Mesenchymal stem cells effectively reduce surgically induced stenosis in rat carotids†
To the memory of Prof. Antonino Cascino, deceased 6 April 2008.
Abstract
Restenosis following vascular injury remains a pressing clinical problem. Mesenchymal stem cells (MSCs) promise as a main actor of cell-based therapeutic strategies. The possible therapeutic role of MSCs in vascular stenosis in vivo has been poorly investigated so far. We tested the effectiveness of allogenic bone marrow-derived MSCs in reduction of stenosis in a model of rat carotid arteriotomy. MSCs were expanded in vitro retaining their proliferative and differentiation potentiality. MSCs were able to differentiate into adipocyte and osteocyte mesenchymal lineage cells, retained specific antigens CD73, CD90, and CD105, expressed smooth muscle alpha-actin, were mainly in proliferative phase of cell cycle and showed limited senescence. WKY rats were submitted to carotid arteriotomy and to venous administration with 5 × 106 MSCs. MSCs in vivo homed in injured carotids since 3 days after arteriotomy but not in contralateral uninjured carotids. Lumen area in MSC-treated carotids was 36% greater than in control arteries (P = 0.016) and inward remodeling was limited in MSC-treated carotids (P = 0.030) 30 days after arteriotomy. MSC treatment affected the expression level of inflammation-related genes, inducing a decrease of IL-1β and Mcp-1 and an increase of TGF-β in injured carotids at 3 and 7 days after arteriotomy (P < 0.05). Taken together, these results indicate that allogenic MSC administration limits stenosis in injured rat carotids and plays a local immunomodulatory action. J. Cell. Physiol. 217: 789–799, 2008. © 2008 Wiley-Liss, Inc.
Restenosis is a pathophysiological phenomenon that can occur in patients submitted to revascularization procedures (bypass, endartherectomy, angioplasty), possibly resulting in new narrowing of injured vessels. Vascular restenosis remains a pressing clinical problem, despite the therapeutic strategies and devices developed so far (Lal, 2007).
The disruption of endothelial cells (ECs) in the intima by vascular injury is of particular importance in stenosis progression, since it is the cause of a concomitant reduction of vasculoprotective mediators (e.g., nitric oxide and prostacyclin). EC disruption also triggers a number of signaling cascades that lead to inflammation, platelet adhesion and cell proliferation. Also the intraoperative damage to adventitia and to vasa vasorum and nervous fibers it contains can have deleterious effects on restenosis (Khurana et al., 2004).
Stem cells hold a great potential for the regeneration of damaged tissues in cardiovascular diseases. Recent studies clearly indicated that different bone marrow-derived stem cell populations, characterized by different markers and with distinct behaviors, contribute to vascular remodeling after injury. Nevertheless, the exact role of vascular cell precursors in restenosis pathophysiology is not yet well defined, as heterogeneous and contrasting data are currently available (Tanaka et al., 2003; Forte et al., 2007a), probably in relation to the different kind of injury, to the animal models and to the heterogeneity of stem cell populations.
Mesenchymal stem cells (MSCs) are non-hematopoietic multi-potent stem-like cells able of differentiating into both mesenchymal and non-mesenchymal lineages. MSCs offer a series of advantages: (a) they can be isolated from a small aspirate of bone marrow; (b) extensively proliferate in vitro while preserving a normal karyotype and telomerase activity on several passages (Pittenger et al., 1999); (c) express low immunogenicity (Beggs et al., 2006), and hence their use in animal models should not require a pharmacological immunosuppression.
MSCs have an intrinsic ability to differentiate into functional cell types able to repair the diseased or injured tissue in which they are localized. This trend to adopt the local cellular identity may be correlated to tissue cytokines and matrix factors, as well as to adequate contact with host cells. For this reason, MSCs are currently under scrutiny for treatment of different cardiovascular diseases. In the concern of heart valve surgery, for example, tissue-engineered porcine heart valves created from MSCs in a lamb model, injected in a decellularized xenograft scaffold, exhibited satisfactory hemodynamic and histological aspects (Vincentelli et al., 2007).
It has not yet been clearly determined whether MSCs can substantially contribute to a positive resolution of restenosis after vascular injury. As a matter of fact, limited and contrasting results are currently available in experimental models. In more detail, Chen et al. (2007) revealed in rats submitted to aortic angioplasty that the local infusion of MSCs induced higher restenosis than controls. Another study also linked the homing of MSCs to an excessive repair process of the vessel in a model of mice femoral artery wire injury (Wang et al., 2008). Conversely, an ex vivo model of endothelium repair developed in rabbit revealed the positive role played by MSCs in prevention of smooth muscle cell (SMC) proliferation and in differentiation to ECs (Wu et al., 2005). Moreover, MSCs have been successfully applied in engineered vascular grafts, showing excellent long-term patency (Hashi et al., 2007). Finally, a recent study demonstrated that MSCs seeded on polyurethane patch restore endothelium in rat aorta and induce complete SMC differentiation, restoring a media-like thick wall (Mirza et al., 2008).
MSCs are able to suppress both B and T cell function (Rasmusson et al., 2003; Corcione et al., 2006). Allogenic MSCs suppress T cells activation by increasing the CD8(+) subpopulation and decreasing the CD25(+) T lymphocyte subset. One of the major mediators of T-cell suppression by MSCs is their production of nitric oxide (NO) by inducible NO synthase, that is not induced in T-cells (Sato et al., 2007). This observation can have potential positive repercussions on application of MSCs for restenosis reduction, since it has been demonstrated that inflammation plays a major role in restenosis, as lymphocytes recruited at the injury site secrete growth factors that activate vascular cell proliferation. Moreover, NO production by MSCs can have positive effects on vascular remodeling, since it is a mediator of vasorelaxation and has effects on the modulation of the SMC phenotype, maintaining them in a contractile status. MSCs are known to inhibit the response of allogeneic T lymphocytes in vitro through different pathways. Two different mechanisms, either cell contact-dependent or independent, have been proposed to account for this immunosuppression (Chen et al., 2007), based on the expression of soluble factors, including IL-10 and TGF-β, after contact with T lymphocytes (Nasef et al., 2007). These observations imply that allogenic MSC transplantation may be accomplished without the need for host immunosuppression.
In this study we aimed to verify the role, positive or negative, played by MSCs in stenosis progression induced by a previously characterized model of rat vascular injury based on carotid arteriotomy (Forte et al., 2001). We demonstrated that a systemic administration of allogenic bone marrow-derived MSCs injected via tail vein are able to home at the injury site and to limit lumen stenosis. Our data also reveal that MSCs are able to affect the local expression of inflammation-related genes. These findings add new information about the possible therapeutic role in restenosis for this peculiar population of stem cells.
Methods
Animals
Studies were carried out on 12-week-old male Wistar Kyoto (WKY) rats (230–250 g) (Charles Rivers, France). All animals were handled in compliance with the “Ethical principles and guidelines for scientific experiments on animals” of the Swiss Academy of Medical Sciences. All protocols were approved by the Animal Care and Use Committee of the Second University of Naples. Rats were acclimatized and quarantined for at least one week before undergoing surgery.
MSC cultures
MSCs have been harvested from the bone marrow of the femurs and tibias of adult WKY rats. WKY rats have been anaesthetized with an intraperitoneal injection of ketamine hydrochloride and MSCs have been harvested from the bone marrow by inserting a 21-gauge needle into the shaft of the bone and flushing it with complete α-modified Eagle's medium (αMEM) containing 20% fetal bovine serum (FBS), 2 mM L-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin. Cells from one rat have been plated into two 100 mm dishes. After 24 h, non-adherent cells have been discarded, and adherent cells have been washed twice with phosphate buffered saline (PBS). The cells have been then incubated for 5–7 days to reach confluence. Finally, MSCs have been extensively propagated for further experiments. All cell culture reagents have been obtained from Invitrogen (Milan, Italy).
MSC differentiation
Differentiation of isolated MSCs into the mesenchymal osteogenetic and adipogenetic lineages was induced using different protocols.
Adipocyte differentiation: cells were grown for 14 days in αMEM containing 10% FBS, 100 µM indomethacin, 1 µM hydrocortisone, 1 µg/ml insulin, and 10% horse serum. Adipocytes were detected by standard Oil Red-O staining.
Osteocyte differentiation: cells were grown for 14 days in αMEM containing 10% FBS, 100 mM dexamethasone, 0.2 mM ascorbic acid, and 10 mM β-glycerophosphate. Osteocytes were detected with Alizarin Red staining.
Senescence-associated β-galactosidase staining
Cultured MSCs were fixed for 10 min with 2% formaldehyde/0.2% glutaraldehyde at room temperature (RT). Cells were then washed twice with PBS and 2 ml of senescence-associated β-galactosidase staining solution per 35 mm dish were added. Cells were incubated at 37°C until blue staining was clearly detectable under a light microscope. The percentage of senescent cells was calculated by the number of β-galactosidase positive cells out of at least 500 cells in different microscope fields. Just before using the senescence-associated β-galactosidase staining solution, we added 20 ml of citric acid/sodium phosphate in a volume of 100 ml, pH 6.0, 150 mM NaCl, 2 mM MgCl2, 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide and 1 mg/ml X-Gal.
TRAP assay
The telomere repeat amplification protocol (TRAP) assay was performed according to Kim and Wu (1997). Briefly, 5 × 105 MSCs or control HEK cells were lysed in 10 mM Tris–HCl, pH 7.5, 2.5 mM MgCl2, 1 mM EGTA, 0.5% CHAPS, 10% glycerol, 5 mM β-mercaptoethanol, and 1 mM AEBSF for 30 min at 4°C. The lysates were then centrifuged for 10 min at 10,000g at 4°C. After centrifugation, protein concentration was determined by the Bradford assay. The reaction mixture for the TRAP assay was done as follows: 1× Taq DNA polymerase buffer (Promega, Madison, WI), 1.5 mM MgCl2, 50 µM dNTPs, primer M2(TS) (5′-AATCCGTCGAGCAGAGTT-3′) and TRAP internal control (5′AATCCGTCGAGCAGAGTTAAAAGGCCGAGAAGCGAT-3′). This mixture was incubated for 30 min at 25°C; we then added primer ACX (5′-GCGCGG[CTTACC]3CTAACC-3′), primer NT (5′-ATCGCTTCTCGGCCTTTT-3′) and 2.5 U Taq DNA polymerase (Promega). The reaction was denatured for 3 min at 94°C, then amplified for 30–35 cycles (94°C for 15 sec, 60°C for 15 sec, 72°C for 15 sec). Primers ACX and M2(TS) amplify the telomerase products, whereas primers M2(TS) and NT amplify the TRAP internal control. PCR products were resolved on 20% polyacrylamide gels stained with 1× GelStar nucleic acid stain (Lonza, Basel, Switzerland).
Cell cycle analysis
For each assay, 3 × 105 MSCs were collected and resuspended in 500 µl of a hypotonic buffer (0.1% Triton X-100, 0.1% sodium citrate and 50 µg/ml propidium iodide/RNAse A). Cells were incubated in the dark for 30 min and then analyzed. Samples were acquired on a FACSCalibur flow cytometer using the Cell Quest software (Becton Dickinson, Fanklin Lakes, NJ) and analyzed using the Cell Quest software and the ModFitLT software version 3 (Becton Dickinson).
Fluorescence immunocytochemistry
MSCs (1.5 × 104) were used for immunofluorescence analysis of the expression of surface antigens CD73, CD90, and CD105, and of smooth muscle (SM) alpha-actin. Cultured MSCs grown in slide chambers (BD Falcon) were fixed in 4% buffered paraformaldehyde for 10 min at RT, incubated with blocking solution (1% BSA) for 1 h and then with the primary antibody (mouse anti-rat CD73, BD Biosciences, San Jose, CA, 1:100; mouse anti-rat CD90, BD Biosciences 1:250; goat anti-rat CD105, Santa Cruz, Santa Cruz, CA, 1:100; mouse anti-rat SM-alpha actin, Sigma-Aldrich, Milan, Italy, 1:250) in blocking solution overnight at 4°C. Negative controls were incubated with blocking solution only. Subsequently, the samples were incubated with monoclonal anti-mouse or anti-goat FITC- or TRITC-conjugated secondary antibodies (dil 1:100, Jackson ImmunoResearch, Suffolk, UK) for 1 h at RT. The nuclei were stained with Hoechst 33258 (Sigma-Aldrich) for 5 min and the samples examined under a fluorescence microscope (Leica, Wetzlar, Germany).
RNA extraction and RT-PCR analysis
Total RNA was extracted from cultured MSCs, from injured rat carotids at 3 and 7 days after arteriotomy (n = 4 DiI-MSC-treated rats and n = 4 DiI-DMEM-treated rats, for each time point) and from uninjured rat carotids (n = 3) using the RNAeasy minikit (Qiagen, Hilden, Germany) according to manufacturer's instructions. RNA was treated with DNase (Qiagen) to remove DNA contamination. RNA concentration was measured using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE). RNA integrity was verified by electrophoresis on denaturing 1% agarose gel. Absence of residual DNA was verified by PCR on total RNA without reverse transcription.
cDNA was generated from 200 ng of each RNA sample. Reverse transcription was done at 42°C for 1 h in presence of random examers and Moloney-Murine Leukemia Virus (M-MULV) reverse transcriptase (Finnzymes, Espoo, Finland). GeneBank sequences for rat mRNAs and the Primer Express software (Applied Biosystem) were used to design primer pairs for the genes PPAR-γ, osteopontin, IL-6, IL-1α, IL-1β, TGF-β, TNF-α, IFN-γ, Mcp-1, SM22, vWF and the house keeping gene GAPDH. Primer sequences are available upon request.
Primer pairs were chosen to yield 100–150 bp PCR products and were validated running the PCR products on agarose gel to confirm a single band. Melting curves (65–94°C) were also generated to determine whether there were any spurious amplification products. Each RT-PCR reaction was repeated at least three times. A semi-quantitative analysis of mRNA levels was performed by the GEL DOC UV system (Bio-Rad, Hercules, CA) on agarose gels. When minimal differences in gene expression were detected, experiments were repeated using the real-time PCR assays, run on an Opticon 4 machine (Bio-Rad). Reactions were performed according to the manufacturer's instructions using the SYBR Green PCR master mix (Stratagene, La Jolla, CA). Relative quantitative RT-PCR was used to determine the fold difference for genes. The real time PCR efficiency was calculated for each primer pair using a dilution series and the MJ Opticon II analysis software.
Vascular injury and MSC treatment
Arteriotomy of rat common carotid artery was performed as already published (Forte et al., 2001). Briefly, a plastic Scanlom clamp for coronary artery grafting was placed for 10 sec on the carotid causing a crushing lesion to the vessel. At the same point where the clamp was applied, a 0.5 mm longitudinal incision was done on the full thickness of the carotid. The incision did not cross to the other side of the vessel. Haemostasis was obtained with a single adventitial 8.0-gauge polypropylene stitch. Once bleeding stopped, the carotid artery was carefully examined and blood pulsation was checked distally to the incision.
For morphometric analysis, WKY rats submitted to arteriotomy were administered with 5 × 106 MSCs resuspended in 200 µl DMEM via tail vein injection (n = 8), while control rats were administered with 200 µl DMEM (n = 8). For homing analysis, 5 × 106 MSCs were labeled with fluorescent Vybrant DiI (Molecular Probes-Invitrogen), according to manufacturer's instructions, and were administered to rats with 200 µl DMEM (n = 3 for each time point) while control rats were administered with 200 µl DiI-DMEM (n = 3 for each time point).
Histological analysis
Carotid arteries were harvested 30 days after arteriotomy and MSC administration for morphological and morphometric analysis. Alternatively, carotid arteries were harvested at 3 and 7 days after arteriotomy for analysis of MSC homing at the injury site. Harvested vessels were fixed in 4% buffered formaldehyde, dehydrated and embedded in paraffin. Five micrometers cross-sections were stained with hematoxylin-orcein for nucleus and elastic fiber staining, respectively. Image screening and photography of serial cross-sections were performed using a Leica IM1000 System. Arterial remodeling, lumen cross-sectional area (CSA) and the CSA of the tunica media + the intima (M + I) were measured using the Leica IM1000 software. Arterial remodeling is defined as a change in arterial size compared with a control artery. In agreement with other studies (Hollestelle et al., 2004), we used the external elastic lamina (EEL) area calculated from the EEL length as a measure of arterial size. Total M + I CSA was measured between the EEL and the lumen. The lumen and M + I CSA of each injured carotid were compared both to the ipsilateral distal region and to contralateral uninjured carotid. Measurements were performed by two independent observers.
For analysis of DiI-labeled MSC homing at the injury site, carotid cross-sections were stained with fluorescent dye Hoechst 33258 (Sigma-Aldrich) for nuclear identification. Image screening and photography of serial cross-sections were then performed using the Leica 4000F software.
Statistical analysis
All statistical analysis was performed using GraphPad software (Prism 4.0). Data are presented as the mean ± SEM. Statistical significance was determined using two-way analysis of variance followed by Bonferroni's multiple comparison test. Values of P < 0.05 were considered significant.
Results
Cultured bone-marrow-derived rat MSCs are able to differentiate to adipocyte and osteocyte phenotypes
One of the minimal criteria to identify multipotent MSCs is their capacity for mesenchymal lineage differentiation (Dominici et al., 2006). Before using MSCs in vivo to test their ability in limiting arterial stenosis, we verified that the MSCs we extracted from rat bone marrow and expanded in vitro for 15 days from passage zero (Fig. 1A) were able to differentiate to osteocytes and adipocytes using standard in vitro culture-differentiating conditions.

Bone marrow-derived MSCs are able to differentiate to adipocyte and osteocyte phenotypes. A–C: Representative micrographs of rat bone marrow MSCs induced to differentiate into mesenchymal lineages with different media. MSCs in conventional culture (A); MSCs induced to differentiate to osteocytes and stained with Alizarin Red (B); MSCs induced to differentiate to adipocytes and stained with Oil red O (C). D: PPAR-γ and osteopontin mRNA analysis by RT-PCR on total RNA extracted from MSCs in conventional culture and induced to differentiate into osteocytes and adipocytes with different media.
Osteogenic differentiation of MSCs was accompanied by morphological changes in cells, by positive staining with Alizarin Red (Fig. 1B) and by the increased expression of the specific marker osteopontin, determined by RT-PCR (Fig. 1D).
Adipogenic differentiation of MSCs was accompanied by morphological changes in cells, by the formation of lipid vacuoles, visualized by staining with Oil Red O (Fig. 1C), and by the increased expression of the specific marker PPAR-γ, determined by RT-PCR (Fig. 1D).
Cultured bone marrow-derived rat MSCs are positive for specific antigens
We verified on MSCs before their injection in vivo the expression of markers recognized as one of the criteria to identify MSCs (Dominici et al., 2006). In particular, we successfully verified by immunocytochemistry that bone marrow-derived MSCs expressed the surface antigens CD73 (also known as ecto 5′ nucleotidase), CD90 (also known as Thy-1) and CD105 (also known as endoglin, a TGF-β receptor subunit) (Fig. 2B–D).
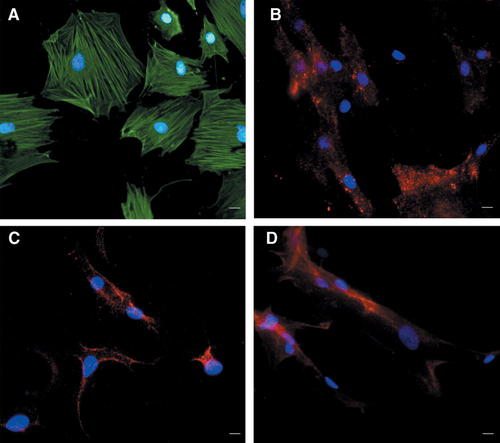
MSCs express lineage-specific antigens at the time of injection in vivo. Representative photomicrographs of MSCs showing immunocytochemistry for SM-alpha actin (A), CD73 (B), CD90 (C), and CD105 (D). The cell nuclei were counterstained with Hoechst 33258 and emitted blue fluorescence. The cells emitting green fluorescence were positive for SM-alpha actin (A); the cells emitting red fluorescence were positive for CD73 (B), CD90 (C) and CD105/Endoglin (D). Scale bar represents 50 µm.
Moreover, we successfully verified that MSCs were positive for SM-alpha actin, another defining feature of MSCs (Fig. 2A) (Ball et al., 2007).
Cultured bone-marrow-derived rat MSCs are mainly in the G1/S phase of cell cycle and show limited senescence at the time of injection in vivo
In order to obtain the sufficient amount of cells to be injected in rats at the time of arteriotomy, MSCs were cultured for 23 days, including 15 days from passage zero. Nevertheless, during this period, MSCs maintained their proliferative capacity and were mainly in G1 and S phase of cell cycle (50% and 49%, respectively), as demonstrated by the FACS analysis (Fig. 3A).
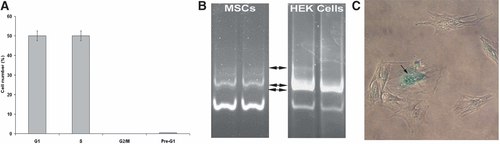
MSCs are in the G1/S phase of cell cycle and show limited senescence at the time of injection in vivo: FACS analysis of MSCs at 15 days of culture from passage zero (A). Polyacrylamide gel electrophoresis of TRAP assay products obtained from protein extracts of MSCs at 15 days of culture from passage zero and of control HEK cells. Each assay has been repeated twice. Black arrows indicate the amplification products. The lower bands represent the internal control of assay (B). Senescence-associated β-galactosidase assay performed on MSCs at 15 days of culture from passage zero, when about 23% of senescent cells were present. Arrow indicates a representative blue stained senescent MSC (C).
Since senescence can hamper cell differentiative ability and stemness, we performed a time-dependent follow-up of cellular senescence both through TRAP assay and in situ β-galactosidase assay.
TRAP assay provides indication about telomere shortening, a marker for cellular senescence. Telomerase activity is measured through a primer extension assay in which telomerase reverse transcriptase (TERT) synthesizes telomeric repeats onto oligonucleotide primers.
β-galactosidase assay is based on the increase in the lysosomal compartment of senescent cells of the expression of β-galactosidase, detected as blue perinuclear staining specifically at acid pH.
Both these assays demonstrated that MSCs preserved their telomerase activity in comparison to control HEK cells (Fig. 3B) and showed a limited 23% of senescent cells at the time of injection in vivo (Fig. 3C).
Finally, the FACS analysis revealed suitable conditions of cell culture, as there was no appreciable peak corresponding to pre-G1 phase of cell cycle (Fig. 3A).
MSCs home at the injury site in arteriotomy-injured carotids
Carotids were harvested from WKY rats at 3 and 7 days after arteriotomy and DiI-MSC injection to verify the homing of MSCs at the injury site. Histological analysis conducted on carotid cross-sections at level of injury site revealed a progressive accumulation of DiI-MSCs in the adventitia and in perivascular tissue, more evident at 7 days after injury (Fig. 4B,C,F,G), when some DiI-MSCs were present also in the tunica media (Fig. 4D) and in the endothelium (Fig. 4H). In particular, DiI-MSCs were present around the polypropylene stitch applied after longitudinal incision of the vascular wall, where reparative phenomena occurred, and in the perivascular tissue. No DiI-MSCs were detectable in contralateral uninjured carotids, thus revealing the specificity of MSC engraftment at the injury site (Fig. 4A,E).
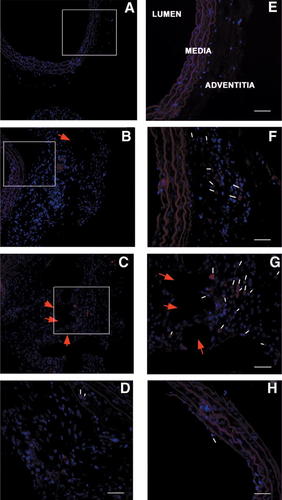
MSCs home at the injury site after arteriotomy. Representative cross-sections of carotids from DiI-labeled MSC-treated rats harvested at 3 and 7 days after arteriotomy. Uninjured carotid (A,E). DiI-MSC-treated rat carotid harvested at 3 days after arteriotomy (B,F). DiI-MSC-treated rat carotid harvested at 7 days after arteriotomy (C,G). Representative DiI-MSCs in the tunica media at 7 days after arteriotomy (D). Representative DiI-MSCs in the endothelium at 7 days after arteriotomy (H). Subparts (E–G) represent 40× magnification of the area enclosed in the white perimeter in A–C (20× magnification). D–H: 40× magnification. Representative DiI-labeled MSCs emitting red fluorescence are indicated by small white arrows. Red arrows indicate the point in the adventitia where the suture polypropilene stitch was applied after arteriotomy. The cell nuclei were counterstained with Hoechst 33258 and emitted blue fluorescence. Scale bar in (D–H) represents 50 µm.
MSCs are able to reduce arteriotomy-induced carotid (re)stenosis
Rat common carotids were submitted to arteriotomy and administered with 5 × 106 MSCs (n = 8) or with DMEM (n = 8) soon after carotid arteriotomy and skin suture.
The morphological and morphometric analysis of left common carotid arteries was performed 30 days after arteriotomy and treatment with MSCs or DMEM. Each carotid was compared to its contralateral uninjured artery and to distal ipsilateral uninjured region. Qualitative morphological analysis revealed that the surgical injury induced marked neoadventitia formation along with extracellular matrix and elastic lamina accumulation in both groups of rats, with only limited focal neointima in a few cases. In addition, internal and external elastic lamina and media disruptions at the injury site were evident, often with media substitution by fibrotic tissue. Quantitative analysis performed 30 days after injury showed a significantly 36% larger carotid lumen CSA with MSC treatment in comparison to control DMEM-treated rats (P < 0.05) (Fig. 6A). Also the carotid area encompassed by the EEL, an index of arterial remodeling, resulted to be significantly decreased in DMEM-treated rats in comparison to MSC-treated rats (Fig. 6B), indicating that MSC treatment strongly reduces the deleterious inward remodeling, a major determinant of luminal narrowing in restenosis. Finally, the CSA of the M + I significantly increased after injury both in MSC- and in DMEM-treated rat carotids in comparison to contralateral uninjured carotids, but no significant difference was detectable between the two groups of arteriotomy-injured carotids (Fig. 6C).
MSCs affect the expression of inflammation-related genes and of the differentiation marker SM22
We extracted total RNA from injured and contralateral uninjured carotids harvested at 3 and 7 days after arteriotomy from MSC- (n = 4 for each time point) or DMEM-treated rats (n = 4 for each time point) and from carotids of uninjured rats (n = 3). We analyzed through RT-PCR the differential expression of the inflammation-related genes IL-1α, IL-1β, IL-6, IL-10, Mcp-1, TGF-β, TNF-α, and IFN-γ. Expression data obtained in injured were compared to expression levels in carotids from uninjured rats.
Mcp-1 mRNA was detectable only in arteriotomy-injured carotids and it showed a significant 1.5- and 1.82-fold decrease in MSC-treated rats in comparison to DMEM-treated rats at 3 and 7 days after arteriotomy, respectively (P < 0.05) (Fig. 7A). Similarly, IL-1β mRNA was detectable only in arteriotomy-injured carotids both at 3 and 7 days after arteriotomy, and it showed a significant threefold decrease in MSC-treated rats in comparison to DMEM-treated rats 7 days after injury (P < 0.05) (Fig. 7B).
Of note, basal levels of TGF-β mRNA observed in uninjured carotids did not change after arteriotomy in DMEM-treated rat carotids, but it showed a significant 1.68- and 1.37-fold increase only in MSC-treated rats at 3 and 7 days after arteriotomy, respectively (P < 0.05) (Fig. 7C).
IL-6 and TNF-α mRNAs were detectable only in arteriotomy-injured carotids harvested 3 days after arteriotomy, but without significant differences between MSC- and DMEM-treated rats.
We were unable to detect a signal for mRNAs coding for IL-1α, IL-10, and IFN-γ both in uninjured and in arteriotomy-injured carotids.
Finally, we examined by RT-PCR the differential expression of von Willebrandt Factor (vWF), a marker of ECs, and of SM22, a marker of differentiated SMCs. The mRNA coding for vWF showed a progressive 4.3- and 6.6-fold decrease at 3 and 7 days after arteriotomy respectively in comparison to carotids from uninjured rats, but without significant differences between MSC- and DMEM-treated carotids. SM22 mRNA also showed a marked 19- and 17-fold decrease at 3 and 7 days after arteriotomy respectively, in comparison to carotids from uninjured rats, but it revealed to be 2.7-fold higher in MSC-treated carotids in comparison to DMEM-treated rats at 3 days after arteriotomy (P < 0.05) (Fig. 7D).
MSCs do not affect the expression of inflammation-related genes in contralateral uninjured carotids
Our previous studies highlighted relevant changes of mRNA levels not only in injured carotids but also in contralateral uninjured carotids, related to systemic inflammatory reaction and to vasocompensative reactions (Forte et al., 2008). Consequently, we investigated the effect of MSC administration on mRNA expression also in contralateral uninjured carotids at 3 and 7 days after arteriotomy. RT-PCR analysis revealed a significant increase of inflammation-related factors Mcp-1, TGF-β and IL-1β in comparison to carotids harvested from uninjured rats, but no significant difference between MSC- and DMEM-treated rats (data not shown).
Discussion
Bone marrow-derived rat MSCs retain multipotent potentiality, high proliferative activity and low senescence before injection in vivo
The application of stem cells for therapeutic purposes requires an in-depth knowledge of their biological characteristics, a rigorous application of standardized methods and a characterization of cells before administration in vivo. Multipotent MSCs promise to be a valuable therapeutic tool but, due to their low number, require considerable in vitro expansion before use in experimental models of disease and in clinical trials. This can lead to in vitro senescence and subsequently to a decreased potential for proliferation and differentiation (Bonab et al., 2006). For these reasons, before MSC administration in rats submitted to arteriotomy, we verified their biological characteristics in order to set up suitable culture conditions aimed at limiting MSC senescence while retaining their differentiation potential. Moreover, we applied the minimal criteria for defining multipotent MSCs, as suggested by the International Society for Cellular Therapy (ISCT) (Dominici et al., 2006). In agreement with these criteria, we isolated MSCs on the basis of their adherence to plastics and we verified that rat bone marrow-derived MSCs were able to differentiate into bone and fat cells (Fig. 1B–D) and expressed the surface markers CD73, CD90, and CD105 (Fig. 2B–D). We also verified that MSCs expressed SM-alpha actin (Fig. 2A), another feature of MSCs that provides them with contractile ability (Ball et al., 2007).
MSCs are able to home at the injury site and to reduce surgically-induced inward remodeling of rat carotids
We successfully treated rats with allogenic MSCs without any immunosuppressive protocol, in agreement with previous studies revealing their low immunogenicity (Patel et al., 2008), probably related to the lack on cell surface of the major histocompatibility complex II (MHC II), that is responsible for immune rejection (Le Blanc et al., 2003). Some studies revealed that a subset of MSCs is positive for MHC II but they equally exerted a veto-like activity on immune response (Potian et al., 2003).
New evidence suggests that the artery wall is a recipient and source of MSCs (Abedin et al., 2004; Hoshino et al., 2008). Our data indicate that DiI-labeled MSCs injected via tail vein in rat were able to home in the carotid vascular wall since 3 days after vascular injury, with a preferential localization in the adventitia and in the perivascular tissue (Fig. 4F,G). Our previous report (Forte et al., 2007b) revealed the up-regulation of mRNAs involved in stem cell homing, and in particular a maximal 4.73-fold increase of the chemokine Cxcl12 (or SDF-1α), able to mediate the mobilization of bone marrow-derived SMC progenitor cells through the corresponding receptor Cxcr4, that also increased. No DiI-labeled MSCs were detectable in contralateral uninjured carotids (Fig. 4A,E), neither an increase of Cxcl12 and Cxcr4 mRNAs (Forte et al., 2008), thus suggesting that engraftment of MSCs requires tissue injury.
It is well known that cell trapping in the pulmonary microvasculature can significantly reduce the number of MSCs that can access injured organs, as demonstrated by interesting studies in mice (Schrepfer et al., 2007). These authors demonstrated that the vast majority of MSCs, whose diameter is about 15–19 µm, are trapped in lung capillaries within seconds after intravenous injection, and that this phenomenon can be reduced by pretreatment with sodium nitroprusside, a source of nitric oxide. Nevertheless, we demonstrated in our study that the MSCs that were able to escape pulmonary trapping were sufficient to limit the negative remodeling in arteriotomy-injured rat carotids, in agreement with other studies underlining that the number of MSCs necessary in regenerative medicine protocols do not necessarily need to be high, as MSCs are presumed to play their reparative and anti-inflammatory action mainly through soluble factors, playing a paracrine action (Psaltis et al., 2008). Moreover, the rate of post-surgery death in MSC-treated rats was very low (about 5%), indicating that injected MSCs did not cause relevant pathophysiological problems. Many other therapeutic protocols in animal models and in patients based on intravenous administration of MSCs revealed to be effective and safe, despite the possible cell trapping in the pulmonary microvasculature (Ma et al., 2005; Lee et al., 2008).
The morphological and morphometric data resulting from this study support the therapeutic potentiality of MSCs in vascular (re)stenosis. In agreement with our previous studies (Forte et al., 2001), the morphological analysis of rat carotids at 30 days after rat carotid arteriotomy highlighted relevant differences in comparison to carotids submitted to balloon angioplasty, since we observed a marked lumen stenosis mainly to due neoadventitia and to negative or inward remodeling, while the role of intima hyperplasia proved quite limited (Fig. 5B,D). Inward remodeling is a phenomenon that involves extracellular matrix and, in particular, collagen synthesis and spatial redistribution, as well as elastic fiber accumulation. Inward remodeling is currently considered to be the main cause of restenosis (Pasterkamp et al., 2000). The morphometric results reveal a significant reduction of carotid inward remodeling together with an increase of lumen CSA (Fig. 6A) but not of the M + I CSA in MSC-treated rats (Fig. 6C), indicating that the difference in inward remodeling between MSC- and DMEM-treated rats is most probably related to adventitial cicatrization, as previously demonstrated by porcine angioplasty studies (Zalewski and Shi, 1997), involving changes in matrix composition and conformation that can induce vessel shrinkage.
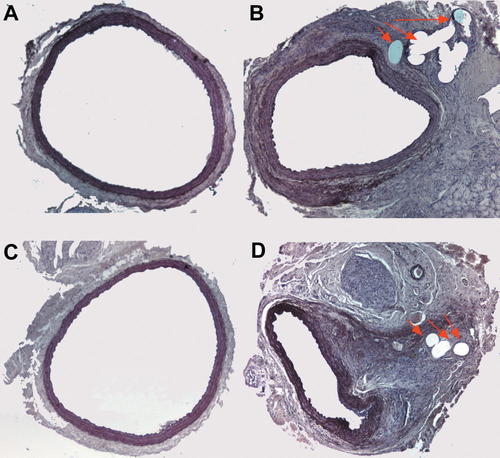
MSCs are effective in reducing arteriotomy-induced stenosis. Representative cross-sections of carotids from MSC- and DMEM-treated WKY rats, harvested 30 days after arteriotomy. Contralateral uninjured carotid from MSC-treated rat (A). Injured carotid from MSC-treated rat (B). Contralateral uninjured carotid from DMEM-treated rat (C). Injured carotid from DMEM-treated rat (D). Red arrows indicate the point in the adventitia where the suture polypropilene stitch was applied after arteriotomy. Hematoxylin-orcein staining, 10× magnification.
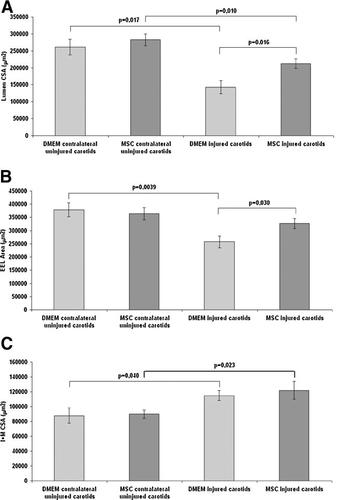
Morphometric measurements on cross-sections of MSC-treated rat carotids 30 days after the surgical injury. Lumen CSA (A), EEL area (B), and I + M CSA (C) in injured and in contralateral uninjured carotids harvested 30 days after arteriotomy and MSC or DMEM treatment. Data are presented as the mean ± SEM. P < 0.05 versus DMEM-treated carotids was considered statistically significant.
MSCs are able to modulate the expression of inflammation-related genes and of SM22 marker at the injury site in rat carotids
Three main hypotheses can be proposed to explain the positive role played by MSCs in limitation of negative remodeling induced by arteriotomy of rat carotid: (1) circulating MSCs could be recruited at the injury site by chemokines released after arteriotomy and then differentiate to SMCs and/or to ECs, thus inducing a rapid regeneration of vascular tissue and consequently limiting vascular cell proliferation. In this context, studies based on co-culture experiments revealed that a direct contact between MSCs and SMCs is required in the differentiation of MSCs into SMCs (Wang et al., 2006); (2) recruited MSCs could produce a variety of cytokines, including VEGF and bFGF, that could exert a paracrine action on resident vascular cells at the injury site and promote a rapid recovery of damage; (3) MSCs could home at the injury site and exert a local and/or systemic immunosuppressive action thus limiting the inflammatory reaction and other related processes, including reactive oxygen species production and cell proliferation and migration. These three hypotheses are not mutually exclusive but could coexist in this pathophysiological process. The microarray analysis of functional pathways revealed a marked inflammatory reaction in arteriotomy-injured carotids (Forte et al., 2007b). On the basis of the immunomodulatory properties of MSCs and of the key role that inflammation plays in vascular stenosis, we aimed to gain insight in the mechanism of action of MSCs in vascular stenosis by verifying the differential expression of some of the most significant markers of inflammation.
TGF-β is an immunosuppressive cytokine whose mRNA increased at 3 and 7 days after injury only in MSC-treated carotids (Fig. 7C). The increased production of TGF-β by MSCs homed at the injury site could induce a suppression of the local immune response, and in particular of T-lymphocyte proliferation (Di Nicola et al., 2002; Nasef et al., 2007). TGF-β has pleiotropic effects on cardiovascular cells (Ruiz-Ortega et al., 2007). In particular, it acts as a potent antiproliferative mediator on SMCs (Seay et al., 2005). Moreover, the increase of TGF-β mRNA in MSC-treated carotids in comparison to control DMEM-rats could have an effect also on myofibroblast differentiation, as it is well known that TGF-β plays a major role in the wound healing process (Hinz, 2007).
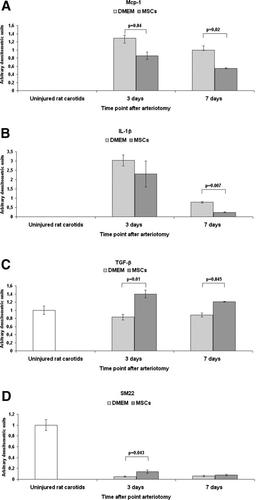
MSCs affect the expression of mRNAs coding for molecules involved inflammatory-immune reaction and for SM22 in arteriotomy-injured carotids. Mcp-1 (A), IL-1β (B), TGF-β (C) and SM22 (D) mRNA analysis by RT-PCR on total RNA extracted at 3 and 7 days after arteriotomy and MSCs or DMEM treatment and from carotids from uninjured rats. All measurements were normalized with respect to endogenous GAPDH levels. The values are expressed in arbitrary units as relative changes over the normalized uninjured control. Data are presented as the mean ± SEM. P < 0.05 versus DMEM-treated carotids was considered statistically significant.
IL-1β is a proinflammatory cytokine able to induce the expression of cyclooxygenase 2 and type 2 phospholipase A, and also to act as a hematopoietic growth factor (Dinarello, 2002). IL-1β expression is activated by vascular injury and the reduction of its mRNA at 7 days after injury and MSC treatment (Fig. 7B) could positively affect the local inflammatory reaction in injured carotids, in agreement with other studies (Guo et al., 2007; Koide et al., 2007).
Similarly, Mcp-1 is a proinflammatory chemokine able to stimulate SMC proliferation and macrophage infiltration in injured vascular tissue (Schober and Zernecke, 2007). Mcp-1 expression in carotids was activated by vascular injury; the reduction of its mRNA at 3 and 7 days after MSC treatment (Fig. 7A) is in agreement with other observations (Ohnishi et al., 2007) and could positively affect the outcome of carotid surgical injury. This hypothesis is supported by previous studies demonstrating that Mcp-1 inhibition reduces restenosis in different models of injury (Furukawa et al., 1999; Schober et al., 2004).
On the basis of these RT-PCR preliminary results we can hypothesize that MSCs reduce carotid stenosis at least in part through a synergic inhibition of the inflammation by macrophage invasion and proinflammatory cytokine expression and through the activation of anti-inflammatory/antiproliferative pathways.
The increase of inflammation-related mRNAs we detected not only in arteriotomy-injured rat carotids but also in contralateral uninjured carotids when compared to arteries from uninjured rats is in agreement with our previous observations (Forte et al., 2008) and can be related to a systemic inflammatory reaction triggered by carotid arteriotomy. The injection of MSCs in rats affects the expression of inflammation-related genes (Mcp-1, IL-1β, and TGF-β) only at the injury site where they home, and not at distal uninjured vascular beds, thus revealing, at least in our experimental protocol, that they do not affect the immune reaction at systemic level. Further studies on serum factors will be necessary to further analyze this aspect.
SMCs within adult blood vessels retain remarkable plasticity and can undergo profound and reversible changes in phenotype in response to environmental influences, such as vascular injury (Owens et al., 2004). Characterization of contractile and proliferative SMCs has lead to the identification of a number of differentiation markers under the control of the Serum Response Factor, including SM22 (Shanahan and Weissberg, 1998; Regan et al., 2000). The decrease of SM22 mRNA in arteriotomy-injured carotids in comparison to carotids from uninjured rats is in agreement with our previous observations (Forte et al., 2005) and suggests an arteriotomy-induced shift of SMCs from a contractile to a proliferative phenotype. MSC treatment transiently limits the loss of SM22 expression (Fig. 7D), possibly contributing to a limitation of SMC proliferation.
The decrease of vWF we observed at 3 and 7 days after injury is in agreement with previous microarray data (Forte et al., 2007b) and with a partial apoptosis-mediated loss of ECs after arteriotomy revealed by TUNEL assay (Forte et al., 2005). The MSC treatment did not contribute to limit the vWF mRNA decrease, thus suggesting that the mechanism of action of MSCs, at least in this model of vascular injury, does not involve a rapid endothelial recovery.
Conclusions
MSCs are multipotent self-renewing cells that retain the flexibility to differentiate into various lineages. Owing to these features, and to the ease of ex vivo expansion, there is a huge expectation from the use of MSCs for therapeutic applications.
This study represents a first step for the comprehension of the role of rat bone marrow-derived allogenic MSCs in surgically induced vascular stenosis. Our results indicate that a systemic intravenous administration of allogenic MSCs is safe, as we did not detect any evident adverse effect in MSC-treated rats and the rate of post-surgery death was very low.
MSCs resulted to home at the injury site in a model of carotid arteriotomy and were able to reduce lumen stenosis. The molecular events leading to tissue repair, including vascular wall repair after arteriotomy, remain a mysterious and complex process. Our data indicate that MSCs are effective in reducing arteriotomy-induced inward remodeling, in contrast with other studies conducted in rats arteries submitted to angioplasty (Chen et al., 2007) and in mice arteries submitted to wire injury (Wang et al., 2008), respectively, thus demonstrating that the effectiveness of a MSC-based therapy is strongly related to the kind of vascular injury to be repaired.
Among all the possible pathways that led to this positive outcome, we provided information about a local immunomodulatory role for MSCs and their contribution to maintain at least in part SMCs in a differentiated phenotype.
Several clinical trials with MSCs are already ongoing for different diseases. They have been used for the first stem/progenitor cell-based clinical trials in patients with osteogenesis imperfecta (Horwitz et al., 2001) and then in patients with mucopolysaccharidosis (Koç et al., 2002). Subsequently, trials were initiated for graft-versus-host disease, that are based on the ability of the MSCs to suppress immune reactions. Our findings can have clinical implications for prevention or reduction of restenosis in patients submitted to vascular injury involving the full wall thickness, such as carotid endarterectomy, arterial grafting or transplant vasculopathy. Nevertheless, a deeper understanding of the mechanisms of action of MSCs in (re)stenosis in other pre-clinical studies is required before trials in patients.
Acknowledgements
We are grateful to Ms. Maria Rosaria Cipollaro, Dr. Tiziana Squillaro and Dr. Nicola Alessio for skilful assistance. SHRO 2007–2008 “Role of cell cycle-related genes in the biology of stem cells” grant to U.G.; Legge 5 Regione Campania 2003 “Modello sperimentale di iperplasia fibrointimale post-chirurgica in modelli di ratto affetti da patologie dell'apparato cardiovascolare” to M.D.F.; Progetto Finalizzato Sanità 2003 “Patologie infettive e insulto chirurgico: studi di genomica e proteomica nel remodeling vascolare” to A.C.




