The angiopoietin-2 gene of endothelial cells is up-regulated in hypoxia by a HIF binding site located in its first intron and by the central factors GATA-2 and Ets-1
Abstract
Angiopoietins are ligands of the endothelial cell tyrosine kinase receptor Tie2. Angiopoietin-1 (Ang-1) is widely expressed in human normal adult tissues and promotes blood vessel maturation and stabilization by inducing Tie2 receptor phosphorylation. In contrast, the antagonistic ligand Angiopoietin-2 (Ang-2) is up-regulated by hypoxia, expressed only at sites of vascular remodeling and plays a crucial role in destabilizing vessels for normal or pathological angiogenesis. Ang-2 expression is tightly regulated at transcriptional and post-transcriptional levels. To characterize the regulatory sequences of the human Ang-2 gene we cloned a fragment of around 8.5 kb upstream of the Ang-2 coding sequence and analyzed the luciferase reporter activity of constructs of various lengths in endothelial and non-endothelial cells. We isolated a minimal promoter sequence sufficient to promote significant Ang-2 non-cell type specific transcription. Moreover, we identified sequences conferring endothelial specificity. Indeed, sequence analysis of the fragment revealed the presence of several potential binding sites for specific endothelial regulatory factors like GATA or Ets. Using GATA-2 and Ets-1 co-transfection and overexpression assay, we showed that these two factors are able to induce Ang-2 promoter activation. We also show that hypoxic regulation of Ang-2 is HIF-dependent and demonstrate that HIF-1α binds in human microvascular endothelial cells (HMVEC) to an evolutionary conserved Hypoxia-Responsive Element (HRE) located in the first intron of the Ang-2 gene. In conclusion, our study provides new elements in favor of HIF involvement in Ang-2 hypoxic regulation and identifies Ets-1 and particularly GATA-2 as central factors in endothelial specific Ang-2 expression. J. Cell. Physiol. 217: 809–818, 2008. © 2008 Wiley-Liss, Inc.
Angiogenesis, the formation of new blood vessels is tightly regulated by a number of factors (Carmeliet, 2003). The Angiopoietin (Ang)/Tie2 receptor system has been shown to regulate vessel stability and maintenance (Thurston, 2003). The angiopoietin family is composed of four members where Ang-1 and Ang-2 are among the most extensively studied. Ang-1 was the first ligand to be identified for the endothelial cell tyrosine kinase receptor Tie2. Through binding to Tie2 it induces Tie2 phosphorylation and downstream signaling that promotes vascular remodeling, maturation and stabilization by mediating interactions between endothelial cells and perivascular cells. Ang-1 is mainly expressed in perivascular cells during development and in adult tissue, where expression seems to be constitutive (Maisonpierre et al., 1997; Thurston, 2003). The role of Angiopoietin-2 (Ang-2), the second Tie2 binding partner, appears to be more complex. In contrast to Ang-1, Ang-2 is only minimally expressed or not expressed in most normal adult tissues but is strongly and rapidly up-regulated in endothelial cells at sites of intensive vessel remodeling such as in the female reproductive system and in tumors (Holash et al., 1999). Depending of the presence of VEGF, Ang-2 might play a role in both vessel sprouting and regression (Hanahan, 1997). Indeed, expression analysis showed that Ang-2 is rapidly induced together with VEGF in setting angiogenic sprouting whereas in the absence of VEGF, Ang-2 is induced only in setting vascular regression (Maisonpierre et al., 1997; Korff et al., 2001; Hackett et al., 2002; Lobov et al., 2002; Visconti et al., 2002; Oshima et al., 2004). Both Ang-1 and Ang-2 bind to Tie2 and depending on the context Ang-2 could activate or block the phosphorylation of Tie2 receptors (Maisonpierre et al., 1997; Kim et al., 2000; Mochizuki et al., 2002). Moreover, Ang-2 knock-out has shown that Ang-2 is required for both postnatal angiogenic remodeling and lymphatic patterning. Remarkably, Ang-1 completely rescues the lymphatic phenotype in mice lacking Ang-2 but not the vascular remodeling defect. This suggests that Ang-2 could act as a Tie2 agonist in the lymphatic setting and as an antagonist in the vascular setting (Gale et al., 2002). Together these data indicate that Ang-2 acts as an agonist as well as an antagonist and is a complex regulator of vascular remodeling. In addition, recent studies have shown that Ang-2 sensitizes endothelial cells to TNFα and plays a crucial role in the induction of inflammation (Fiedler et al., 2006). Thus, the regulation of Ang-2 expression seems to be an essential step in normal and pathological angiogenesis. A model has been proposed in which a balanced Ang-1/Ang-2 ratio would determine the status of the vasculature (Fiedler and Augustin, 2006). In quiescent vessels where the level of Ang-2 is low, the ratio is in favor of Ang-1 and the vessels are maintained quiescent by constitutive Tie2 activation by Ang-1. In sites of endothelium activation the Ang-2 protein, which is stored in Weibel-Palade bodies, is rapidly released (Fiedler et al., 2004) and Ang-2 transcription is up-regulated in endothelial cells, turning around the ratio in favor of Ang-2. This induces an Ang-2 antagonist effect on Tie2 and depending on the presence of other stimuli such as VEGF or TNFα, this possibly promotes further vessel destabilization and remodeling. Ang-2 expression in endothelial cells is tightly controlled by several angiogenic inducers including VEGF, bFGF and TNFα and by environmental changes such as a high glucose level and hypoxia, a reduced level of oxygen (Mandriota and Pepper, 1998; Oh et al., 1999; Mandriota et al., 2000; Huang et al., 2002; Fiedler et al., 2004; Yao et al., 2007). Hypoxia arises also as a consequence of the development of solid tumors and is one of the key driving forces for tumor progression. Ang-2 is considered to be a critical component of neo-vascularization in tumors. Endothelial cells are the main source of Ang-2 and the production of Ang-2 is strictly regulated at the transcriptional level at sites of neo-angiogenesis. The goal of our study was to better understand mechanisms that sustain Ang-2 transcriptional expression in endothelial cells and especially under hypoxia. Hypoxia, through the hypoxia-inducible factor (HIF), leads to the activation of a number of genes implicated in angiogenesis (Manalo et al., 2005; Wenger et al., 2005; Hirota and Semenza, 2006). HIFs are heterodimers of an oxygen-regulated HIFα subunit and a constitutive HIFβ/ARNT subunit that binds to Hypoxia-Responsive Elements (HRE) located in the regulatory sequences of target genes. To study the regulatory sequences implicated in Ang-2 transcription, we characterized the Ang-2 promoter region. During the course of this study, two interesting reports by Hasegawa et al. (2004) and Hegen et al. (2004) were published about the Ang-2 promoter sequences. However here, not only we confirm these results, but we extend the characterization of this promoter to the specific regulation by hypoxia. We identified sequences containing elements that confer endothelial specificity and show that the GATA-2 and Ets-1 transcription factors act as regulators of Ang-2 transcription. Moreover, we show that hypoxic up-regulation of Ang-2 is HIF-dependent and identify for the first time, a HIF-1 binding HRE located in the first intron of Ang-2 gene.
Materials and Methods
Materials
Opti-MEM and Lipofectin Reagent were from Invitrogen (San Diego, CA); the pGL3 vector, pRL-TK vector, prL null vector and Dual Luciferase reporter assay system were from Promega (Madison, WI); rabbit polyclonal anti-human HIF-1α antibody was produced in our laboratory. Anti-human HIF-2α antibodies were from ABcam (Cambridge, UK); anti-human tubulin antibodies were from Zymed (San Fransisco, CA). The GATA-2 expression plasmid was kindly supplied by Dr. S. Chrétien (Institut Cochin, Paris, France) and Ets-1 expression plasmid was provided by Dr. G. Pages of our laboratory.
Cells
Bovine aortic endothelial (BAE) cells were kindly supplied by Dr. H. Drexler (Max Planck Institute, Bad Nauheim, Germany), Human Micro Vascular Endothelial Cell line (HMEC-1) by Dr. FJ Candal (Centers for Disease Control and Prevention, Atlanta, GA) and the Human Micro Vascular Endothelial Cell line (HMVEC) by Dr. Xing Guo (Duke University Medical Center, Durham, USA).
Cell culture
BAE cells were cultured in DMEM 1,000 mg/L glucose (Gibco, Invitrogen, San Diego, CA) and 7.5% FBS from passage 12–16. HeLa and NIH3T3 cells were cultured in DMEM 4,500 mg/L (Gibco) and 5% FBS for HeLa and 10% BS for NIH3T3. Human Micro Vascular Endothelial Cell (HMVEC and HMEC) were cultured in MCDB131 (Gibco) supplemented with 12% Hyclone FCS (Perbio Science, Hyclone, Cramlington, UK), 10 mM glutamine (Invitrogen), 100 mg/ml heparin, 10 ng/ml FGF2, 10 ng EGF/ml (Sigma, St. Louis, MO), 1 µg/ml hydroxycortisone (Sigma), 50 U/ml penicillin and 50 mg/ml streptomycin.
Hypoxic conditions
Cells were incubated in normoxia (21% O2) or hypoxia (1% O2) during 24 h. Hypoxic treatment was performed in an anaerobic workstation (Ruskinn Technology, Biotrace International Plc, Jouan).
Cloning of the angiopoietin-2 promoter region and DNA constructs
The human Ang-2 promoter region was cloned by PCR using human genomic DNA from HUVEC as template. Oligonucleotide primers (Table 1) were designed based on the human Ang-2 gene sequence published in the human genome mapping database. Using primers scattered from −1,486 to +304 upstream of the Ang-2 coding region (Table 1) we amplified and cloned a series of cDNA, of various length, into the pRL-null luciferase (renilla) reporter plasmid. DNA sequence analysis was determined with an A310 sequencer using the ABI PRISM dye terminators (Applied Biosystems, Foster City, CA).
| Name | Sequence |
|---|---|
| Primers used for RT-PCR analysis | |
| 36B4 fd | GGCGACCTGGAAGTCCAACT |
| 36B4 rev | CCATCAGCACCACAGCCTTC |
| Beta actin fd | GCCAACCGCGAGAAGATGACCCAG |
| Beta actin rev | CTCGAAGTCCAGGGCGACGTAGC |
| bAng-2 Afd | CTGACTCTGATGTCAACATC |
| bAng-2 Drev | CTTAGGTGTATTTTCAGCACG |
| hAng-2 fd | CAGTTCTTCAAAAGCAGCAACATG |
| hAng-2 rev | TGGGATGTTTAGAAATCTGCT |
| GATA-2 fd | GACTATGGCAGCAGTCTCTTCC |
| GATA-2 rev | GGTGGTTGTCGTCTGACAATT |
| Ets-1 fd | GATGTCCCACTATTAACTCCAAGCAGC |
| Ets-1 rev | CGTCTGATAGGACTCTGTGATGAAGC |
| Intron 1 | |
| INS1 fd | AGACCGTGACAACCATGCTGAT |
| INS1 rev | AACTGTCAGACAGACCGTCCTGT |
| Primers used for ChIP experiments | |
| 1282 S2 fd TAAGGGCTTGCCGTCTGTAA | |
| 1282 AS rev | AGCTCCCATGTTTACATGTG |
| 1282 N fd | CTCTTGGTAAACGAGAGGAA |
| 1282 N rev | TCTAGGAAACAGTGGGATCA |
| 9351 fd | TCACCTGAGGATACAGAGAC |
| 9351 rev | AGCGACAGGCAAATCTATCCA |
| PHD3 fd | TTCTCTGGTGACTGGGGTAGAGAT |
| PHD3 rev | GAGCCCATGCAATTAGGCACAGTA |
| Primers used for promoter cloning | |
| PROAECO fd | CGGAATTCTCATTGATAAGGGAAAGGCTGT |
| PROBECO fd | CGGAATTCAGAGTGAATATCCAAGTGGAGT |
| PROEFCO fd | CGGAATTCTTCTTTCTTCAGTAATAAACCA |
| BTERECO rev | CGGAATTCGGCAGGCATTCTGCTCTGATTT |
| DTERECO rev | CGGAATTCGAAAGGAAAGTGATTGATTCGG |
| Primers used for RNAi experiments | |
| HIF1bis fd | CUGAUGACCAGCAACUUGATT |
| HIF1bis rev | UCAAGUUGCUGGUCAUCAGTT |
| HIF2bis fd | CAGCAUCUUUGAUAGCAGUTT |
| HIF2bis rev | ACUGCUAUCAAAGAUGCUGTT |
Cells transfection and reporter assays
Depending of the cell type, cells were transfected at about 80% confluence using either the calcium phosphate method or lipofectin reagent.
For the Ets-1 and GATA-2 overexpression assay, HeLa cells were grown in 6-well plates and transfected for 6 h at 37°C using the calcium phosphate method with about 5 µg of Ets-1 and/or GATA-2 expression plasmid. A GFP expression plasmid was used as a transfection control. Ang-2, Ets-1, and GATA-2 mRNA levels were then determined by RT-PCR analysis with corresponding primers (Table 1).
Reporter assays were performed with cells seeded in 12-well plates. Cells were transfected for 6 h at 37°C using the calcium phosphate method with 1–2 µg of the appropriate reporter construct and 0.1–0.2 µg of pGL3-TKluc (Promega) as a normalization control. HMVEC were transfected using the lipofectin reagent for 1 h. BAE, HMVEC, HeLa, and NIH3T3 cells were transiently co-transfected with appropriate pRL constructs (expressing renilla luciferase) and pGL3-TKluc plasmid (expressing firefly luciferase) as a normalization control. The pRL-TKluc plasmid was used as a positive control of transfection. The luciferase activity was determined with the dual luciferase assay system from Promega. After normalization, the luciferase activity obtained with the different constructs was expressed as fold induction compared to the promoter less plasmid (pRL-null) expression. Each condition was measured in triplicate. The effect of progressive 5′ deletion on the Ang-2 promoter activity was tested in various cell types. To investigate the role of potential HRE the same constructs were tested under hypoxic versus normoxic conditions.
siRNA assay
HIF-1α, SIMA (also known as drosophila HIF-α) and HIF-2α mRNA silencing using siRNA was performed as previously described (Sowter et al., 2003; Bilton et al., 2005). HIF-1α, HIF-2α, and SIMA siRNA were synthesized by Eurogentec. HMVEC were transfected with siRNA at 50–60% confluence using the calcium phosphate method. After 4 h of transfection, cells were washed and the day after exposed to either hypoxia (1% oxygen) or normoxia (21% oxygen) during 20 h.
RT-PCR assay
Total RNA was prepared using “RNeasy plus” kit from Qiagen (Courtaboeuf, France). Semi-quantitative RT-PCR analysis was performed with indicated primers (Table 1) using the “one step RT-PCR” kit supplied by Qiagen. RNA levels were quantified with gene tools software and signals were normalized using the 36B4 RNA level.
Chromatin immunoprecipitation (ChIP)
ChIP experiments were performed using a ChIP assay kit from Upstate (Lake Placid, NY). Briefly, HMEVC cells were grown on 10-cm plates until they reached about 70% confluence and exposed to hypoxia or left in normoxia for about 20 h (5–10 × 106 cells were used per condition). Cells were then fixed with 1% (v/v) formaldehyde (final concentration) for 10 min at 37°C and the action of the formaldehyde then stopped by the addition of 125 mM glycine (final concentration). Next, cells were washed in cold PBS containing a protease inhibitor cocktail (Roche, Basel, Switzerland), scrapped into the same buffer and centrifuged. The pellets were re-suspended in lysis buffer, incubated on ice for 10 min, and sonicated to shear the DNA into fragments of between 200 and 1,000 bp. Insoluble material was removed by centrifugation and the supernatant was diluted 10-fold by addition of ChIP dilution buffer and pre-cleared by addition of salmon sperm DNA/protein A agarose 50% slurry during 1 h at 4°C. About 5% of the diluted samples was stored and constituted the input material. Immunoprecipitation was then performed by addition of anti-HIF-1α, anti-HIF-2α or anti-tubulin as IgG control antibodies for 24 h at 4°C. Immunocomplexes were recovered by adding a 50% slurry of salmon sperm DNA/protein A agarose and washed sequentially with low salt buffer, high salt buffer, LiCl buffer and TE. DNA complexes were extracted in elution buffer, and cross-linking was reversed by incubating overnight at 65°C in the presence of 200 mM NaCl (final concentration). Proteins were removed by incubating for 2 h at 42°C with proteinase K and the DNA was extracted with phenol/chloroform and precipitated with ethanol. Immuno-precipitated DNA was amplified by PCR with the primers indicated in Table 1.
Results
Cloning and characterization of the human Ang-2 promoter sequences
We first determined the transcriptional start site by 5′ RACE PCR analysis (data not shown). We confirmed the previously published location of the transcription start site (TS) in the human genome mapping site (ref NT_023736 Homo sapiens chromosome 8 gi: 37552484) and identified a new one 35 bp upstream (1′) (Fig. 1A). The two sites display good homology to the initiator (Inr) consensus sequence (Javahery et al., 1994).
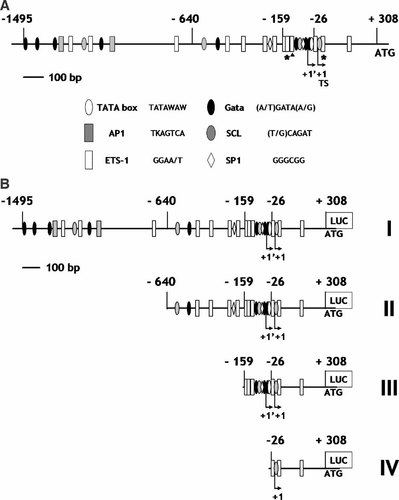
A: Schematic of the Ang-2 promoter structure showing potential regulatory elements. The two asterisks and the triangle indicate previously described Ets binding sites (Hasegawa et al., 2004; Hegen et al., 2004). B: Truncated Ang-2 promoter cDNA constructs. 5′ deletion fragments were amplified and cloned into the pRL-null luciferase (renilla) reporter plasmid. The dark line represents 100 bp. +1 indicates the transcriptional start site (TS) published in the NCBI human genome database. +1′ indicates a newly identified transcriptional start site. All locations are numbered taking the TS (+1) as a reference.
We then isolated an 8,443 bp genomic DNA fragment, 8,135 bp upstream of the (TS) and 308 bp downstream up to the ATG. Sequence analysis using Genomatix software, identified the presence of a consensus TATA box sequence located 34 bp upstream of the MTS, and several putative binding sites for transcription factors such as GATA, Ets, SCL/Tal, AP1 and SP1, scattered along a 1.8 kb zone upstream of the ATG (Fig. 1A).
The human Ang-2 promoter is active in endothelial cells
The 8,443 bp fragment (−8,135 to +308) and two smaller fragments, a 3,433 bp fragment (−3,125 to +308) and a 1,803 bp fragment (−1,495 to +308) (Fig. 1A,B) were fused to the GFP or luciferase gene and tested for promoter activity in transient transfection assays in endothelial (BAE and HMVEC) and non-endothelial cells (HeLa and NIH3T3). Neither the 8,433 bp fragment, nor the 3,433 bp fragment were capable of driving GFP expression in any of the cell lines used (data not shown). These results suggest that these sequences contained silencing elements and confirm results published by Hegen et al. (2004). However, the smaller 1,803 bp fragment (−1,495 to +308) was able to drive luciferase expression in endothelial cells (depending on the experiments, luciferase activity was increased four- to sevenfold compared to the promoter less vector) but not in non-endothelial HeLa cells (Fig. 2, construct I). These data indicate that the (−1,495 to +308) sequence promotes transcription in an endothelial cell specific manner.
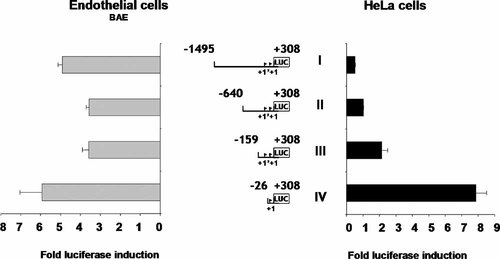
Comparative effect of 5′ deletions on Ang-2 promoter activity in endothelial and non-endothelial cells. Endothelial or HeLa cells were transiently transfected with each promoter construct and the luciferase activity was determined with a dual luciferase assay. After normalization, the luciferase activity obtained with the different constructs was expressed as fold induction compared to the promoter less plasmid (pRL-null) expression.
To identify endothelial specific sequences, 5′ deletion fragments were constructed from the 1,803 bp fragment (Fig. 1B) and tested in a luciferase transient transfection assay. Fourteen 5′ deletion fragments were constructed and tested. The four most informative were selected for further analysis (Fig. 1B).
Luciferase activity analysis showed that the minimal fragment (−26 to +308) induces basal promoter activity (six- to sevenfold compared to the promoter less vector) in both endothelial and non-endothelial cells (Fig. 2, construct IV) and is sufficient to promote non-cell type specific transcription. The addition of the −159 to −26 sequences dramatically decreases the promoter activity in non-endothelial cells (Fig. 2, construct III) and the addition of (−640 to −159) or (−1,495 to −159) sequences practically abolishes activity (Fig. 2, constructs I and II). In contrast, in endothelial cells the addition of the −1,495 to −26 sequences had no or little effect on basal Ang-2 promoter expression. This strongly suggests that the fragment (−1,495 to −26) and mainly the (−640 to −26) fragment contain elements which confer endothelial expression specificity and are able to negatively regulate the Ang-2 promoter activity in non-endothelial cells. Indeed, structural analysis of the (−1,495 to −26) fragment revealed, in addition to AP1 and SP1 sites, the presence of several binding sites for specific endothelial regulatory factors such as GATA and Ets.
GATA-2 and Ets-1 activate the Ang-2 promoter
Sequence analysis identified 13 putative Ets-binding sites (EBS) and 8 GATA-binding sites within the −1,495 to +308 Ang-2 promoter fragment (Fig. 1A).
GATA-2 and Ets-1 transcription factors are predominantly expressed in endothelial cells or in their precursors (Sato, 2001), and are involved in the transcriptional regulation of several endothelium-specific genes such as Tie1, Tie2, VEGFR1 and R2 and the human EPCR (Kappel et al., 2000; Han et al., 2003). To study Ets-1 or GATA-2 involvement in Ang-2 promoter activity, we co-transfected the four Ang-2 promoter constructs (I, II, III, and IV) with either Ets-1 or GATA-2 expression plasmids into endothelial (BAE) and non-endothelial (HeLa) cells. The results (Fig. 3A) show that Ets-1 overexpression has no significant action on the basal promoter activity of Ang-2 constructs I, II, III, and IV in endothelial cells. In contrast, in HeLa cells, forced expression of Ets-1 was able to increase respectively; fivefold the basal promoter activity of construct I, sixfold the activity of construct II, threefold the activity of construct III and about twofold the activity of construct IV.
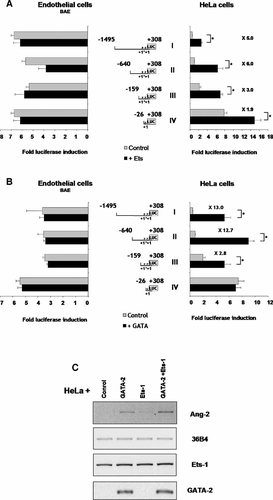
A: Ets-1 activates the Ang-2 promoter. Endothelial or HeLa cells were transiently co-transfected with each promoter construct with Ets-1 expression vector (black bars) or without (gray bars). P < 0.05 versus control values. B: GATA-2 activates the Ang-2 promoter. Cells were transiently co-transfected with each promoter construct with GATA-2 expressing vector (black bars) or without (gray bars). P < 0.05 versus control values. C: Ang-2 mRNA de novo expression in Ets-1 and GATA-2 transfected HeLa cells. Ets-1 and GATA-2 expressing plasmids alone or both were transiently transfected into HeLa cells and Ang-2 expression was analyzed by RT-PCR (the 36B4 signal shows equal reverse transcription).
In the same way, GATA-2 overexpression had no effect on the Ang-2 basal promoter activity in BAE cells but strongly increased this activity in HeLa cells when the GATA-2 expression plasmid was co-transfected with the Ang-2 promoter construct I (13.0-fold), construct II (12.7-fold), and construct III (2.8-fold) (Fig. 3B). GATA-2 expression has no effect on the minimal construct IV activity in HeLa cells. These results show that GATA-2 and Ets-1 overexpression has no or little effect on the Ang-2 basal promoter activity in endothelial cells but that these two factors are able to re-initiate Ang-2 promoter activity when they are expressed in non-endothelial HeLa cells.
We then studied the effect of GATA-2 and Ets-1 overexpression on endogenous Ang-2 expression. Using RT-PCR analysis we show that the GATA-2 factor and to a lesser extent the Ets-1 factor are able to re-activate Ang-2 mRNA expression when they are transiently expressed in Ang-2 non-expressing HeLa cells (Fig. 3C). Moreover, we observed that the Ang-2 mRNA level detected when GATA-2 and Ets-1 are both overexpressed was slightly higher than the level obtained with GATA-2 or Ets-1 alone, indicating that the two factors could cooperate in Ang-2 regulation. However, it should be noted that while untransfected HeLa cells do not express any GATA-2 mRNA, these cells express already significant level of endogenous Ets-1 mRNA without any Ang-2 mRNA detection (Fig. 3C, control). This could indicate that the level of endogenous Ets-1 mRNA detected in HeLa cells does not allow production of enough functional Ets-1 protein, either because Ets-1 mRNA translation is blocked or because Ets-1 proteins are inactivated. So, it could be hypothesized that in transfected HeLa cells, exogenous Ets-1 mRNA overexpression could restore functional Ets-1 proteins production.
Taken together, these data indicate that forced expression of GATA-2 and Ets-1 are able to re-induce Ang-2 transcriptional activity in non-endothelial cells and suggest that the GATA-2 and Ets-1 factors act as Ang-2 transcriptional activators in endothelial cells. In non-endothelial cells such as HeLa cells our data suggest that Ang-2 expression is negatively regulated by a complex mechanism including Ets-1 gene down-regulation and/or protein inactivation. Such a mechanism is yet to be determined. Future experiments using GATA-2 and Ets-1 siRNA assays should allow us to validate these hypotheses.
Finally, our results show that GATA-2 and Ets-1 target sequences are mainly located in the (−640 to −26) fragment and confirm that this fragment could contain sequences controlling Ang-2 endothelial specific expression.
Hypoxia induces Ang-2 gene transcription
Published data (Mandriota and Pepper, 1998; Mandriota et al., 2000; Pichiule et al., 2004) and our own data show that hypoxia up-regulates Ang-2 expression in endothelial cells. Indeed, using RT-PCR analysis (Fig. 4A), we show that hypoxic conditions (1% O2) increase significantly the Ang-2 mRNA level in BAE cells (bovine aortic endothelial), and in HMEC and HMVEC (Human Micro Vascular Endothelial Cells). Moreover, to examine the effect of hypoxia on Ang-2 transcription we performed an alternative to run-on assay as previously described by Gerald et al. (2004). Using amplification of an intronic sequence (intron 1, Table 1), we measured the level of Ang-2 primary transcripts (precursor RNA) in hypoxia and normoxia. The results (Fig. 4B) indicate that hypoxia induces both the Ang-2 mRNA level and the Ang-2 primary transcript level and show that hypoxic accumulation of Ang-2 mRNA could be proceeded by both transcriptional induction and post-transcriptional stabilization.
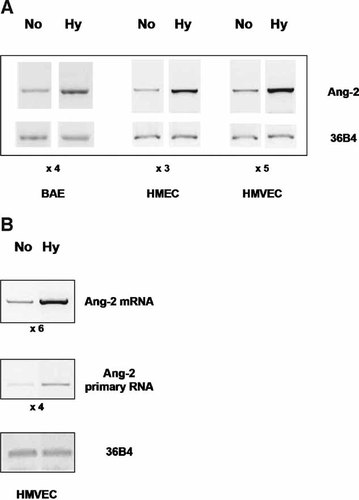
Cells were exposed to normoxia or hypoxia during 24 h. Total RNA was isolated for RT-PCR analysis. Ang-2 mRNAs levels were quantified and signals normalized using the 36B4 signal as a control. A: Hypoxia increases the Ang-2 mRNA level. Relative levels of Ang-2 mRNA in BAE, HMEC and HMVEC exposed to normoxia (No) or hypoxia (Hy). B: Hypoxia regulates Ang-2 expression at the transcriptional level. Relative levels of Ang-2 mRNA and its primary transcript in HMVEC exposed to normoxia (No) or hypoxia (Hy).
Hypoxic Ang-2 gene regulation is HIF-dependent
To assess the role of HIF in hypoxic Ang-2 transcriptional up-regulation, we ablated HIF-α subunit expression with siRNA (Fig. 5A). It should be noted that transfection conditions have an effect on their own on Ang-2 expression since the addition of transfection buffer alone (Buf) decreases Ang-2 hypoxic induction when compared to the untransfected cells (C). Calcium phosphate transfection buffer may have a general impact on cells viability and slightly reduce their capacity to answer to environmental changes such as hypoxic conditions. However, as compared to cells transfected with buffer alone or compared to the control SIMA siRNA, suppression of HIF-1α and HIF-2α in HMVEC lead to a significant shutdown of Ang-2 hypoxic induction. These data indicate that hypoxia up-regulates Ang-2 expression in a HIF-dependent manner.
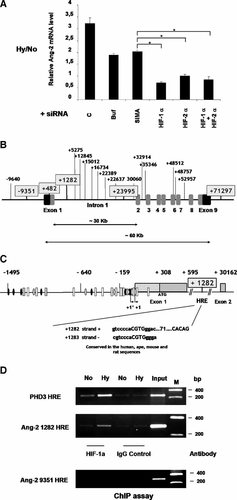
A: siRNA to HIF-α decrease Ang-2 mRNA hypoxic regulation. HMVEC were transiently transfected with nothing (C), transfection buffer alone (Buf) or with siRNA targeting respectively SIMA, HIF-1α, HIF-2α, and HIF-1α + HIF-2α. After transfection, cells were exposed to either normoxia or hypoxia during 20 h. Total RNA was isolated for RT-PCR analysis and signals were quantified and normalized using the 36B4 signal as a control. Results are expressed as a ratio of Ang-2 mRNAs level detected in Hypoxia (Hy) on Ang-2 mRNAs level detected in normoxia (No). P < 0.05 versus SIMA values. B: Analysis of potential Ang-2 HRE. Schematic representation of Ang-2 genomic structure. Exons are indicated by gray boxes, black boxes represent exons 1 and 9 un-translated sequences. Numbers indicate the position of potential HRE. Selected HRE are boxed. C: HRE 1,282 is localized in the first intron of the Ang-2 gene. Exons 1 and 2 are represented by dark and light gray boxes, respectively. The HRE 1,282 is boxed and its sequence is shown on the two DNA strands. D: HIF-1α binds to the Ang-2 gene. Chromatin Immunoprecipitation (ChIP) was performed on extracts from HMVEC cells exposed to either normoxia (No) or hypoxia (Hy). Immunoprecipitation was performed using a specific anti-HIF-1α antibody or anti-tubulin antibody as a control. Immuno-precipitated DNA was amplified by PCR using primers specific for the Ang-2 1,282 site, for the Ang-2 9,351 site as a negative control and for the PHD3 HRE as a positive control (Table 1). A control for PCR amplification was performed using samples of fragmented genomic DNA purified before immunoprecipitation (Input).
Identification of a putative HRE in the Ang-2 gene sequence
To identify HIF binding sites in the Ang-2 sequence, we performed comparative sequence analysis on a large genomic region of the human, ape, mouse and rat Ang-2, for the presence of HRE sites (Fig. 5B).
Eighteen putative HREs were identified on the Ang-2 gene sequence. No canonical HRE was identified in the −1,495 to +308 sequence. Among the 18 putative HRE sites, five were selected (framed, Fig. 5B) mainly on the basis of their sequence, and location in the vicinity of the HRE canonical sequence (G/T/C) ACGTG (C/G) (T/G/C) of an additional sequence CACACAG often associated with functional HRE sites (Tazuke et al., 1998). The most interesting HRE was located into the Ang-2 intron 1 at +1,282 bp from the TS. It displays a HRE canonical sequence in both plus and minus strands and is evolutionary conserved in human, ape, mouse, and rat sequences (Fig. 5C).
HIF-1α binds to the +1,282 HRE in cellulo
The binding of HIF-1 and HIF-2 factors to these five putative HRE was investigated by ChIP. ChIP experiments were performed on human endothelial cells, HMVEC, grown under normoxia (21% O2) or hypoxia (1% O2) (Fig. 5D). Chromatin was immuno-precipitated with anti-HIF-1α or anti-HIF-2α antibodies or an IgG control. Bound DNA sequences were identified by PCR using specific primers (Table 1) for the five selected sites or for the positive control PHD3 HRE. Our results show that in hypoxia, HIF-1α binds to the control PHD3 HRE as well as to the (+1,282) Ang-2 HRE. Interestingly no binding could be detected for the four other selected sites such as the (−9,351) site. Moreover, no binding could be detected for HIF-2α⋅ Normoxia strongly decreased the binding of HIF-1α both to the PHD3 HRE and to the 1,282 HRE site. Taken together these results strongly suggest that the (+1,282) Ang-2 site is a functional HRE.
Discussion
Ang-2 is considered to be a crucial regulator of angiogenesis. Ang-2 is expressed almost exclusively in endothelial cells and activated only at sites of neo-angiogenesis. Quiescent endothelial cells express a low, basal Ang-2 level that is up-regulated following endothelial activation. This regulation occurs at a transcriptional and post-transcriptional level. To better understand the mechanisms that sustain Ang-2 activation, we became interested in sequences that regulate Ang-2 transcription. Here, we report new data concerning cell specific Ang-2 expression and its regulation by hypoxia.
We first determined the transcriptional start site (TS) by 5′ RACE PCR analysis. Our findings are in agreement with recent data listed on the NCBI human genome site. Then, sequence analysis allowed us to identify a consensus TATA box located 34 bp upstream of the TS.
Our analysis showed that Ang-2 promoter sequences −1,495 to +308 are active only in endothelial cells and not in other cell types suggesting the presence of endothelial cell specific elements. Structural analysis revealed the presence of 13 putative Ets-binding sites (EBS) and 8 putative GATA-binding sites. Among them, three functional EBS (see Fig. 1A) were reported to be essential for Ang-2 promoter activation (Hasegawa et al., 2004; Hegen et al., 2004). Our experiments identified Ets-1 and particularly GATA-2 as critical regulators in Ang-2 transcriptional activation. Ets-1 and GATA-2 overexpression re-initiate Ang-2 promoter activity in non-endothelial HeLa cells. Moreover, GATA-2 and Ets-1 are able to re-activate endogenous Ang-2 mRNA expression when they are transfected into Ang-2 non-expressing HeLa cells. In addition, our results indicate that GATA-2 and Ets-1 target sequences are mainly located between −640 and −26 on the Ang-2 promoter and suggest that these sequences contain elements controlling Ang-2 endothelial specific expression.
So, we propose that in endothelial cells, the endogenous GATA-2 and Ets-1 factors constitutively bind to GATA and Ets motif clusters located in the Ang-2 promoter and interact to maintain chromatin in an open conformation. This conformation could allow basal Ang-2 transcription. In contrast, in non-endothelial cells, where endothelial factors such as Ets-1 or GATA-2 are absent or inactive, the Ang-2 promoter chromatin would remain in a folded and closed conformation avoiding transcription. In such a model GATA-2 and Ets-1 constitute essential elements in the cell specific regulation of Ang-2 expression. Several studies have underlined the combinatorial involvement of Ets and GATA factors in endothelial cell specific expression (Kappel et al., 2000; Gottgens et al., 2002; Mollica et al., 2006). A recent report (Mollica et al., 2006) identified in the 5′ of the hEPCR promoter a 130 bp enhancer sequence which drives endothelial cell specific expression of this gene. It was demonstrated that this specific enhancer activity is conferred by the interplay of transcription factors interacting with Ets, GATA, and SLC motifs. Future experiments including Ets and GATA binding site mutations and ChIP should allow us to validate the hypothesis that a GATA-2/Ets-1/chromatin complex is involved in Ang-2 cell specific regulation.
Published data suggest that Ang-2 could be an important regulator of hypoxia-induced angiogenesis but the mechanisms sustaining its expression are not well known. Several studies showed that Ang-2 mRNA and protein expression are up-regulated in endothelial cells in hypoxia (Mandriota and Pepper, 1998; Pichiule et al., 2004). Our study and another (Pichiule et al., 2004) indicate that hypoxia induces Ang-2 expression through both transcriptional and post-transcriptional regulation but the involvement of the HIF transcription factor in this regulation required clarification. HIFs are heterodimers of an oxygen-regulated HIF-α subunit and a constitutive HIF-β/ARNT subunit that bind to HRE located in target gene regulatory sequences. Until now, no HRE had been identified in the Ang-2 regulatory region. Two articles (Kelly et al., 2003; Yamakawa et al., 2003) reported that infection of primary endothelial cells with an adenovirus expressing a constitutively stable hybrid HIF-1α/V-16 protein, increases Ang-2 mRNA and protein levels and promotes invasion and tube formation in these cells. This strongly suggests that HIF-1 mediates the hypoxic regulation of Ang-2 during angiogenesis. However, another study showed that CoCl2 and DFO, which mimic hypoxia through the stabilization of HIF-1α, did not increase Ang-2 expression in human endothelial cells (Pichiule et al., 2004). This led them to conclude that hypoxic up-regulation of Ang-2 is HIF-1-independent.
To clarify these contradictory data we knocked-down HIF-α with siRNA and showed that both HIF-1α and HIF-2α down-regulation decreased significantly Ang-2 up-regulation in HMVEC in hypoxia. Moreover, we identified a well conserved HIF-1α binding site in the Ang-2 sequence by using exhaustive DNA sequence and ChIP analysis. The results strongly suggest that Ang-2 hypoxic transcriptional regulation is directly mediated by HIF-1. Although siRNA data suggest that HIF-2α is involved in hypoxic Ang-2 up-regulation, HIF-2α does not bind on Ang-2 sequence. Thus, we could hypothesize that HIF-2 acts in an indirect manner on Ang-2 hypoxic up-regulation. So, our results are in agreement with those published by Kelly et al. (2003) and Yamakawa et al. (2003) but in contradiction with the study presented by Pichiule et al. (2004). Their conclusion of the absence of HIF-1 regulation of Ang-2 transcription is in part based on results obtained with CoCl2 or DFO treatment. Although these agents increase HIF-1α protein levels and induce some HIF-target genes, we argue that this treatment does not fully reflect hypoxic conditions.
Two reports by Hasegawa et al. and Hegen et al. investigated the Ang-2 promoter sequences. They identified endothelial cell specific sequences on Ang-2 promoter (Hegen et al., 2004) and showed that Ets-1 acts as regulator of Ang-2 expression in endothelial cells (Hasegawa et al., 2004; Hegen et al., 2004). Our data are in agreement with these results and in addition we show that GATA-2 is a major actor of Ang-2 transcriptional regulation in endothelial cells. Moreover, exhaustive genomic sequence analysis allowed us to localize an evolutionary conserved HRE on Ang-2 gene. In conclusion, our study provides new and convincing elements in favor of HIF involvement, especially HIF-1α, in Ang-2 hypoxic regulation. Using siRNA down-regulation we show that hypoxic up-regulation of Ang-2 is HIF-dependent and identify for the first time, a HIF-1α binding HRE located in the first intron of the Ang-2 gene. In addition, our results identify Ets-1 and particularly GATA-2 as central factors in endothelial specific Ang-2 activation.
Some data have investigated the complementing roles of HIF-1α, HIF-2α and GATA or Ets factors in hypoxia specific gene regulation. Particularly, Elvert et al. (2003) have shown that HIF-2α co-operates with Ets-1 in activating transcription of the vascular endothelial growth factor receptor 2 (VEGFR-2) gene. Using RNAi-mediated inactivation of HIF-1α and HIF-2α and microarrays analysis, Aprelikova et al. (2006) showed that in MCF7 a small group of HIF-2α dependent genes had at least one Ets binding site in their promoter at the vicinity of a potential HRE site. In addition they showed that Ets-1 inactivation abolished or significantly reduced the hypoxic induction of the majority of these genes. Moreover, Yamashita et al. (2001) report that regulation of endothelin-1 gene promoter by hypoxia in endothelial cells required cooperation and interaction between HIF-1α, GATA-2, AP1, and p300/CPB. Finally, Makita et al. (2005) demonstrated that HIF-1α and GATA factors are required for high level of Epo gene expression in the early fetal liver. These data suggest that interaction between HIF and others transcriptional factors particularly with GATA and Ets factors in hypoxic gene regulation could be a general phenomenon. Nothing is known about a possible HIF co-operation with GATA-2 or Ets-1 in Ang-2 transcription regulation. Further studies should allow us to determine if such interactions play a role in Ang-2 hypoxic up-regulation in endothelial cells.
Acknowledgements
We thank Dr. MC Brahimi-Horn for revising the manuscript and for helpful discussion. The laboratory is funded by the Ligue Nationale Contre le Cancer (Equipe Labellisée), the Centre National de la Recherche Scientifique, the Ministère de l'Education, de la Recherche et de la Technologie, the National Cancer Institute, the Canceropôle PACA and the Centre A. Lacassagne.




