution and inhibition of the current by 20 U/ml apyrase are shown in (A,B). A typical time course of inhibition of the current by apyrase is shown in (C). I–V relationships are presented in (D) (mean ± standard error of six cells). *P < 0.05 (vs. control).
Activation of chloride currents by extracellular ATP
The above results indicate that the activation of background current is associated with the purinergic pathway. To study further, the effects of extracellular ATP in micromolar scales were tested (Fig. 6). As shown in Figure 6A,B, extracellular applications of ATP (0.25, 0.5, and 1 µM) activated a chloride current in a concentration-dependent manner. The density of the current activated by 0.25, 0.5, and 1 µM ATP was 25.3 ± 0.6, 35.3 ± 5.0, and 37.4 ± 7.3 pA/pF at +80 mV, respectively (n = 6).
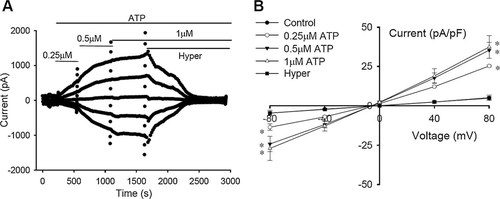
Chloride currents activated by extracellular ATP. A: Typical activation process of the chloride current induced by extracellular applications of ATP (0.25, 0.5, and 1 µM) and inhibition of the current by 47% hypertonic bath solution (Hyper). B: I–V relationships under different treatments (mean ± standard error, n = 6). *P < 0.05 (vs. control).
Similar to the background current, the ATP-activated current exhibited almost linear current–voltage (I–V) relationship (Fig. 6B) and negligible time-dependent inactivation (Fig. 7E). Furthermore, exposures of the cells to 47% hypertonic solution (Fig. 6A,B) and extracellular application of the chloride channel blocker NPPB (100 µM, Fig. 7A) inhibited the ATP-activated current.
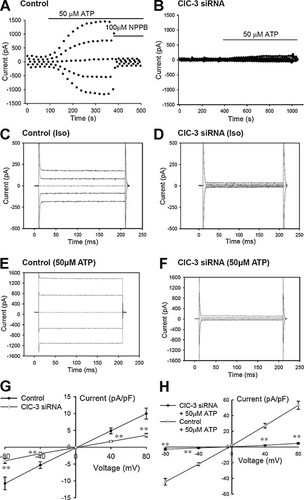
Inhibitions of the background and ATP-activated chloride currents by ClC-3 siRNA treatments. A,B: Representative time courses of ATP-activated chloride currents in control cells (A) and in the cells transfected with ClC-3 siRNA (100 nM, B) in the presence of lipofectamine 2000 (1/500 µl) for 48 h. The typical current traces of the background current under the isotonic condition (Iso) and the ATP-activated chloride current (50 µM ATP) are presented, respectively, in (C,E) for the control cells and in (D,F) for the cells treated with ClC-3 siRNA for 48 h. G,H: I–V relationships of the background (G) and ATP-activated (H) chloride currents in the control cells and in the cells treated with ClC-3 siRNA (mean ± standard error of 8–12 cells). **P < 0.01 (vs. control).
Suppression of background and ATP-activated chloride currents by ClC-3 siRNA treatments
To investigate the role of ClC-3 in the activation of the background current, the effects of knockdown of ClC-3 expression by ClC-3 siRNA were tested. Cells were treated with FAM carboxyfluorescein-labeled ClC-3 siRNA (100 nM) and lipofectamine 2000 (1 µl in 500 µl medium) for 6 h and incubated in normal 1640 RPMI medium for 48 h before current measurements. Cell fluorescence, which indicates successful transfection of ClC-3 siRNA, was monitored with a florescent microscope. The whole cell currents of the cells with fluorescence were recorded. The results show that the background chloride current was attenuated significantly by ClC-3 siRNA treatments (Fig. 7B,D), compared with that recorded in the control cells (Fig. 7A,C). The current was decreased from 10.0 ± 1.6 pA/pF (+80 mV, n = 8) in control cells to 3.4 ± 0.5 pA/pF (+80 mV, n = 12, P < 0.01) in the cells transfected with ClC-3 siRNA (Fig. 7G). The remaining background current of the cells with fluorescence could not be inhibited further by chloride channel blockers or by hypertonicity-induced cell shrinkage (data not shown). The results indicate that activation of the cell volume-sensitive background chloride current is associated with the expression of ClC-3 proteins.
ClC-3 siRNA treatments not only decreased the background chloride current but also inhibited or prevented the activation of the ATP-induced chloride current (Fig. 7B,F), compared with that recorded in the control cells (Fig. 7A,E). In the control cells, 50 µM ATP activated a large chloride current (53.1 ± 5.1 pA/PF at +80 mV, n = 5; Fig. 7H). However, only a very weak chloride current was induced by 50 µM ATP in the cells transfected with ClC-3 siRNA (4.7 ± 1.0 pA/PF at +80 mV, n = 5; Fig. 7H).
Analysis of cell images indicates that the cells treated with ClC-3 siRNA were larger in size than the control cells. Cell volume was increased from 6.8 ± 0.2 pl in control cells (n = 67) to 9.3 ± 0.6 pl in the cells treated with ClC-3 siRNA (n = 66, P < 0.01).
Treatments with the negative control siRNA plus the transfection agent lipofectamine 2000 did not affect significantly the background and ATP-activated chloride currents and cell volume.
Knockdown of ClC-3 protein expression by ClC-3 siRNA
The knockdown of ClC-3 protein expression was confirmed by the Western blotting. Western blotting experiments demonstrated that the expression of ClC-3 proteins was inhibited significantly by the ClC-3 siRNA treatments (100 nM) in the presence of lipofectamine 2000 (1/500 µl). As shown in Figure 8, transfection of ClC-3 siRNA for 48 h decreased the expression of ClC-3 proteins by about 60%. Because the transfection efficiency of ClC-3 siRNA in different cells varied, the decrement of total ClC-3 proteins in a cell population treated with ClC-3 siRNA would not be very large. In the patch clamp experiments, only the cells with strong fluorescence (indicating high transfection) were recorded.
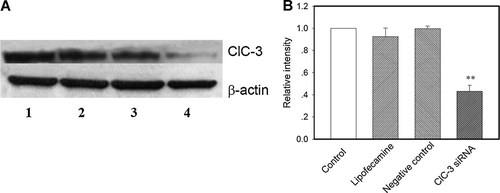
Inhibitions of ClC-3 protein expression by ClC-3 siRNA. Cells were incubated in the control medium (one, control) or the medium containing 1 µl lipofectamine 2000 (two, lipofectamine) or transfected with 100 nM negative control siRNA (three, negative control) or 100 nM ClC-3 siRNA (four, ClC-3 siRNA) in the presence of 1 µl lipofectamine 2000 for 48 h. A: Typical Western blotting and (B) presents the relative intensity of ClC-3 protein expression of different treatments (mean ± standard error of five experiments). **P < 0.01 (vs. control).
Transfection of cells with the negative control siRNA (100 nM) plus lipofectamine 2000 (1/500 µl) or with lipofectamine 2000 alone did not significantly alter ClC-3 expression (Fig. 8).
Inhibitions of the background Cl− currents by GdCl3
Hydrolysis of extracellular ATP with apyrase depressed the background Cl− current. However, the source of extracellular ATP in CNE-2Z cells under isotonic conditions is still not clear. GdCl3 (50 µM), which can inhibit ATP release from cells (Liu et al., 2008b), was applied to explore the possible mechanism of ATP release.
The results indicate that GdCl3 suppressed the background Cl− current under isotonic conditions. Typical background Cl− current traces recorded under the normal isotonic condition and after the treatment with 50 µM GdCl3 are shown in Figure 9A,B. The inhibition of the current was irreversible (Fig. 9C). The outward (at +80 mV) and inward (at −80 mV) background currents were decreased by 67.40 ± 5.62% and 74.79 ± 4.59%, respectively (n = 9; P < 0.01, vs. control; Fig. 9D).
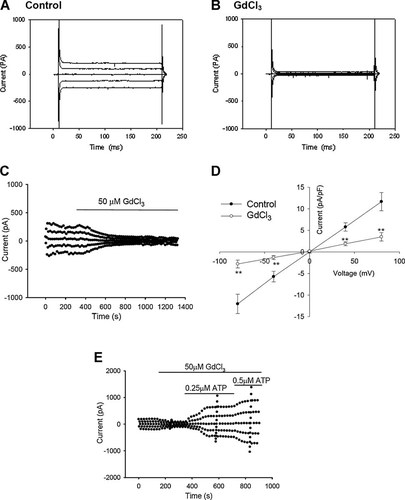
Inhibitions of the background Cl− current by GdCl3 under isotonic conditions. Typical samples of the background Cl− current recorded in the normal isotonic bath solution and after the treatment with 50 µM GdCl3 are shown in (A,B). The typical time course of inhibition of the background current by GdCl3 is shown in (C). I–V relationships are presented in (D) (mean ± standard error, n = 9). The inhibitory effect of GdCl3 was reversed by extracellular applications of ATP (0.25 and 0.5 µM, E). **P < 0.01 (vs. control).
The above results indicate that GdCl3 (50 µM), which can inhibit ATP release from cells, could inhibit the background current. Could the inhibitory effect of GdCl3 be reversed by external ATP? As shown in Figure 9E, ATP was added to the bath when the inhibitory effect of GdCl3 on the background current reached the maximum. Extracellular ATP (0.25 and 0.5 µM) activated a chloride current in the presence of 50 µM GdCl3 (Fig. 9E), indicating that the gadolinium block could be reversed by external ATP.
Discussion
In this study, a background chloride current was recorded in human nasopharyngeal carcinoma CNE-2Z cells. The current exhibits the properties similar to those of the volume-activated chloride current recorded by us in the same cell line (Chen et al., 2002). The current reversed at a potential close to the equilibrium potential for Cl− and was inhibited by high concentrations of ATP and by the chloride channel blockers tamoxifen and NPPB. The permeability of the background chloride channel to anions was I− > Br− > Cl−. In the gluconate replacement experiments, apart from the shift in reversal potential, both the inward and outward currents were reduced, suggesting a partial blockage of the chloride channels by sodium gluconate.
We demonstrated also for the first time in CNE-2Z cells that the background chloride current was volume-sensitive. Cell shrinkage induced by exposures to hypertonic solutions suppressed the current. These data imply that some of the volume-sensitive chloride channels are activated under isotonic conditions and are involved in regulation of basal cell volume. This hypothesis is confirmed by our observations on cell volume changes induced by chloride channel blockages. It was shown that cell volume was increased significantly by exposures of the cells to the chloride channel inhibitors.
How do the background chloride channels be activated under normal isotonic conditions? We found in this study that extracellular perfusion of P2Y purinergic receptor antagonist reactive blue 2 reversibly inhibited the background Cl− current, indicating that P2Y purinergic receptors are involved in activation of the Cl− channels under isotonic conditions in the nasopharyngeal carcinoma CNE-2Z cells. P2Y purinergic receptors belong to the super family of G-protein-coupled receptors (GPCRs), and the P2Y family is composed of eight human subtypes that have been cloned and functionally defined (von Kugelgen, 2006). However, we could not exclude the possible involvement of other purinergic receptors in the activation of the background channels. It was reported that reactive blue 2 (cibacron blue) could also inhibit P2X2 receptors (Zhong et al., 2000). How are the purinergic receptors associated with the appearance of the background Cl− current? What is the nature molecule that acts on the purinergic receptor to activate the background Cl− channel? It was demonstrated by us previously and in this study that extracellular ATP of micromolar scales could activate a chloride current in CNE-2Z cells (He et al., 2004). Furthermore, our present results demonstrate that hydrolysis of ATP by apyrase suppresses the background Cl− current. These data indicate that ATP, one of the most important signaling molecules, is involved in activation of the background Cl− current.
However, where does ATP come from? It has been proposed that cells can release nucleotides which are involved in autocrine and paracrine regulation of organ function via stimulation of purinergic receptors (Praetorius and Leipziger, 2009). Constitutive basal release of ATP has been shown to be sufficient to stimulate extracellular P2Y purinergic receptors (Lazarowski et al., 2000). However, the mechanism responsible for ATP release is not yet defined clearly. The maxi-anion channel has been proposed to be responsible for ATP releasing from cells (Dutta et al., 2004, 2008; Sabirov and Okada, 2005; Liu et al., 2008a,b; Bell et al., 2009; Shirley et al., 2009). The maxi-anion channel forms a wide nanoscopic pore suitable for nucleotide transport and possesses an ATP-binding site in the middle of the pore lumen to facilitate the passage of the nucleotide (Sabirov and Okada, 2005). ATP and its Mg2+ and/or H+ salts exist in anionic forms at physiological pH and may exit cells via the maxi-anion channel (Sabirov and Okada, 2005). In this study, we found that gadolinium chloride, an inhibitor of the maxi-anion channel (Liu et al., 2008a), inhibited the background Cl− current. The result suggests that the background Cl− channel in CNE-2Z cells can be activated by ATP released from cells in the form of autocrine or paracrine under isotonic conditions.
What is the physiological function of the background Cl− current activated by autocrine or paracrine ATP? Our results in present study show that the background Cl− current activated under isotonic conditions is volume-sensitive and is suppressed by hydrolysis of ATP with apyrase; the P2Y purinergic receptor antagonist reactive blue 2 and chloride channel blockers can inhibit the background Cl− current and induce cell swelling under isotonic conditions. It was also demonstrated previously by us that extracellular applications of low concentrations of ATP shrank the CNE-2Z cells (He et al., 2004). These data indicate that autocrine and/or paracrine ATP plays an important role in control the basal cell volume. ATP released from cells under the basal isotonic condition acts on P2Y purinergic receptors, resulting in activation of the background Cl− channel. Opening of the background Cl− channel allows outflow of chloride and thus prevents cell from swelling.
As discussed above, our data in this study indicate that the volume-sensitive background current is activated by autocrine and/or paracrine ATP via purinergic receptors under isotonic conditions and is associated with maintaining basal cell volume. What is the channel protein responsible for the formation of the current? ClC-2, a voltage-gated chloride channel, has been reported to constitute part of the background conductance and assist chloride extrusion in pyramidal cells of the hippocampus (Rinke et al., 2010). However, the properties of the background current recorded in this study are different from those of ClC-2 current. It has been demonstrated by us and others that the voltage-gated chloride channel ClC-3 is associated with the activation of the volume-activated chloride current induced by hypotonic challenges and has been proposed to be the candidate of volume-activated chloride channels although there is much debate on it (Duan et al., 1997b; Wang et al., 2000, 2003; Hermoso et al., 2002; Jin et al., 2003; Do et al., 2005; Mao et al., 2008; Yoshise et al., 2009; Xiong et al., 2010). The properties of the background chloride current recorded in present study are similar to those of the volume-activated chloride current activated by hypotonicity-induced cell swelling in the same CNE-2Z cell line. Furthermore, the background current is volume-sensitive. These data imply that the ClC-3 may also be the channel responsible for the formation of the background chloride current. This is confirmed by our results of ClC-3 silencing experiments. We found that suppressed expression of ClC-3 by the siRNA technique attenuated the background current and increased cell size. These data suggest that ClC-3 may be a main component of background chloride channels and is involved in the maintenance of the basal cell volume under isotonic conditions. Furthermore, our study demonstrates that knockdown of ClC-3 protein expression prevented activation of the ATP-induced chloride current. The results imply that ClC-3 may work as an ATP-sensitive chloride channel or a channel regulator and can be activated by ATP via the purinergic signaling pathway.
As shown in this study or reported by us previously, the isotonic background Cl− current, the ATP-activated Cl− current and the volume-activated Cl− current share similar characteristics, including almost linear current–voltage relationships among others, on most occasion. However, there are a few occasions that these currents display the property of clear outward rectification. The underlying mechanism for the variation is not clear. We demonstrated previously that the expression of volume-activated chloride channels were cell cycle-dependent (Chen et al., 2002). The difference of the cells situating at cycle stages might affect the current characteristics.
In conclusion, the results of this study suggest that a volume-sensitive background chloride current is activated under isotonic conditions by autocrine/paracrine ATP through purinergic receptor pathways. The background current is involved in maintenance of basal cell volume. The ClC-3 protein is associated with the activation of the background chloride current and may be a main component of background chloride channels.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (30771106, 30870567, 30871267, 90913020, and U0932004).




