Mospd1, a new player in mesenchymal versus epidermal cell differentiation
Abstract
Mospd1 codes for a small protein with unknown physiological function, which is part of a family of genes, including Mospd2 and Mospd3, defined by the presence of the major sperm protein domain and two transmembrane domains. This work characterizes the Mospd1 gene, the intracellular location of the protein and its expression in different mouse tissues and mesenchymal cell lines during differentiation. The role of Mospd1 in mesenchymal cellular differentiation was studied by siRNA knockdown experiments in mouse osteoblastic MC3T3-E1 cells. Transfection experiments of the targeted cDNA show MOSPD1 located in the endoplasmatic reticulum and in the Golgi apparatus. Removal of the last exon of the gene resulted in localization of the protein in the nucleus, which was attributed to a nuclear export sequence in the N-terminal part. In mouse tissues the gene was generally strongly expressed while mesenchymal tissues showed the highest expression. In mesenchymal cell lines Mospd1 mRNA was higher expressed in cells with advanced differentiation status. In osteoblastic, myoblastic, and adipocytic cell lines Mospd1 was up-regulated during differentiation. Genome-wide gene expression analysis after knockdown of Mospd1 by siRNA in MC3T3-E1 cells revealed a shift in the gene expression pattern from mesenchymal to epithelial genes featuring up-regulation of the epithelial cadherin Cdh1 and down-regulation of its inhibitors Snail1 and 2 and the mesenchymal cadherin Cdh11, suggesting a mesenchymal to epithelial transition. From these data we conclude that Mospd1 plays a pivotal role in the developmental regulation at the switch between mesenchymal and epithelial cells. J. Cell. Physiol. 226: 2505–2515, 2011. © 2010 Wiley-Liss, Inc.
Mospd (motile sperm domain) genes code for small proteins whose physiological function is largely unknown. The number of Mospd genes varies between the species. Thus, higher primates like humans or chimpanzees carry three Mospd genes (Mospd1 to Mospd3) whereby rats or mice carry four Mospd genes (Mospd1 to Mospd4) in their genome. Gene similarities were reported between Mospd1 and Mospd3 in humans as well as in mice. In that context, Mospd3 was shown to be involved in the development of the right ventricle of the heart in mice. Human MOSPD1 was found induced in an epithelial ovarian cancer in vitro in correlation to cell invasion, proliferation, and spheroid formation (Puiffe et al., 2007). However, until now no function was addressed as well as any characterization was made for the Mospd1 gene.
The Mospd1 gene is localized on chromosome X in humans, chimpanzees, rhesus monkeys, cattle, mice and rats while in chicken and zebrafish it is localized on chromosome 4 and 14, respectively. At the protein level the genes are highly conserved between humans, chimpanzees (100%), rhesus monkeys (99%), mice (97%), and rats (97%). Protein sequence analysis revealed the presence of a motile sperm domain (MSD, aa 17–112) followed by two putative transmembrane domains (THM, aa 157–179 and aa 189–211) and a putative nuclear export signal (NES, aa 205–208) consisting of four hydrophobic residues (LITM, LLTM in zebrafish). These domains were found being highly conserved between all species (Fig. 1). Based on the domain organization of MOSPD1 (as also of MOSPD3), this protein is to classify into the group of the type IIc MSP-domain proteins (MDPs), which are predominantly found in mammals (Tarr and Scott, 2005).
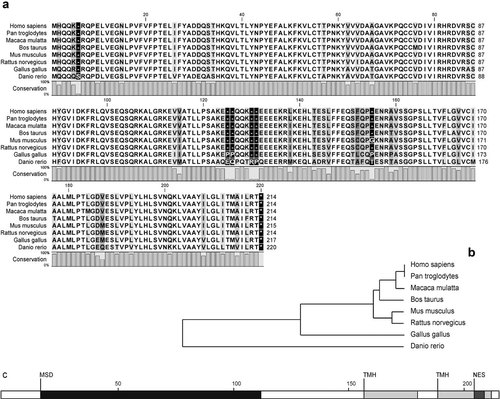
MOSPD1 represents a small, among the species highly conserved protein. a: Using CLC bio software the protein sequence of human, chimpanzee, rhesus monkey, cattle, mouse, rat, chicken, and zebrafish MOPSD1 was aligned. b: Phylogenetic tree made from the above alignment. c: Analysis of MOSPD1 protein sequence suggested the presence of a MSD (motile sperm domain, from aa 17 to aa 112, black bar), of two transmembrane helices (TMH, from aa 157 to aa 179 and from aa 189 to aa 211, light gray bar) and of a NES (nuclear export sequence) situated in the last small exon (from aa 205 to aa 208, dark gray bar) including four hydrophobic residues, namely Leu, Isol (Leu in zebrafish), Thr and Met.
Cellular differentiation is an essential event during tissue development, where cells significantly change size, shape, metabolic activity and function accompanied by changes of the gene expression pattern. In embryonic development mesenchymal cells derive from a primitive epithelium. This process, commonly known as epithelial-to-mesenchymal transition (EMT), is also suggested in tumor progression when epithelial cells lose their characteristic polarity, disassemble cell–cell junctions and become more migratory metastasizing into proper tissue. In EMT, a key event is the switch from epithelial cadherin-1 (Cdh1) to N-cadherin (Cdh4) followed by Cdh11 expression in osteoblasts (Wheelock et al., 2008). Down-regulation of Cdh1 is mediated by up-regulation of its repressors SNAIL (SNAI1), SLUG (SNAI2), ZEB1, ZEB2 or/and TWIST (Baranwal and Alahari, 2009). This represents a central mechanism regulating EMT (Thiery, 2002; Kokudo et al., 2008; Yang and Weinberg, 2008; Thiery et al., 2009).
Depending on the microenvironment, mesenchymal stem cells (MSCs) have the potential to differentiate into adipocytes, myocytes, chondrocytes, and osteoblasts. During osteoblastic differentiation, a process regulated by bone morphogenetic protein-2 (BMP2) and the extracellular matrix (ECM), MSC start to express osteoblast-specific transcription factors like runt-related transcription factor 2 (Runx2) and osterix (Sp7), which control the expression of many bone structural genes like collagen type I (Col1a1, Col1a2), fibronectin (Fn1) or osteocalcin (Bglap2) (Lian et al., 2006). Furthermore, genes mediating specific interactions with the ECM (integrins) or with other cells (OB-cadherin, Cdh11) are up-regulated during differentiation (Luegmayr et al., 2000; Di Benedetto et al., 2010).
In addition to the four cell lineages described above, MSCs have recently been reported to give rise to several other cells including epithelial and neuroectodermal cells (Spees et al., 2003; Phinney and Prockop, 2007; Charbord, 2010). Although these data must be evaluated critically, they indicate that adult mesenchymal cells could differentiate into cells with epithelial character, suggesting mesenchymal–epithelial transition (MET), a process, which is also common during development (Prindull and Zipori, 2004; Thiery et al., 2009). Regulation of this reversal process implicates down-regulation of mesenchymal genes and of the transcription factors SNAIL/SLUG to release the block of expression of Cdh1 as well as of epithelial genes.
In the present study, we characterize Mospd1 gene structure, cellular localization and analyze its expression in several murine tissues as well as in murine cell lines having different phenotypes. Knockdown of Mospd1 by siRNA suggests a developmental role for this protein.
Materials and Methods
Cell culture
For the experiments the murine cell lines C3H10T1/2, ST2, MC3T3-E1, 3T3-L1, and C2C12 were used. For propagation all cell lines were cultured in humidified air under 5% CO2 at 37°C and were sub-cultured twice a week using 0.001% pronase E (Roche, Basel, Switzerland) and 0.02% EDTA in Ca2+- and Mg2+-free phosphate-buffered saline (PBS) before achieving confluence. For MC3T3-E1 cells (kindly donated by Dr. Kumegawa, Meikai University, Department of Oral Anatomy, Sakado, Japan), a clonal preosteoblastic cell line derived from newborn mouse calvariae, C3H10T1/2 embryonic MSCs, ST2 bone marrow stromal cells (kindly donated by R. Gruber, School of Dentistry, Department of Oral Surgery, University of Vienna), alpha-minimum essential medium (α-MEM; Biochrom, Berlin, Germany) supplemented with 5% fetal calf serum (FCS, Biochrom), and 10 µg/ml gentamycin (Sigma–Aldrich, Solms, Germany) was used as culture medium. Osteoblastic differentiation was induced by addition of 50 µg/ml ascorbic acid (Sigma) in culture medium and osteocalcin mRNA expression was selected as differentiation marker.
C2C12 myoblastic cells and 3T3-L1 adipocytic cells (kindly donated by Dr. Somoza, research platform of molecular food science, University of Vienna, Austria) were maintained in high-glucose DMEM, supplemented with 10% FCS, 10 µg/ml gentamycin (Sigma) and 4 mM L-glutamine (Sigma). C2C12 cells were differentiated at 90% confluence by switch of 10% FCS to 2% of horse serum (Sigma) in the culture medium. The CO2 content was increased to 10% during myoblastic differentiation and culture medium was daily renewed until myotube formation was reached (after 10 days of culture). Differentiation of adipocytic 3T3-L1 cells were started at 80% confluence by adding 0.86 µM human insulin, 0.25 µM dexamethasone, and 0.5 mM isobutylmethylxanthine (IBMX; all Sigma) for 4 days to the culture medium. Thereafter, cells were incubated for further 2 days with 0.86 µM human insulin in medium and without any additives for the last for days. After 10 days about 95% of the cells showed accumulation of fat droplets. According to literature, fat droplets formation as well as myotube formation was chosen as marker for adipocytic (Sommer et al., 2008) or myoblastic (Ferri et al., 2009) differentiation, respectively.
In most experiments, cells were seeded at 20,000 cell/cm2, differing seeding densities are noted in the text. Culture time is noted in the figures.
Determination of adipocytic differentiation
Differentiation of 3T3-L1 adipocytes was assessed by Oil Red O Staining. After cell culture, cell monolayers were rinsed twice with PBS and then fixed with 4% paraformaldehyde in PBS for 30 min at room temperature. Thereafter fixed cells were rinsed three times with distilled water and incubated with 2% Oil Red O (Sigma) in 60% isopropanol solution for 30 min. Subsequently, 3T3-L1 adipocytes were rinsed five times with distilled water and dried for 10 min at 32°C. Finally Oil Red O stain was extracted with isopropanol and absorbencies were measured at 490 nm with a multiwell plate reader.
Mouse bone marrow stromal cells (BMSC)
OF-1 mice were obtained from the approved animal breeding institution of the Medical University of Vienna (Himberg, Austria), and sacrification was performed according to the regulatory guidelines of the Medical University of Vienna.
Eight-week-old OF-1 mice (Himberg, Austria) were euthanized by carbon dioxide asphyxiation and the femur and tibia removed under aseptic conditions. The marrow cavities were flushed with sterile medium using a 25-gauge needle and the culture established in αMEM supplemented with 10% FCS and 10 µg/ml gentamycin. After 48 h of culture at 5% CO2 and 37°C, the non-adherent cells were removed by gentle rinsing with PBS. At confluence, cells were harvested and seeded at a density of 1.5 × 103/cm2 for cell experiments.
For osteogenic differentiation cells were cultured in αMEM supplemented with 10% FBS, 10 µg/ml gentamycin, 100 nM dexamethasone, 10 mM β-glycerophosphate, and 50 µg/ml ascorbic acid. For adiopogenic differentiation of BMSC cells were cultured in αMEM supplemented with 10% FBS, 10 µg/ml gentamycin, 0.5 mM IBMX, 1 µM dexamethasone, 5 µg/ml insulin, and 50 µM indomethacin. Culture medium was renewed twice a week and cells were cultured for the periods indicated in the results. Again, as differentiation markers, osteocalcin mRNA expression was selected for the osteoblastic lineage and fat droplet formation was chosen for the adipocytic lineage.
Cellular MOSPD1 localization
Full-length cDNA and a cDNA without the last exon of the Mospd1 gene were cloned into the pcDNA 3.1 V5-His-TOPO vector (Invitrogen, Carlsbad, CA) using primers listed in Table 1. For cellular localization, MC3T3-E1 cells were seeded on cover slips at 20,000 cells/cm2 in 6-well multiwell plates. Six hours after seeding, cells were transfected with 0.15 µg/cm2 of the expression vector construct using 1 µg/cm2 DOSPER transfection reagent (Roche) following the supplier's protocol. After 4 days, cells were washed three times with ice cold PBS and fixed for 20 min with 4% of paraformaldehyde in PBS at room temperature. Next, cells were permeabilized for 3 min with 0.5% Triton X-100 and blocked for 2 h in 1% BSA in PBS. Afterwards, cells were incubated overnight with the V5-antibody (Invitrogen) diluted 1:200 in 0.5% BSA in PBS. Next day cells were incubated for 1 h with FITC labeled secondary antibody (IgG-FITC) diluted 1:300 in 0.5% BSA in PBS.
| Gene symbol | Forward primer (5′–3′) | Reverse primer (5′–3′) | Tm (°C) |
|---|---|---|---|
| Primer for qRT-PCR | |||
| Mospd1 | GTGTGGATATTGTGATTCGT | AGTGGAGGTAGAGAGGC | 61 |
| Myod1 | GCCGTGGCAGCGAGCACTACAG | CATTGGGGTTTGAGCCTGCAG | 60 |
| Trp63 | TACTGCCCCGACCCTTACAT | GCTGAGGAACTCGCTTGTCTG | 60 |
| Emp1 | TTGGTGCTACTGGCTGGTCT | CATTGCCGTAGGACAGGGAG | 60 |
| Cdh1 | CAGGTCTCCTCATGGCTTTGC | CTTCCGAAAAGAAGGCTGTCC | 62 |
| Cdh11 | CTGGGTCTGGAACCAATTCTTT | GCCTGAGCCATCACTGTGTA | 62 |
| Primer for Mospd1 cloning in pcDNA 3.1 V5-His-TOPO vector | |||
| Full length | CACCATGCATCAACAAAAAAGAC | TGTTCTAAGTATAGCCATTGTGATAAG | 62 |
| Rev primer for Mospd1 version lacking last exon | ACCTAAGATATAAGCAGCCACTAG | 62 | |
Isolation of RNA from cell lines and murine tissues and mRNA expression analysis by qRT-PCR
RNA from cell cultures was extracted using a RNA Isolation Kit (Qiagen, Hilden, Germany). Tissues were isolated from 6-day-old mice (strain HIM OFF-1; Institute of Experimental Animal Research, University Vienna), which were sacrificed by cervical dislocation. Total RNA from ribs, calvariae, heart, liver, thymus, spleen, kidney, muscle, and lung was isolated with TRIZOL reagent (Life Technologies, Inc., Gaithersburg, MD). Briefly, 100 mg of each tissue were homogenized with a Dounce homogenizer in the presence of 1 ml TRIZOL reagent, centrifuged, and the aqueous phase was extracted once with phenol/chloroform and once with chloroform. The RNA in the aqueous phase was then precipitated with isopropanol. RNA from cell lines was extracted using a total RNA Isolation Kit (Promega, Madison, WI) following supplier's instructions. cDNA was synthesized from 0.5 µg RNA using the 1st Strand cDNA Synthesis Kit as described by the supplier (Roche). The obtained cDNA was subjected to PCR amplification with a real-time cycler using FastStart SYBR-Green Master Mix (Roche) for the genes Mospd1, Myod1 (MyoD), Cdh1, Cdh11, Trp63, and Emp1 (for primer design see Table 1). SYBR-Green PCR was started with 10 min of an initial denaturation step at 95°C and then continued with 45 cycles consisting of 30 sec denaturation at 95°C, 30 sec annealing at primer-specific temperatures and extension at 72°C. For amplification of Runx2 and osteocalcin (Bglap 2) we used TaqMan gene expression probes (Mm00501578_m1 and X04142-EX2, respectively, Applied Biosystems, Rotkreuz, Switzerland) and for normalization we used a 18S RNA TaqMan probe (4319413E, Applied Biosystems) or a Gapdh TaqMan Probe (Mm4352339E, Applied Biosystems) as indicated, all in combination with TaqMan Gene expression Master Mix (Applied Biosystems) with an initial denaturation at 95°C for 10 min followed by 45 cycles alternating 60°C. All PCRs were performed in triplicate and expression was evaluated using the comparative quantization method (Pfaffl, 2001). For each experiment, the triplicate results of the qRT-PCR were averaged and this mean value was treated as a single statistical unit. The data of the experiments are presented as means ± standard deviation (s.d.).
Mospd1 gene and protein sequence comparison was performed with CLCbio Main Workbench software using EMBL/GenBank database sequences (reference sequences chosen: NW_001842400, NW_001252131, NW_001218189, NW_001508692, NW_001035169, NM_001014107, NW_001471681, NM_213369 for human, chimpanzee, rhesus monkey, cattle, mouse, rat, chicken, and zebrafish, respectively). For analysis of putative functional sites in the protein sequence the open source software Pfam (Wellcome Trust Sanger Institute, Hinxton, GB) and TMHMM (CBS, Technical University of Denmark) were used.
Mospd1 siRNA transfection
For Mospd1 depletion by siRNA, MC3T3-E1 cells were seeded at 20,000 cells/cm2 in a six multi-well plate. Six hours after seeding, cells were transfected with 40 pmol of Mospd1 siRNA (Sigma) by using X-tremeGENE siRNA Transfection Reagent (Roche) as described by the supplier. After 4 days of incubation, mRNA was isolated as described above and subjected to qRT-PCR or to Affymetrix gene chip analysis.
Affymetrix GeneChip analysis
Total RNA was isolated using a RNA Isolation Kit (Qiagen). Quality control of the RNA's as well as labeling, hybridization, and scanning of the hybridized arrays was performed by the “Kompetenzzentrum fuer Fluoreszente Bioanalytik (KFB)” (Regensburg, Germany) using the mouse 430 2.0 chip (Affymetrix).
Statistical analysis
Statistical analyses were performed using either ANOVA or Student's t-test using Prism 4.03 (GraphPad Software, San Diego, CA). P ≤ 0.05 was considered as significant. For each experiment, the triplicate results of the qRT-PCR were averaged and this mean value was treated as a single statistical unit. The data of the triplicate experiments are presented as means ± s.d.
Results
Mospd1 is localized in cytoplasm and nucleus depending on the presence of the NES
The presence of a putative NES at the carboxy-terminal end of the protein suggested a temporarily localization of MOSPD1 in the nuclei. To proof this suggestion two cDNA with V5-tags were constructed and localized after transfection into MC3T3-E1 cells. As shown in Figure 2a, the full-length MOSPD1 was localized in the endoplasmatic reticulum of the cells with enhanced perinuclear localization. Transfection of a shorter cDNA (without the last exon, containing NES) into the MC3T3-E1 cells resulted in a localization of the protein in the nucleus of the cells, excluding nucleoli. Furthermore, these cells showed coarse granula in the cytoplasm (Fig. 2b).
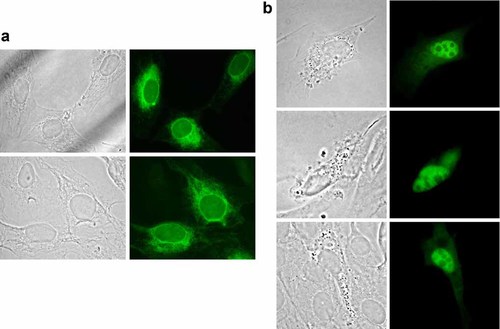
Intracellular localization of MOSPD1. a: MOSPD1-V5 localizes in cytoplasm, presumably in the ER (a) and in nucleus (b). Using immunostaining techniques against the V5-target, full-length protein was detected in the cytoplasm, adjacent to the nucleus (a). Lack of the last, NES-sequence carrying exon allocates MOSPD1-V5 in the nucleus. Furthermore, forced expression of this nuclear form induces the formation of coarse granula in the cytoplasm of the affected cells (b).
Expression of Mospd1 mRNA murine tissues and cell lines
Analysis of Mospd1 mRNA expression in murine tissues from 6-day-old mice was performed by qRT-PCR. mRNA was isolated from ribs, calvariae, heart, liver, thymus, spleen, kidney, muscle, and lung. As shown in Figure 3a, Mospd1 was expressed in all tissues analyzed. Among these, Mospd1 expression was found to be lowest in liver and in spleen. A higher expression was detected in calvariae, kidney, thymus, muscle and heart and the highest expression was measured in ribs.
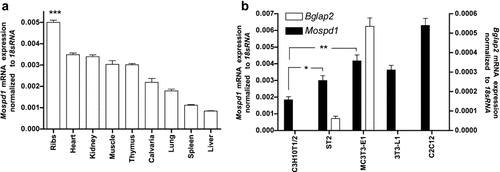
Mospd1 mRNAexpression in some murine tissues and diverse cell lines. a: Mospd1 is highly expressed in bone, heart, kidney, muscle, and thymus. Its expression is significantly at highest expressed in Rib bones when compared to each other tissue. b: Among the osteoblastic lineage, Mospd1 expression correlates with osteoblastic commitment (compare to osteocalcin (Bglap2) mRNA expression in the cell lines C3H10T1/2, ST2 and MC3T3-E1). Cells were seeded at 20,000 cells/cm2 and cultured for 48 h (until confluence). To analyze mRNA expressions, RNA was isolated and analyzed by qRT-PCR. Gene expression was normalized to 18S rRNA. Values are represented as mean ± s.d.; *P ≤ 0.05; **P ≤ 0.01; n = 3.
Mospd1 expression was analyzed in immortalized mouse mesenchymal cell lines: three osteoblastic cells with increasing differentiation status (C3H10T1/2, ST2, and MC3T3-E1), an adipocytic cell line (3T3-L1) and C2C12 myoblasts. Between the osteoblastic cell lines Mospd1 expression increased with increasing differentiation status (shown by increased osteocalcin expression) showing the highest expression in the preosteoblastic cell line MC3T3-E1 (Fig. 3b). The preadipocytic 3T3-L1 showed nearly the same expression level as MC3T3-E1 cells while the myoblasts showed the highest expression. C2C12 cells and 3T3-L1 cells also have a differentiated phenotype.
Regulation of Mospd1 mRNA expression during myoblastic, adipocytic, and osteoblastic differentiation
To address Mospd1's role in cell differentiation we analyzed its expression pattern in differentiating C2C12, 3T3-L1, and MC3T3-E1 cells as well as in BMSC differentiated into adipocytes and osteoblasts in vitro. Interestingly, all three cell lines showed an increase of Mospd1 expression during the first 10 days of differentiation (Fig. 4). During this time C2C12 cells showed the typical myotube formation with decreased Myod1 expression (Fig. 4a,b), 3T3-L1 produced an increasing amount of fat droplets, which incorporated increasing amount of Red Oil O dye (Fig. 4c) and osteoblastic MC3T3-E1 cells increasingly expressed the osteoblastic marker gene osteocalcin (Fig. 4d). During further differentiation, however, Mospd1 expression decreased in MC3T3-E1 cells. Mospd1 expression showed a similar expression pattern in mouse BMSC when either differentiated into adipocytes with increasing number of fat droplets (Fig. 5a,b) or into osteoblasts, showing increasing osteocalcin expression (Fig. 5c).
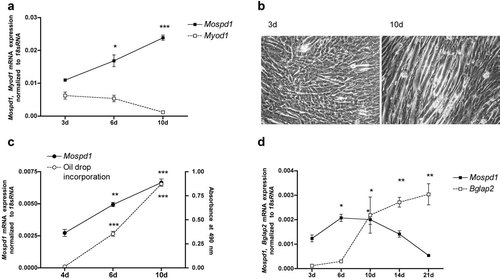
Mospd1 mRNA expression in differentiating C2C12 myoblasts (a) 3T3-L1 adipocytes (c) and MC3T3-E1 osteoblastic cells (d). C2C12 and 3T3-L1 cells show a continuous increase in Mospd1 mRNA expression until differentiation is reached. In MC3T3-E1 cells Mospd1 expression increases in the first differentiation phase and decreases while up-regulation of the osteoblastic differentiation marker osteocalcin (Bglap2). To define differentiation status of C2C12 cells, mRNA expression of the gene Myod1 (a) as well as myotube formation was analyzed (after 3 days and 10 days of cell culture) (b). Differentiation of 3T3-L1 cells was measured by the incorporation of fat droplets in the adipocytes by red oil O staining (c). Differentiation status of MC3T3-E1 cells was defined by Bglap2 mRNA expression (d). To analyze mRNA expressions, RNA was isolated and analyzed by qRT-PCR. Gene expression was normalized to 18S rRNA and referred to the first measured time. Values are represented as mean ± s.d. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; n = 3.
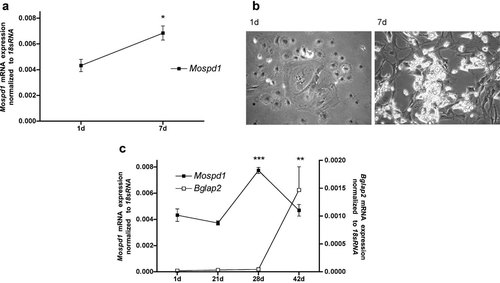
Mospd1 mRNA expression in differentiating adipocytes (a) and osteoblasts (c) from primary murine MSC. Similar as in differentiating 3T3-L1 cells, in adipocytes differentiating from MSC Mospd1 expression correlates with the differentiation status of the cells (shown by oil drop incorporation, b). Also in osteoblasts differentiating from MSC Mospd1 expression shows a similar expression pattern as in differentiating MC3T3-E1 cells: Mospd1 expression reaches its maximum before the osteoblastic differentiation marker osteocalcin (Bglap2) is up-regulated. To analyze mRNA expressions, RNA was isolated and analyzed by qRT-PCR. Gene expression was normalized to 18S rRNA and referred to the first measured time. Values are represented as mean ± s.d. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; n = 3.
Mospd1 mRNA was up-regulated in confluent cultures
MC3T3-E1 behavior and differentiation is strongly influenced by cell density (Varga et al., 1999, 2010). Therefore MC3T3-E1 and C2C12 cells were seeded at different densities and tested for expression of Mospd1. Figure 6 demonstrates that Mospd1 was up-regulated in dense cultures of both MC3T3-E1 and C2C12 when compared to sparse seedings indicating the involvement of cell–cell contact in the regulation of Mospd1 expression.
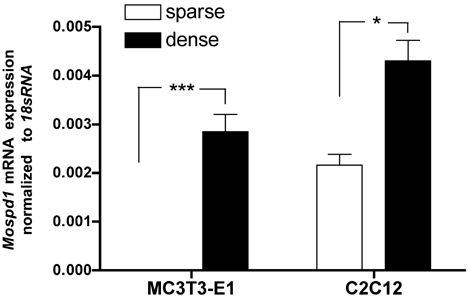
Mospd1 mRNA expression in dependence of cell confluence. Between the two cell lines analyzed, Mospd1 was dramatically up-regulated by cell–cell contacts in MC3T3-E1 osteoblasts and strongly in C2C12 myoblasts. For this purpose, cells were seeded at 2,500 cells/cm2 (sparse) to avoid cell–cell contact in culture or at 50,000 cells/cm2 (dense) to guarantee full cell confluence and cultured for 24 h. To analyze mRNA expressions, RNA was isolated and analyzed by qRT-PCR. Gene expression was normalized to 18S rRNA. Values are represented as mean ± s.d.; *P ≤ 0.05; ***P ≤ 0.001; n = 3.
Knockdown of Mospd1 resulted in a global change of the genome-wide gene expression pattern in MC3T3-E1 cells
Transfection of Mospd1-siRNA resulted in down-regulation of—as expected—Mospd1 (Fig. 7a) but, surprisingly of both, Runx2 (Fig. 7b), the indispensable osteoblastic transcription factor and osteocalcin (Bglap2, Fig. 7c), the osteoblastic differentiation marker gene. These findings suggested the evaluation of Mospd1 depletion on MC3T3-E1 cells by a genome-wide expression screen. Affymetrix gene chip analysis demonstrated that with Mospd1 repression, numerous osteoblastic and mesenchymal genes were down-regulated (Table 2) as well as osteoblast-related growth- and differentiation factors (GDFs) like insulin-like growth factors (Igf1, 2) and transforming growth factor beta (Tgfb1, 2, 3) and their receptors. Except Igf2r, Tgfbr1 and Tgfbr3, all genes considered were down-regulated (Table 2). Surprisingly, concomitant to down-regulation of the osteoblastic cell adhesion gene OB-cadherin (Cdh11, Fig. 8a) a significant up-regulation of the endothelial cadherin-1 was found (Cdh1, Fig. 8b). Genome-wide expression analysis revealed further up-regulation of epithelial genes and regulation of genes involved in EMT (Table 3). Thus we found an up-regulation of p63 (Trp63, Fig. 8c), a member of the p53 tumor suppressers, which is required for skin and limb development (Wolff et al., 2009) and epithelial membrane protein 1 (Emp1, Fig. 8d, Table 4), a specific epithelial marker protein. Moreover, of 51 keratin and 58 keratin-associated genes found on the Affimetrix chip, 42 and 49 were up-regulated, respectively (Table 4).
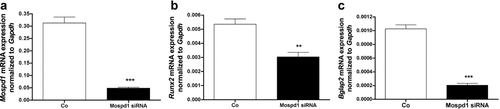
Mospd1 knockdown by siRNA down-regulated osteoblastic genes. Mospd1 (a), Runx2 (b), and osteocalcin (Bglap2) (c) expression in MC3T3-E1 cells after 4 days of Mospd1 depletion by siRNA. Mospd1 mRNA expression was significantly down-regulated as well as the expression of two typical osteoblastic genes Bglap2 (4.99-fold) and its regulator Runx2 (1.76-fold). To analyze mRNA expressions, RNA was isolated and analyzed by qRT-PCR. Gene expression was normalized to Gapdh. Values are represented as mean ± s.d. siRNA transfected probes are referred as fold change to control; **P ≤ 0.01; ***P ≤ 0.001; n = 3.
| Gene symbol | Log 2 signal | Signal ratio | Gene description |
|---|---|---|---|
| Runx2 | 4.79 | −1.32 | Runt related transcription factor 2, transcript variant 2 |
| Sp7 | 7.16 | −1.27 | Sp7 transcription factor 7 |
| Col1a1 | 13.52 | −1.55 | Collagen, type I, alpha 1 |
| Col1a2 | 13.54 | −1.52 | Collagen, type I, alpha 2 |
| Fn1 | 13.04 | −1.07 | Fibronectin 1 |
| Alpl | 6.66 | −1.62 | Alkaline phosphatase, liver/bone/kidney |
| Cdh11 | 11.93 | −1.39 | Cadherin 11 |
| Col3a1 | 12.18 | −1.60 | Collagen, type III, alpha 1 |
| Ogn | 10.64 | −1.85 | Osteoglycin |
| Dlx5 | 8.15 | −1.51 | Distal-less homeobox 5, transcript variant 1 |
| Msx2 | 6.45 | −1.18 | Homeobox, msh-like 2 |
| Ibsp | 6.72 | −3.19 | Integrin binding sialoprotein |
| Aspn | 11.61 | −4.71 | Asporin |
| Satb2 | 8.92 | −1.44 | Special AT-rich sequence binding protein 2 |
| Hoxa2 | 5.21 | −1.14 | Homeo box A2 |
| Atf4 | 8.72 | −1.21 | Activating transcription factor 4 |
| Sfn | 5.38 | 1.09 | Stratifin |
| Nt5e | 6.45 | −1.15 | 5′-nucleotidase, ecto |
| Cd44 | 9.17 | −1.45 | CD44 antigen, transcript variant |
| Vim | 12.93 | −1.20 | Vimentin, mRNA |
| Acta2 | 12.21 | −2.62 | Actin, alpha 2, smooth muscle, aorta |
| S100b | 7.58 | −2.68 | S100 protein, beta polypeptide, neural |
| S100a4 | 9.82 | −1.37 | S100 calcium binding protein A4 |
| Igf1 | 8.20 | −4.65 | Insulin-like growth factor 1, transcript variant 1 |
| Igf1r | 8.91 | −1.18 | Insulin-like growth factor 1 receptor |
| Igf2 | 6.96 | −1.37 | Insulin-like growth factor 2, transcript variant 3 |
| Igf2r | 9.64 | 1.08 | Insulin-like growth factor 2 receptor |
| Tgfb1 | 7.84 | −1.17 | Transforming growth factor, beta 1 |
| Tgfb1i1 | 8.38 | −1.64 | Transforming growth factor beta 1 induced transcript 1 |
| Tgfb2 | 7.48 | −1.33 | Transforming growth factor, beta 2 |
| Tgfb3 | 9.30 | −1.50 | Transforming growth factor, beta 3 |
| Tgfbr1 | 8.56 | 1.73 | Transforming growth factor, beta receptor I |
| Tgfbr2 | 9.25 | −1.28 | Transforming growth factor, beta receptor II, transcript 1 |
| Tgfbr3 | 10.09 | 1.17 | Transforming growth factor, beta receptor III |
- Down-regulated genes were colored in green, up-regulated in red.
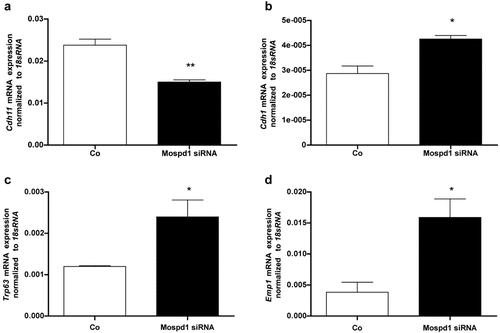
Mospd1 knockdown by siRNA down-regulated mesenchymal genes and up-regulated epithelial genes. Osteoblastic Cdh11 (a) and epithelial Cdh1 (b) Trp63 (c) and Emp1 (d) expression in MC3T3-E1 cells after 4 days of Mospd1 depletion by siRNA. Repression of Mospd1 mRNA expression decreases significantly the expression of the osteoblastic cadherin Cdh11 (1.58-fold) and enhances significantly the expression of the epithelial cadherin Cdh1 (1.48-fold). Furthermore, the transcription factor Trp63 was 2.00-fold and Emp1 was 4.13-fold up-regulated; both genes are involved in epithelial tissue development and maintenance. To analyze mRNA expressions, RNA was isolated and analyzed by qRT-PCR. Gene expression was normalized to 18S RNA. Values are represented as mean ± s.d. siRNA transfected probes are referred as fold change to control; *P ≤ 0.05; **P ≤ 0.01; n = 3.
| Gene symbol | Log 2 signal | Signal ratio | Gene description |
|---|---|---|---|
| Bmi1 | 9.17 | −1.51 | Bmi1 polycomb ring finger oncogene |
| Cdh1 | 4.83 | 1.19 | Cadherin 1 (Cdh1), mRNA |
| Cdh16 | 4.96 | 1.20 | Cadherin 16 |
| Col6a1 | 11.19 | −1.60 | Collagen, type VI, alpha 1 |
| Col6a2 | 10.36 | −1.71 | Collagen, type VI, alpha 2 |
| Foxc2 | 5.08 | 1.46 | Forkhead box C2 |
| Gsc | 6.70 | −1.74 | Goosecoid homeobox |
| Hnf1b | 4.43 | 1.33 | HNF1 homeobox B |
| Klf8 | 3.64 | 1.37 | Kruppel-like factor 8 |
| Ren1 | 4.49 | 1.26 | Renin 1 structural |
| Smad3 | 8.48 | −1.00 | MAD homolog 3 (Drosophila) |
| Snai1 | 8.13 | −1.11 | Snail homolog 1 |
| Snai2 | 9.45 | −1.73 | Snail homolog 2 (Drosophila) |
| Snai3 | 5.17 | −1.09 | Snail homolog 3 (Drosophila) |
| Tcf3 | 7.82 | −1.25 | Transcription factor 3 |
| Tcf4 | 9.89 | −1.68 | Transcription factor 4 |
| Twist1 | 9.23 | −1.37 | Twist homolog 1 (Drosophila) |
| Twist2 | 6.64 | −1.08 | Twist homolog 2 (Drosophila) |
| Zeb1 | 9.42 | −1.29 | Zinc finger E-box binding homeobox 1 |
| Zeb2 | 4.93 | 1.27 | Zinc finger E-box binding homeobox 2 |
- Down-regulated genes were colored in green, up-regulated in red.
| Gene symbol | Log 2 signal | Signal ratio | Gene description |
|---|---|---|---|
| Trp63 | 8.63 | 1.26 | Transformation related protein 63, transcript variant 1 |
| Emp1 | 10.49 | 1.47 | Mus musculus epithelial membrane protein 1 |
| Flg2 | 2.88 | 1.14 | Filaggrin family member 2 |
| Lor | 4.24 | 1.11 | Loricrin |
| Thbd | 8.55 | 1.68 | Thrombomodulin |
| Perp | 4.15 | 1.20 | PERP, TP53 apoptosis effector |
| Krt31 | 4.92 | 1.38 | Keratin 31 |
| Krt8 | 5.50 | 1.52 | Keratin 8 |
| Krt2 | 3.15 | 1.56 | Keratin 2 |
| Krt39 | 3.64 | 1.56 | Keratin 39 |
| Krt17 | 4.32 | 1.76 | Keratin 17 |
| Krtap2–4 | 6.62 | 1.46 | Keratin associated protein 2–4 |
| Krtap4–2 | 4.23 | 1.49 | Keratin associated protein 4–2 |
| Krtap6–1 | 6.10 | 1.53 | Keratin associated protein 6–1 |
| Krtap10–10 | 5.33 | 1.56 | Keratin associated protein 10–10 |
| Krtap5–1 | 6.49 | 1.72 | Keratin associated protein 5–1 |
| Krtap31–2 | 3.84 | 1.76 | Keratin associated protein 31–2 |
- Moreover, most Keratins and their associated proteins were up-regulated (colored in red) when Mospd1 was down-regulated by siRNA. Each of the five strongest regulated genes is listed.
Discussion
The MSP (major sperm protein)-domain is highly conserved between organisms. Homologies were found from bacteria (E. coli, PapD) via nematodes, to mammals (Tarr and Scott, 2005). Nematodes sperm use a crawling motility where the locomotion is mediated by a dynamic cytoskeleton composed of the most abundant motile sperm protein, where the MSP-domain enables the dynamic protein–protein interaction (King et al., 1994; Smith and Ward, 1998; Tarr and Scott, 2005). Forced expression of targeted cDNA of MOSPD1 in MC3T3-E1 osteoblasts demonstrated localization of the protein in the Golgi apparatus and in the ER. Additionally, in silico analysis (Jensen et al., 2002) of the protein sequence revealed beneath the well-known MSP-domain and two transmembrane domains, classifying the MOSPD1 as type IIc MDP (Tarr and Scott, 2005), a NES in the last exon. Deletion of the NES from the targeted cDNA and forced expression in MC3T3-E1 cells revealed a nearly solely localization of the truncated protein in the nucleus with a weak diffuse staining of the Golgi apparatus and ER. The nuclear localization suggests involvement in nuclear matrix formation but it also supports a further finding of the in silico analysis (Jensen et al., 2002) that suggested involvement of the protein in regulation of gene expression.
As Mospd1 is highly expressed in bone as well as in MC3T3-E1 cells, and as Mospd1 expression is strongly regulated by confluence and differentiation in MC3T3-E1 cells, this preosteoblastic cell line was chosen for Mospd1 knockdown experiments by siRNA. Knockdown of Mospd1 expression by siRNA revealed significant down-regulation of two genes specific for osteoblasts: the indispensable transcription factor Runx2 and the most abundant protein of the osteoblastic phenotype, osteocalcin. Time course experiments of preosteoblastic MC3T3-E1 cells, where they differentiate to mature, mineralizing osteoblasts, suggested a differentiation-dependent up-regulation of Mospd1 with a maximum before the strong up-regulation of osteocalcin. This experiment further suggests that Mospd1 expression was up-regulated by confluence of the culture as found with osteocalcin (Varga et al., 1999, 2010). Comparison of Mospd1 basal expression in three mesenchymal cell lines having different differentiation status on the way to osteoblasts demonstrated clearly a differentiation dependent expression when compared to the osteoblastic marker osteocalcin. Culture density dependent expression of Mospd1 was demonstrated in sparse versus dense seedings, which could reach about 100-fold stimulation when single cell expression of MC3T3-E1 cells was compared to confluent cultures. After 24 h, the confluence of these dens seeded cells was comparable to those reached after about 3 days of culture presented in the differentiation experiments of MC3T3-E1 cells. This suggests that not only density but also early differentiation processes modulate Mospd1 expression.
Mospd1 was expressed in all tissues tested with higher expression in mesenchymal cells and not surprisingly, Mospd1 was also expressed in cell lines derived from adipocytes and muscle cells. Moreover, in theses cell lines a differentiation dependent expression was found as well, at least during the first 10 days. According to the literature, as differentiation markers we used fat droplets generation, myotube formation and osteocalcin expression for adipocytes, myoblasts, or osteoblasts, respectively, which requires different culture times among the different cell lines. Although, we cannot exclude down-regulation of Mospd1 expression during prolonged culture time of adipocytes and myoblasts on the way to terminal differentiation. Osteoblastic BMSC differentiation needs longer culture periods because for their commitment to osteoblasts. Therefore, the increase in Mospd1 expression started later, in the early phase of osteoblastic differentiation when osteocalcin was only weakly increased (days 21–28). This could be compared to the first culture days of the already committed MC3T3-E1 preosteoblasts (days 3–6). All these data clearly indicate a differentiation dependent regulation of this gene in mesenchymal cells.
Knockdown of Mospd1 by means of siRNA and genome-wide expression analysis revealed down-regulation of a plethora of osteoblastic and mesenchymal genes and up-regulation of numerous epithelial genes. Thus, inspection of cadherins, genes entailed in formation of cell–cell contacts, disclosed a general change of the phenotype of the preosteoblasts: the osteoblastic cadherin Cdh11 was down-regulated while, in contrast, the epithelial Cdh1 was up-regulated. Moreover, genes of the Snail/Twist-family which prevent Cdh1 expression, thus playing a central role in EMT regulation, were down-regulated indicating that Mospd1 could be involved in up-regulation of the epithelial inhibitors Snail/Slug and thus play a role in EMT (Ireton et al., 2002; De Craene et al., 2005; Wheelock et al., 2008; Baranwal and Alahari, 2009; Guarino et al., 2009; Thiery et al., 2009; Yang et al., 2010). Very recently, it was demonstrated that SNAIL down-regulates thrombomodulin (Thbd) and induces tumorigenesis through epithelial–mesenchymal transition. Again, in our experiments we found a clear inverse regulation of thrombomodulin to Snail (Yang et al., 1998; Mills et al., 1999; Wolff et al., 2009; Kao et al., 2010).
Epidermis development is regulated by the transcription factor p63 (Trp63) a member of the p53 tumor suppressor gene family. Trp63, which encodes multiple variants with transactivating, death-inducing, and dominant-negative activity variants is expressed in many epithelial tissues (Yang et al., 1998). The alpha/beta carboxy-terminal domains of p63 are required for skin and limb development (Wolff et al., 2009). Trp63 regulates proliferation and differentiation of developmentally mature keratinocytes. Down-regulation of two variants of Trp63 (TAp63, ΔNp63) by siRNA knockdown experiments showed down-regulation of more than 200 genes, among them were 23 found to be strongly regulated (Truong et al., 2006). Consequently, up-regulation of TRP63 should up-regulate those genes. Mospd1 knockdown experiments up-regulated Trp63 and as expected most of those 23 genes, although, some of them only weak. Our finding supports the assumption that Trp63 is a regulator of epithelial differentiation (Yang et al., 1998; Mills et al., 1999; Wolff et al., 2009) and suggests that Mospd1 is acting upstream of Trp63 in inhibition of epithelial differentiation.
Knockdown of Mospd1 also strongly modulated expression of proteases. Interestingly Mmp13, which is well expressed in osteoblasts, as well as Mmp3 were up-regulated, Mmp9 (gelatinase-B), Mmp14 (MT1-MMP), and Mmp28 (Epilysin) were down-regulated. Recently, it was reported that Mmp28 induces TGFβ mediated EMT in lung carcinoma cells, while Mmp9 and Mmp14 were co-regulated, which suggested involvement in regulation of epithelial cell function (Illman et al., 2006). But also down-regulation of Snai1 by siMospd1 could explain down-regulation of Mmp9 and 14 that is regulated by Snai1 in epithelial cells (Miyoshi et al., 2004). Two proteases, however, were up-regulated: Mmp3 and Mmp13. Mmp3 triggers EMT by increasing the cellular levels of reactive oxygen species (Radisky et al., 2005), which should in turn stimulate Snai1 expression followed by increased Mmp13 transcription that is involved in metastasis. siRNA knockdown experiments, which decreased Snai1 and 2 expression should therefore down-regulate Mmp3 and Mmp13 expression. However, this was not the case; one explanation for our findings could be that under physiological conditions, when MET occurs, the mesenchymal matrix should be removed by these proteases.
During EMT in both normal and cancer cells, CDH1 (E-cadherin) is down-regulated by a lot of transcription factors: Snail and Zeb 1 and 2, Twist, Tcf3 (E47), Tcf4 (E2.2), Gdc (Goosecoid), Klf8, and Foxc2. Except of the last two mentioned, Mospd1-knockdown attenuated all those factors ensuring up-regulation of Cdh1, indicating the inverse process, namely MET (Suppl Fig. 1). Klf8 is inversely regulated to CDH1 in some lymph node positive breast cancers (Wang and Zhao, 2007) while Foxc2 does not affect E-cadherin expression rather it promotes its cytoplasmic localization (Hader et al., 2010).
TGFβ induces EMT in fibrosis via SMAD3 and SNAIL1 in increasing the expression of mesenchymal genes like collagens and down-regulation of epithelial ones (Sato et al., 2003; Boutet et al., 2006). Again, Mospd1 knockdown resulted in the inverse process, down-regulating the three TGFβ genes (Tgfb1,2,3) and one of the three receptors. As expected all mesenchymal genes were down- and the epithelial up-regulated (Suppl Fig. 2). Moreover, the inhibitor of the fibrotic EMT, Bmp7 (Zeisberg et al., 2003), was up-regulated indicating blocking of any residual TGFβ signaling (Suppl Fig. 2). Like TGFβ, many other GDF systems are involved in EMT, when activated. Although, other GDF systems were attenuated (not shown), as an example, we demonstrate that Mospd1-knockdown regulated Igf1 and Igf2 as well their receptor Igf1r; Igf1 via Igf1r and Zeb1 drives EMT (Graham et al., 2008).
We are aware of the fact that most of the epidermal genes were only low expressed and weakly regulated. However, one must consider that expression of these genes happens in an osteoblastic environment. Many transcription factors necessary for full expression of epithelial genes are possibly weak or not expressed in osteoblasts, maybe due to an improper epigenetic configuration of the chromatin. Furthermore, correct cell–cell contacts in combination with a suitable interaction of the cells with a defined ECM are prerequisites for the expression of a fully epithelial phenotype and these conditions may not be fully accomplished in the experiments with MC3T3-E1 cells. However, the expression switch from mesenchymal to epithelial genes shows that the relatively differentiated MC3T3-E1 cells seem to change their phenotype under Mospd1 depletion.
The presented data suggest Mospd1 as potent regulator in EMT, very upstream of the regulatory chain, possibly acting during development. These considerations are supported by the observation that a part of this protein is highly conserved phylogenetically, indicating the importance of the MSP-domain of MOSPD1 for the development of organisms. Furthermore, MOSPD2 the second protein of this family, highly conserved as well, was found expressed in early development (pregastrula) of zebrafish (Hong et al., 2010). Moreover, the third member of this family Mospd3 plays a role in right ventricle development of the heart (Pall et al., 2004).
All these data suggest a pivotal role of Mospd1 in the developmental regulation at the switch between epithelial and mesenchymal cells.
Acknowledgements
This study was supported by the Fonds zur Foerderung der wissenschaftlichen Forschung (FWF; The Austrian Science Fund) Project P20646-B11, the WGKK (Social Health Insurance Vienna), and the AUVA (Austrian Social Insurance for Occupational Risks).




