Parathyroid hormone-related protein (107–139) increases human osteoblastic cell survival by activation of vascular endothelial growth factor receptor-2†
Verónica Alonso and Arancha R. de Gortázar contributed equally to this work.
Abstract
Parathyroid hormone-related protein (PTHrP) (107–139), in contrast to the N-terminal fragment PTHrP (1–36), has been shown to interact with the vascular endothelial growth factor (VEGF) system to modulate human osteoblast differentiation. In this study, we evaluated whether this interaction might affect human osteoblastic cell survival. Pre-incubation with PTHrP (107–139) for 1–24 h dose-dependently (0.1–100 nM) inhibited dexamethasone- or etoposide-induced cell death in human osteoblastic MG-63 cells and human osteoblast-like cells from trabecular bone. This effect, but not that elicited by PTHrP (1–36), was abolished by the VEGF receptor (VEGFR)-2 inhibitors SU5614 and SU1498 or VEGFR-2 siRNA transfection in these cells. PTHrP (107–139), but not PTHrP (1–36), at 100 nM, rapidly (within 2 min) increased VEGFR-2 tyrosine-phosphorylation in MG-63 cells; an effect unaffected by several inhibitors of metalloproteinases, neutralizing VEGF165 or VEGFR-2 antibodies, or the VEGF binding inhibitor CBO-PP1. The latter two antagonists also failed to affect 125I-[Tyr116] PTHrP (107–115) binding to these cells. Consistent with its effect on VEGFR-2 activation, PTHrP (107–139) rapidly induced extracellular signal-regulated kinase (ERK) 1/2 and Akt activaton, and both ERK and phosphatidylinsositol-3 kinase (PI3K) inhibitors abolished its pro-survival effect in human osteoblastic cells. In addition, SU5614 and the latter two types of inhibitors abrogated Runx2 activation by this peptide in MG-63 cells. Transfection with a dominant-negative Runx2 construct abolished the pro-survival effect of PTHrP (107–139), associated with a decrease in Bcl-2/Bax protein ratio. Our findings demonstrate that PTHrP (107–139) interacts with VEGFR-2 to promote human osteoblastic cell survival by a mechanism involving Runx2 activation. J. Cell. Physiol. 217: 717–727, 2008. © 2008 Wiley-Liss, Inc.
Osteoporosis, a major problem in elderly populations, is characterized by bone mass loss and microarchitectural deterioration of skeletal structure leading to fragility fractures. The pathogenesis of osteoporosis involves a relative deficit of bone formation to compensate the excessive bone resorption associated with increased bone remodeling units (Raisz, 2005). An increase of osteoblast apoptosis has been shown to occur in osteoporosis associated with both estrogen deficiency and glucocorticoid administration (Tomkinson et al., 1997; Weinstein et al., 1998; Hock et al., 2001; Jilka et al., 2007). Apoptosis is an active form of cell death which can be susceptible to therapeutic manipulation. In this regard, intermittent administration of parathormone (PTH) (1–34)—which was recently approved as the first anabolic treatment of osteoporosis (Tashjian and Gagel, 2006)—has been shown to expand the osteoblast pool by increasing osteoblast life-span in normal and osteopenic mice (Jilka et al., 1999, 2007; Bellido et al., 2003).
PTH-related protein (PTHrP) is a widespread factor in mammalian tissues which can be considered as a string of cytokines rather than a single cytokine, due to the complex autocrine/paracrine and even intracrine actions displayed by its different domains. Alternative splicing of PTHrP gene transcripts and processing of their translation products generate various peptide fragments with different activities: two secretory forms, one containing the N-terminal 1–36 domain, and a C-terminal fragment which includes the highly conserved 107–111 sequence (called osteostatin); and a less defined mid-region fragment(s) containing a nuclear localization signal in the 87–106 domain, and thus can be either secreted or directly transported into the nucleus (Whitfield, 2007). Only the N-terminal fragment of PTHrP which exhibits structural homology to the N-terminal domain of PTH interacts with the PTH type 1 receptor (PTH1R) in target cells; while still uncharacterized receptors seem to mediate the biological effects associated with the mid- and C-terminal PTHrP domains (Whitfield, 2007).
PTHrP is now emerging as an important modulator of bone remodeling, and a stimulator of bone formation by promoting osteoblast differentiation and survival (Bisello et al., 2004; Miao et al., 2005a). Regarding the latter, the N-terminal fragment of PTHrP, through interaction with PTH1R, has been reported to inhibit apoptosis in various cell types including bone cell lines (Amling et al., 1997; Chen et al., 2002; Miyaji et al., 2003; Hastings et al., 2004; Ortega et al., 2006). In mouse osteoblastic cells, this effect seems to occur at the early stages of osteoblast differentiation (Chen et al., 2002). In addition, PTHrP domains other than its N-terminal domain might have important yet poorly defined roles in in vivo bone development and remodeling. Interestingly, a preliminary report has shown that post-natal mice with deletion of the mid- and C-terminal regions of PTHrP have a widespread increase of osteoblast apoptosis associated with decreased bone formation (Miao et al., 2005b). PTHrP (107–139)—or just its 107–111 epitope—can inhibit bone resorption by directly preventing osteoclast activation, and it also interacts with a PTH1R-unrelated receptor in osteoblastic cells (Cornish et al., 1997; Valín et al., 2001). More recently, we found that transient exposure of human osteoblastic cells to either PTHrP (107–139) or PTHrP (1–36) similarly induced cell differentiation (de Gortázar et al., 2006). It was previously reported that each of these PTHrP peptides rapidly induced the expression of VEGF (Esbrit et al., 2000a), an angiogenic factor which has an important role in the process of bone formation (Maes et al., 2002; Peng et al., 2005; Towler, 2007), in human osteoblastic cells. Interestingly, however, the differentiation-inducing effect of PTHrP (107–139) in these cells, in contrast to that of PTHrP (1–36), was abrogated by a VEGF receptor (VEGFR)-2 tyrosine kinase inhibitor (de Gortázar et al., 2006). These recent studies thus suggest that interaction of PTHrP (107–139) with VEGFR-2 might be important in mediating its effects on osteoblast differentiation.
VEGFR-2 signaling is known to promote survival in endothelial cells as well as in other nonbone cell types (Gerber et al., 1998; Santos and Dias, 2004; Villegas et al., 2005). We show here evidence that PTHrP (107–139) is also able to increase human osteoblastic cell viability, and that this effect was related to PTHrP (107–139)-induced activation of VEGFR-2 in these cells.
Materials and Methods
Reagents
Human PTHrP (1–36) was kindly supplied by A.F. Stewart, M.D. (Division of Endocrinology and Metabolism, University of Pittsburgh School of Medicine, Pittsburgh, PA), and human PTHrP (107–139) as well as [Tyr116] PTHrP (107–115) were provided by F. Roncal, Ph.D. (Proteomics Unit, Centro Nacional de Biotecnología, Madrid, Spain). Recombinant human VEGF165 was from R&D Systems (Minneapolis, MN). U0126 was from Alomone Labs (Jerusalem, Israel). LY294002, 2′-amino-3′-methoxyflavone (PD98059), SU5614, the VEGF inhibitor CBO-P11, (2R)-2-[(4-biphenylylsulfonyl)amino]-3-phenylpropionic acid [matrix metalloprotease (MMP)-2/MMP-9 inhibitor I], and the pan-specific MMPs inhibitor GM6001 were obtained from Calbiochem (San Diego, CA). Dexamethasone, etoposide, SU1498, and mouse monoclonal antibody to α-tubulin were from Sigma (St. Louis, MO). T4 polymerase was supplied by Promega (Madison, WI). [γ32P]ATP (3,000 Ci/mmol) and Na125I (2,130 Ci/mmol) were from Amersham (Buckinghamshire, UK) and MP Biomedicals (Solon, OH), respectively. The following polyclonal antibodies to VEGFR-2 (sc-19530, N-terminal, and sc-504, C-terminal), Bax (sc-493), and Bcl-2 (sc-492), as well as anti-phospho(p)-473Ser-Akt (c-7985-R) and anti-Akt (sc-8312) antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse monoclonal antibodies to either recombinant human VEGF165 or p-tyrosine (PY20) (sc-508) were from Sigma and Santa Cruz Biotechnology, respectively. Protein A/G PLUS-agarose (sc-2003) was from Santa Cruz Biotechnology. Rabbit polyclonal anti-p42/p44 extracellular signal-regulated kinases (ERK 1/2) and anti-p-ERK 1/2 (Thr202/Tyr204) antibodies were supplied by Cell Signaling Technology (Beverly, MA).
An expression vector encoding a dominant negative form of Runt homology domain protein Runx2 (dnRunx2) was kindly provided by Dr. Patricia Ducy (Baylor College of Medicine, Houston, TX) (Ducy et al., 1999). Silencer pre-designed siRNAs targeted to VEGFR-2 (ID #: 220, 221, and 222) were from Ambion (Austin, TX). Fugene was from Roche Molecular Biochemicals (Indianapolis, IN)
Cell cultures
MG-63 cells (ATCC CRL 1427), a useful model for their nontransformed counterparts responding to both N- and C-terminal PTHrP peptides (Esbrit et al., 2000a; Guillén et al., 2002; de Gortázar et al., 2006), were grown in RPMI 1640 with 10% fetal bovine serum (FBS), and antibiotics (100 IU/ml of penicillin and 100 µg/ml of streptomycin), in 5% CO2 at 37°C. This culture medium has been shown to increase these cells' sensitivity to pro-apoptotic challenges (Bu et al., 2003). Human osteoblast-like (hOB) cells were isolated from trabecular bone explants obtained from knee or hip samples discarded at the time of surgery on osteoarthritic subjects (aged 61–84 years), as previously reported (Esbrit et al., 2000a; Guillén et al., 2002; de Gortázar et al., 2006). These cells were cultured in Dulbecco's Modified Eagle's Medium with 15% FBS and antibiotics, and used between passages 1 and 4. For experiments, cells were seeded at 20,000 cells/cm2 in culture medium. After 24 h, cells were refed with fresh medium, and incubated with each PTHrP peptide, VEGF165 or vehicle for 1–72 h. Antibodies and inhibitors were added at least 1 h before each peptide (or vehicle). Then, cells were treated with either dexamethasone or etoposide (or the corresponding vehicle) for 18 h (MG-63) or 6 h (hOB) in FBS-depleted medium. To assess changes in VEGFR-2 phosphorylation, MG-63 cells were seeded as described above, and grown up to 80% confluence. They were subsequently incubated in medium with 0.5% FBS for 16 h, and serum-depleted for at least 2 h before being exposed to the agents tested for different time periods.
In some experiments, serum-depleted MG-63 cells were transiently transfected with a mixture of three siRNA duplexes against different target sequences in mouse VEGFR-2 (10 nM each) using fugene (3 µl) for 5 h at 37°C. Efficiency of VEGR-2 silencing was assessed by detecting VEGFR-2 protein by Western blot. Specificity was checked by transfecting MG-63 cells with a negative control siRNA which has no homology to any known human gene sequence (AM4607, Ambion). In other experiments, 2.5 µg of either dnRunx2 construct or empty plasmid (as control) were transfected with fugene (1.5 µl/µg plasmid) as previously mentioned. Transfection efficiency was assessed by RT-PCR amplification of osteocalcin gene containing Runx2 binding sites (Ducy et al., 1999). After transfection, the medium was replaced by fresh culture medium, and cells were treated with different agonists as described above.
Cell death evaluation
Trypan blue staining, a common method to estimate cell viability, was used (Chen et al., 2002; Bellido et al., 2003; Ortega et al., 2006). Following cell stimulation, nonadherent cells were collected and pooled with adherent cells (after gentle trypsinization), and then stained with Trypan blue. Cell suspension was mixed with 0.4% Trypan blue solution (1:2, v/v), and about 200 cells per culture well were examined under light microscopy. The number of total cells and that of those exhibiting intracellular staining (nonviable cells) were counted in a hemocytometer, and the percentage of cell viability was then determined. The number of nonviable cells × 100/total number of viable + nonviable cells in the presence of death inducing agent (1 µM dexamethasone or 50 µM etoposide) was calculated. After treatment with the latter, this represented 12% or 30% in MG-63 and hOBs cells, respectively, compared to 6% or 10% in untreated cells, respectively. To assess the effect of different tested agonists on cell viability, the % of dead cells calculated as above after treatment with either dexamethasone or etoposide only was normalized to 100%.
As a more specific method to assess apoptosis, propidium iodide staining (PI) was performed. Thus, cells grown on round cover slips were prestimulated or not with the tested peptides and then with etoposide for 18 h, and then fixed with Merckofix(R) (Merck, Darmstdt, Germany). Subsequently, cells were incubated in 0.01% Triton X-100 in phosphate-buffered saline (PBS) for 5 min at room temperature, and in 100 µg/ml RNAse A and 2 µg/ml propidium iodide in PBS in the dark for 10 min at room temperature. The number of apoptotic cells—identified by the presence of nuclei with fragmented or condensed DNA—was counted and expressed as percentage of total cells (approx. 350 cells counted) in high power fields.
Caspase-3 activity was analyzed in cell extracts using the CaspACETM Assay System (Promega), following the manufacturer's instructions. Cells were harvested as described above, washed with PBS, and finally resuspended in the lysis buffer provided. Cell extracts were subsequently submitted to three freeze-and-thaw cycles, and then centrifuged at 15,000g at 4°C. Supernatants were then incubated with the colorimetric substrate Ac-DEVD-p-nitroaniline for 4 h at 37°C. Caspase-3 activity was determined by the difference between the absorbance at 405 nm produced in the absence and in the presence of the cell-permeable pan-caspase inhibitor Z-VAD-FMK.
Western blot analysis
Cell extracts were obtained with 50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 1% sodium deoxycholate, and 0.1% SDS (extraction buffer). To analyze ERK, Akt, and VEGFR-2 phosphorylation, a phosphatase-inhibitor cocktail (Set II, Calbiochem, San Diego, CA) was added to this extraction buffer. Protein content was determined by Bradford's method (Pierce, Rockford, IL), using bovine serum albumin (BSA) as standard.
Immunoprecipitation was performed to detect VEGFR-2 phosphorylation. Thus, protein extracts (250 µg) were incubated with 2 µg rabbit IgG for 1 h at 4°C with shaking. Then, 20 µl of protein A/G PLUS-agarose were added to cell extracts, and incubated for 2 h at 4°C with shaking. After centrifugation for 2 min at 500g, pellets were resuspended in 50 µl of 100 mM Tris, 150 mM NaCl, pH 7.5 (TBS buffer), and incubated overnight with two polyclonal anti-VEGFR-2 antibodies, N- and C-terminal specific, respectively (each at 1:200 dilution), at 4°C with shaking. Then, protein A/G PLUS-agarose was added and incubated again, as described. After centrifugation at 500g for 2 min, the resulting pellets were reconstituted in 40 µl of extraction buffer.
Cell protein extracts or the reconstituted immunoprecipitated pellets (for VEGFR-2 analysis) were transferred onto nitrocellulose membranes (Amersham), blocked with 5% defatted milk in TBS buffer, containing 0.05% Tween-20, and then incubated overnight at 4°C with the following antibodies (each at 1:1,000 dilution): Anti-p-473Ser-Akt or anti-Akt antibodies; p-ERK 1/2 or ERK 1/2 antibodies; anti-Bax; anti-Bcl-2; or PY20. As a loading control, an anti-α-tubulin antibody was used. The membranes were subsequently incubated with peroxidase-conjugated goat anti-rabbit or anti-mouse IgG, developed by ECL chemiluminiscence (Amersham), and fluorogram bands were quantified by densitometry.
Semiquantitative RT-PCR
Cell total RNA was isolated using guanidinium thiocyanate-phenol-chloroform extraction (Tri-Reagent©, Molecular Research Center, Cincinnati, OH), according to the manufacturer's instructions. Cell total RNA (10 ng) was reverse-transcribed and the resulting cDNA was amplified using a commercial kit (TitaniumTM One-step RT-PCR kit; Clontech, Palo Alto, CA) with the following specific primers: 5′-CATGAGAGCCCTCACACTCC-3′ (sense) and 5′-CAGCAGAGCGACACCCTAGACC-3′ (anti-sense), corresponding to nucleotides 18–37 and 315–336, respectively, in the human OC gene (Genebank accession number X51699), as previously reported (de Gortázar et al., 2006). Modified 18S primers (QuantumRNATM 18S Internal Standards; Ambion) were used for 18S co-amplification, as an internal control. PCR products were separated on 2% agarose gels, and bands were visualized by ethidium bromide staining.
Electrophoretic mobility shift assay (EMSA)
To determine Runx2 activity, MG-63 cell nuclear extracts were obtained following a commercially available procedure (NE-PER®, Pierce), as previously described (de Gortázar et al., 2006). The double-stranded oligonucleotide 5′-AGCTCCCAACCACATATCCT-3′, containing a consensus sequence specific for Runx2, was 5′-end-labeled with 10µCi [γ32P]ATP and T4 polymerase. Nuclear extracts (3 µg protein) were incubated with 200,000 dpm of 32P-labeled oligonucleotide probe in 20 µl of a reaction mixture containing 10 mM Tris–HCl, pH 7.9, 100 mM NaCl, 1 mM MgCl2, 0.5 mM EDTA, 4% glycerol, and 1 µg poly(dI-dC), for 20 min at 4°C. The binding specificity of the Runx2 oligonucleotide was analyzed by incubating the nuclear extracts with a 100-fold molar excess of unlabeled Runx2 oligonucleotide or NF-kB oligonucleotide for 15 min at 4°C, before the addition of the 32P-labeled probe. Protein-DNA complexes were resolved on native 5% polyacrilamide/0.25 × TBE gels. Gels were then dried and exposed to radiosensitive film.
Radioreceptor assay
[Tyr116]PTHrP (107–115) was radioiodinated with Na125I (2,130 Ci/mmol) by chloramine-T using a standard procedure (Esbrit et al., 2000b). In brief, 5 µg of this peptide was incubated with 1 mCi Na125I and 0.3 mM chloramine T in 50 µl of 0.3 M sodium phosphate buffer, pH 7.4, for 30 s. The radiolabeled peptide was purified by HPLC in a µBondapak C18 column using a linear gradient of 35–45% acetonitrile with 0.1% trifluoroacetic acid, at a flow rate of 1 ml/min. Fractions containing the peak radioactivity were pooled and stored at −20°C.
MG-63 cells were pre-incubated with RPMI medium containing 0.1% BSA for 30 min at 37°C and then incubated with 50,000–70,000 cpm 125I-[Tyr116]PTHrP (107–115), with or without different unlabeled peptides or antagonists, in a total volume of 100 µl of the same medium containing a protease inhibitor cocktail (cat. no. P8340, Sigma) and MMPs inhibitor GM6001 (1 µM) (binding buffer) for 30 min at 22°C with gentle agitation. Preliminary experiments showed that binding was maximal using these conditions. Unbound radioligand was then removed, and cell monolayers were rinsed twice with 600 µl binding buffer. Cell-bound radioactivity was recovered with 1 N NaOH, and counted in a γ-spectrometer.
Statistical analysis
The data throughout the text are mean ± SEM. Statistical analysis was performed by Kruskal–Wallis nonparametric ANOVA followed by Dunn's post hoc test or Mann–Whitney test, as appropriate. P < 0.05 was considered significant.
Results
PTHrP (107–139) promotes human osteoblastic cell survival
To assess the pro-survival effect of PTHrP (107–139) in human osteoblastic cells, we used two well known pro-apoptotic agents, dexamethasone and the chemotherapeutic drug etoposide (Weinstein et al., 1998; Jilka et al., 1999; Bellido et al., 2003). It has been previously reported that PTH (1–34) or PTHrP (1–34)—interacting with the common PTH1R in osteoblasts—blocked the decrease in cell viability triggered by the aforementioned pro-apoptotic agents in mouse osteoblastic and MG-63 cells (Jilka et al., 1999; Chen et al., 2002; Bellido et al., 2003). Thus, we initially tested whether pre-treatment with the native N-terminal fragment PTHrP (1–36) reproduced this protective effect of the aforementioned related peptides in human osteoblastic cells. We found that exposure to PTHrP (1–36), at 100 nM, for 1–24 h prior to either etoposide or dexamethasone increased viability in both MG-63 and hOB cells (Figs. 1A,B and 2A). The same exposure regimen to PTHrP (107–139), at 100 nM, was also found to protect against death induced by these pro-apoptotic stimuli in these cells (Figs. 1A,B and 2A). This pro-survival effect of both PTHrP peptides was similarly dose-dependent, within a concentration range of 0.1–100 nM in MG-63 cells (Fig. 1C). Moreover, the increase in the number of these cells exhibiting apoptotic morphologic appearance—revealed by PI staining—in the presence of etoposide was similarly inhibited by pre-incubation with each PTHrP peptide for the same time period (Fig. 1D). This anti-apoptotic effect was confirmed by measuring caspase-3 activity in MG-63 cells (Fig. 3A).
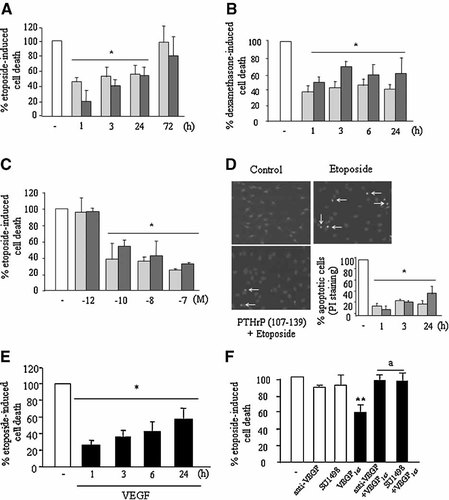
Both PTHrP (107-139) and PTHrP (1–36) and VEGF decreased cell death induced by dexamethasone or etoposide in MG-63 cells. MG-63 cells were pre-treated with either PTHrP (107–139) (light bars) or PTHrP (1–36) (dark bars) (each at 100 nM) for different time periods (A,B,D), with different doses of these peptides for 3 h (C), or VEGF165 (0.5 nM) (black bars) for different times (E). A neutralizing VEGF165 antibody (1 µg/ml) or the VEGFR-2 tyrosine kinase inhibitor SU1498 (1 µM) were added 1 h before treatment with VEGF165 for 3 h (F). Etoposide (50 µM) or dexamethasone (1 µM) (B) was then added, and cells incubated for 18 h in the absence of FBS. Cell death was assessed by either Trypan blue or Propidium iodide (PI) staining (D) Representative images of PI staining in MG-63 cells treated or untreated (control) with etoposide, with or without addition of PTHrP (107–139) for 3 h, are shown. Arrows indicate apoptotic nuclear morphology (D). Values are mean ± SEM from at least three independent experiments in triplicate. *P < 0.05; **0.01 versus corresponding etoposide- or dexamethasone-only value (100%); aP < 0.01 versus corresponding value without the inhibitors.
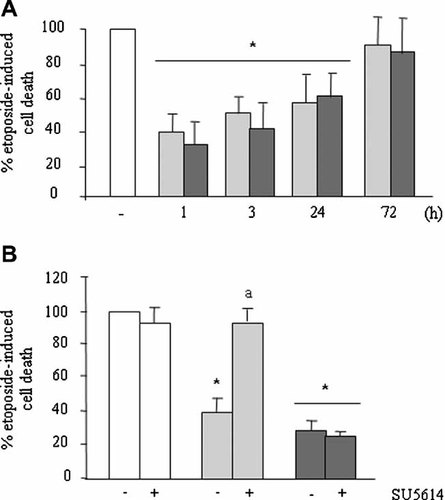
PTHrP (107–139) and PTHrP (1–36) increased cell viability in hOB cells. Cells were pre-treated with either PTHrP (107–139) (light bars) or PTHrP (1–36) (dark bars) (each at 100 nM) for various time periods (A) or 3 h (B), and then etoposide (50 µM) was added, and cells incubated for 6 h in the absence of FBS. The VEGFR-2 tyrosine kinase inhibitor SU5614 (1µM) was added 1 h before the tested peptides (B). Cell viability was assessed by Trypan blue staining as described in the text. Values are mean ± SEM from at least three independent experiments in triplicate. *P < 0.05 versus corresponding etoposide-only value (100%). aP < 0.01 versus corresponding value without the inhibitor.
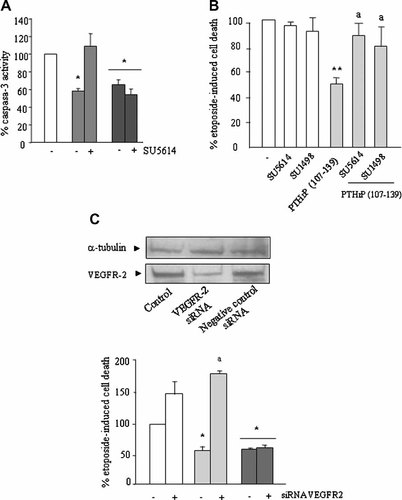
PTHrP (107–139) interacts with VEGFR-2 to decrease human osteoblastic cell death. Serum-depleted MG-63 cells were stimulated or not with either PTHrP (107–139) (light bars) or PTHrP (1–36) (dark bars) (both at 100 nM) for 3 h, in the presence or absence of the VEGFR-2 tyrosine kinase inhibitors SU5614 or SU1498 (each at 1 µM) (A,B). These inhibitors were added 1 h before the tested peptides. VEGFR-2 siRNA-transfected MG-63 cells were also pre-treated with the PTHrP peptides as described in the text (C). Etoposide (50 µM) was then added, cells incubated for 18 h without FBS, and cell death assessed by either analyzing caspase-3 activity (A) or Trypan blue staining (B,C). By Western analysis, using an anti-VEGFR-2 antibody, a single band corresponding to an apparent molecular weight of 160 kDa was detected in these cells' protein extracts; this band was dramatically reduced in VEGFR-2 siRNA-transfected cells, compared to that in untransfected (control) cells and in those transfected with a negative control siRNA (C, top). Values are mean ± SEM from at least three independent experiments in triplicate. *P < 0.05; **0.01 versus corresponding etoposide-only value in control cells (100%). aP < 0.01 versus corresponding value without the inhibitor.
PTHrP (107–139)-increased survival of human osteoblastic cells depends on VEGFR-2 activation
We recently reported that transient exposure to PTHrP (107–139) induced osteoblastic differentiation apparently related to VEGFR-2 activation in both MG-63 and hOB cells (de Gortázar et al., 2006). Thus, we examined whether this activation might mediate the present effect of PTHrP (107–139) on these cells' viability. VEGF165, at 0.5 nM—a dose which increases endothelial viability—was found to elicit a pattern of pro-survival response similar to that induced by 100 nM PTHrP (107–139) in MG-63 cells (Fig. 1E). This effect of VEGF was inhibited by SU1498, at 1 µM, a highly selective VEGFR-2 tyrosine kinase inhibitor which has previously been used to inhibit several VEGF activities in endothelial cells (Tanimoto et al., 2002; Zhang et al., 2003); consistent with the notion that the effects of VEGF on cell survival are mediated by the latter receptor (Gerber et al., 1998) (Fig. 1F). We also found here that either SU1498 or SU5614—another VEGFR-2 tyrosine kinase inhibitor used in our previous study in human osteoblastic cells (de Gortázar et al., 2006), which also shows weak inhibitory activity for platelet-derived growth factor receptor tyrosine kinase—, each at 1 µM, abolished the pro-survival effect of PTHrP (107–139), but not that of PTHrP (1–36), each at 100 nM, in both human osteoblastic cell types studied (Figs. 2B and 3A,B).
To further assess the putative role of VEGFR-2 on this effect of PTHrP (107–139), we used a siRNA approach to knock down VEGFR-2 in MG-63 cells. Under these conditions, VEGFR-2 protein expression was significantly suppressed; whereas MG-63 cell transfection with a negative control siRNA was inefficient in this regard (Fig. 3C, top). We found that VEGFR-2 siRNA-transfected MG-63 cells were more sensitive to etoposide-induced cell death, and this was prevented by PTHrP (1-36) pre-incubation. On the other hand, the protective effect of PTHrP (107–139) on these cells' survival was abrogated in these cells (Fig. 3C, bottom). Taken together, these results strongly suggest that interaction with VEGFR-2 represents a necessary point for the pro-survival action of PTHrP (107–139) in human osteoblastic cells.
ERK and PI3K signal transduction pathways are crucial mechanisms to modulate cell survival through VEGFR-2 activation in several cell types (Gerber et al., 1998; Santos and Dias, 2004). We found here that U0126, at 20 µM, and PD98059, at 10 µM, as well as LY 294002, at 10 µM, abolished the pro-survival effect of PTHrP (107–139) in MG-63 cells, and also in hOB cells (Fig. 4A,B). These data further support the hypothesis that the pro-survival effect of PTHrP (107–139) occurs through VEGFR-2 activation in human osteoblastic cells.
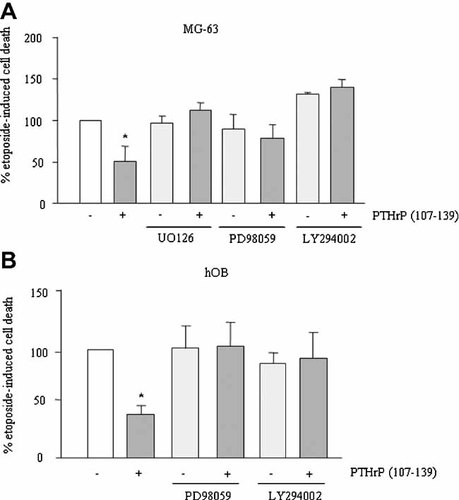
Effect of different inhibitors on the pro-survival effect of PTHrP (107–139) in MG-63 (A) and hOB (B) cells. Serum-depleted cells were stimulated or not with PTHrP (107–139) (100 nM) for 3 h, in the presence or absence of different inhibitors as indicated in the text. Subsequently, etoposide was added, and cell death assessed by Trypan blue staining, as described in the text. Values are mean ± SEM from at least three independent experiments in triplicate. *P < 0.05 versus etoposide-only value (100%).
Since the effects of PTHrP (107–139) on cell survival, as observed herein, and on gene expression, as found elsewhere (Esbrit et al., 2000a; Guillén et al., 2002; de Gortázar et al., 2006), were very similar in both MG-63 and hOB cells, all additional experiments were performed using the former cells.
PTHrP (107–139) increases VEGFR-2 activation in a VEGF-independent fashion in human osteoblastic cells
We next aimed to confirm that PTHrP (107–139) may induce VEGFR-2 activation in MG-63 cells. By Immunoprecipitation of protein cell extracts with two anti-VEGFR-2 antibodies, followed by western analysis with PY20 antibody, we found that PTHrP (107–139), in contrast to PTHrP (1–36), at 100 nM, activates (by tyrosine phosphorylation) VEGFR-2 within 2 min, being maximal at 20 min and decreasing at 60 min (Fig. 5A,C). Similar results were obtained by Immunoprecipitation with PY20 antibody, and subsequent detection of VEGFR-2 by Western blot (not shown). VEGF165 (a positive control), at 0.5 nM, also phosphorylated VEGFR-2, but this phosphorylation showed a greater intensity and more rapid kinetics than those observed with PTHrP (107–139) in these cells (Fig. 5B). In addition, we found that both ERK and Akt phosphorylation were increased by PTHrP (107–139), at 100 nM, with a similar time course to that observed for VEGFR-2 activation by this peptide in MG-63 cells (Fig. 6A,B).
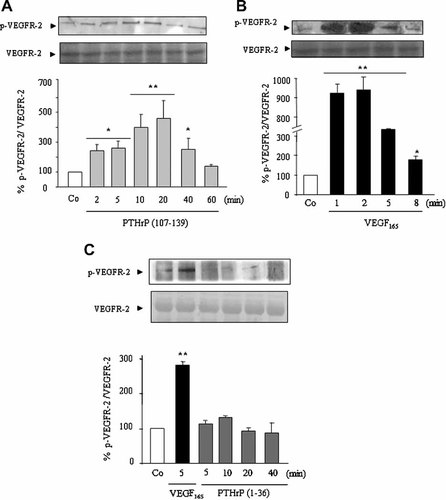
PTHrP (107–139) induces VEGFR-2 tyrosine phosphorylation in MG-63 cells. Serum-depleted MG-63 cells were stimulated or not with either PTHrP (107–139) (A) or PTHrP (1–36) (C) (each at 100 nM), or VEGF165 (0.5 nM) (B,C) for different times. Cell protein extracts were immunoprecipitated with two different anti-VEGFR-2 antibodies, and phosphorylated (p)VEGFR-2 as well as total VEGFR-2 (as loading control, after pVEGFR-2 antibody stripping) was then detected in the reconstituted pellet by Western analysis using PY20 antibody. Values are mean ± SEM from at least three independent experiments in duplicate. *P < 0.05; **0.01 versus corresponding nonstimulated control (Co) value (100%).
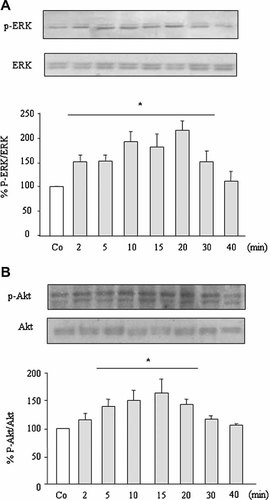
PTHrP (107–139) induces ERK and Akt phosphorylation in MG-63 cells. Serum-depleted cells were stimulated with or without PTHrP (107–139) (100 nM) for different times. Western blot analysis was performed in cell extracts using antibodies against pERK 1/2 and ERK 1/2 (A), or p-473Ser-Akt and total Akt (B). Relative densitometric values corresponding to pERK/ERK and pAkt/Akt changes are mean ± SEM from three independent measurements in duplicate. Co = nonstimulated control (at 15 min). *P < 0.05 versus corresponding Co value (100%).
Some tyrosine kinase receptors can be transactivated through activation of MMPs and subsequent release of their soluble ligands (Ahmed et al., 2003). Our results, however, do not support that such a mechanism would explain the PTHrP (107–139)-induced phosphorylation of VEGFR-2 in MG-63 cells. Thus, an inhibitor of MMP-2 and -9, at 350 nM, and the pan-MMPs inhibitor GM 6001, at 1 µM, failed to affect VEGFR-2 activation by PTHrP (107–139) in MG-63 cells (Fig. 7A). Moreover, pre-incubation with an antibody against VEGF165—the major diffusible VEGF isoform in human osteoblastic cells (Esbrit et al., 2000a)—at a concentration (1 µg/ml) which abolished its pro-survival effect in MG-63 cells (Fig. 1F), did not change VEGFR-2 phosphorylation by PTHrP (107–139) in these cells (Fig. 7A). In addition, this phosphorylation was modified neither by a neutralizing anti-VEGFR-2 antibody (2 µg/ml) nor by the inhibitor CBO-P11 (20 µM), an agent which prevents VEGF binding to VEGFR-2 and its subsequent activation (Zilberberg et al., 2003) (Fig. 7B). To support further that PTHrP (107–139) activation of VEGFR-2 does not occur by binding directly to this receptor, a radioreceptor assay using 125I-[Tyr116] PTHrP (107–115)—containing the epitope responsible for PTHrP (107–139) binding and activity in osteoblastic cells (Cornish et al., 1997; Valín et al., 2001)—as ligand, was performed in MG-63 cells. As shown in Figure 7C, while a saturating dose of PTHrP (107–139) (10 µM) dramatically displaced the bound labeled ligand, either a high VEGF165 concentration (2.5 nM), a neutralizing anti-VEGFR-2 antibody or the inhibitor CBO-P11 were inefficient in this regard.
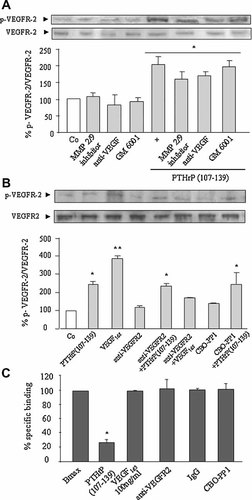
PTHrP (107–139) stimulates VEGFR-2 phosphorylation in a VEGF- independent manner in MG-63 cells. Serum-depleted MG-63 cells were stimulated with or without PTHrP (107–139) (100 nM) or VEGF165 (0.5 nM) for 20 min, in the presence or absence of different MMPs inhibitors and VEGF system antagonists (added 1 h before the tested peptides) as indicated in the text (A,B). 125I-[Tyr116] PTHrP (107–115) binding to MG-63 cells, with or without different unlabeled peptides or VEGF antagonists, was carried out as described in Materials and Methods Section (C). Maximal specific binding (Bmax) ranged from 1.0% to 1.8% of total radioactivity added to MG-63 cells. Nonspecific binding was consistently < 0.5%. Values are mean ± SEM from at least three independent experiments in duplicate. *P < 0.05; **0.01 versus nonstimulated control (Co) value (A,B) or Bmax (C) (100%).
Collectively, these results strongly support that VEGFR-2 can be transactivated by PTHrP (107–139) in human osteoblastic MG-63 cells.
The pro-survival action of PTHrP (107–139), via VEGFR-2, requires the transcription factor Runx-2 in human osteoblastic cells
The transcription factor Runx2 has a key role in osteoblast differentiation (Ducy et al., 1999). Interestingly, recent studies have started to disclose an unpredicted role of Runx2 in the mechanisms associated with the survival of various cell types including osteoblasts (Bellido et al., 2003; Inman and Shore, 2003; Blyth et al., 2006). We found here that Runx2 protein localization into MG-63 cell nuclei was maximally increased by PTHrP (107–139), at 100 nM, within 3–6 h (Fig. 8A); an effect which was prevented by SU5614, and also by both PD 98059 and LY 294002 inhibitors (Fig. 8B). None of these inhibitors significantly affected the basal activity of Runx2 in these nonstimulated cells (Fig. 8B). Of note, MG-63 cells transfected with a dnRunx2 construct, which dramatically decreased osteocalcin mRNA expression as a sign of transfection efficacy (Fig. 8C, top), in contrast to those transfected with the control plasmid, failed to respond to the pro-survival effect induced by PTHrP (107–139) (Fig. 8C, bottom). A recent study in rodent osteoblastic cells has suggested that Runx2 activation might be a mechanism leading to induction of the anti-apoptotic protein Bcl-2 (Bellido et al., 2003). We found here that etoposide treatment for 18 h decreased both Bcl-2 protein levels and the Bcl-2/Bax protein ratio in MG-63 cells (Fig. 8D). On the other hand, addition of PTHrP (107–139), at 100 nM, 3 h prior to etoposide significantly increased the latter protein ratio in these cells; an effect which was blocked by dnRunx2 transfection (Fig. 8D).
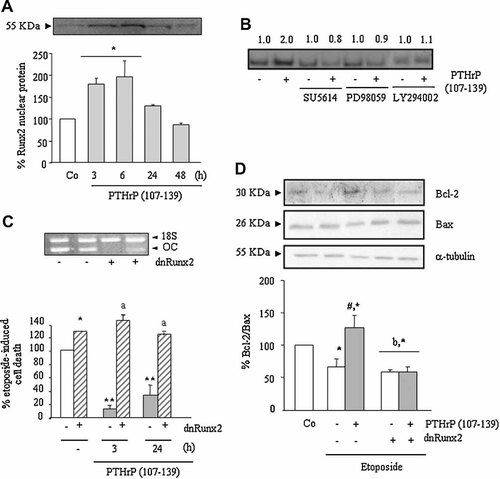
Role of Runx2 on the pro-survival effect of PTHrP (107–139) through VEGFR-2 activation in MG-63 cells. Serum-depleted cells were stimulated or not with PTHrP (107–139) (100 nM) for different times (A,C) or 3 h (B,D), in the presence or absence of different inhibitors (B). PTHrP (107–139) increases Runx2 activation, as determined by Western analysis (A) or EMSA (B) in cell nuclear extracts. Protein loading was similar in each well (A), as assessed by using Ponceau S staining (not shown). Representative autoradiograms are shown (A,B). Relative intensities of Runx2-DNA binding activity from three independent measurements are indicated at the top (fold-induction, means) (B). As specificity controls, the retarded band in some nuclear extracts from cells after PTHrP (107–139) pre-treatment disappeared by pre-incubation with an excess (100×) of the unlabeled Runx2 consensus oligonucleotide, but not by an NF-kB oligonucleotide (not shown). Some cells were transfected with dnRunx2 or empty plasmid before PTHrP (107–139) treatment, followed by exposure to etoposide and cell viability assessment by Trypan blue (C). Representative autoradiogram showing osteocalcin (OC) gene amplification by RT-PCR to evaluate dnRunx2 transfection efficiency in these cells (C, top). Western blot analysis was performed in cell extracts of dnRunx2-transfected or untransfected MG-63 cells using antibodies against Bcl-2 and Bax proteins. Representative autoradiograms are shown (D). Values are mean ± SEM from at least three independent experiments in duplicate. *P < 0.05; **0.01 versus corresponding nonstimulated control (Co) value (A,D) or etoposide-only value (C) (100%); aP < 0.01 versus corresponding value in dnRunx2-untransfected MG-63 cells; #P < 0.05 versus corresponding PTHrP (107–139)-untreated value; bP < 0.05 versus corresponding PTHrP (107–139)-treated value in dnRunx2-untransfected MG-63 cells.
Taken together, these data indicate that the C-terminal domain of PTHrP, via interaction with VEGFR-2, increases human osteoblastic cell survival by a mechanism involving Runx2 activation.
Discussion
In the present in vitro study, we report that the C-terminal domain of PTHrP exerts a pro-survival action in human osteoblastic cells. Pre-treatment with PTHrP (107–139) even for only 1 h was shown to prevent dexamethasone- or etoposide-induced cell death. This effect was also observed after a similar pre-incubation with PTHrP (1–36) in these cells; consistent with previously reported anti-apoptotic effects of this PTH-like domain in various cell types (Amling et al., 1997; Chen et al., 2002; Miyaji et al., 2003; Hastings et al., 2004; Ortega et al., 2006). In vivo studies using mice with osteoblast-specific targeted disruption of PTHrP gene have demonstrated that a defective bone formation was associated with an increase in the number of apoptotic osteoblasts (Miao et al., 2005a). Interestingly, in a preliminary report, a similar relation was found to occur in post-natal mice with deletion of the mid- and C-terminal regions of PTHrP in osteoblasts (Miao et al., 2005b). The present in vitro studies provide a new rationale to explain these previous findings, and strongly support that both N- and C-terminal domains of PTHrP can be similarly efficient at promoting osteoblast survival.
A novel finding here was that interaction with the VEGF system is a key event associated with the pro-survival effect of PTHrP (107–139) in human osteoblastic cells. VEGF is an angiogenic factor playing an important role in coupling angiogenesis to endochondral bone formation (Maes et al., 2002; Towler, 2007). In addition to enhancing angiogenesis, this factor may directly promote bone formation by various mechanisms, such as stimulating osteoblast migration and differentiation, and increasing osteoprogenitor cell survival (Peng et al., 2005). Related to the latter, we found here that VEGF increased cell viability in MG-63 cells. Various local osteogenic factors have been shown to interact with VEGF expression and/or activity associated with their bone anabolic effects (Deckers et al., 2000; Peng et al., 2005). Of note in this regard, recent data indicate that the VEGF/VEGFR-2 system appears to act as a mediator of PTHrP (107–139) to induce differentiation of human osteoblastic cells (de Gortázar et al., 2006). Our present findings demonstrate that the pro-survival effect of PTHrP (107–139), in contrast to that of PTHrP (1–36), was abolished by various maneuvers to inhibit VEGFR-2 in these cells. Specifically, we found that PTHrP (107–139) directly stimulates VEGFR-2 activation, and this does not seem to be a consequence of its putative binding to this receptor in human osteoblastic cells. Moreover, our data using MMP inhibitors or VEGF165 antagonists rule out VEGFR-2 ligands as responsible for this receptor activation by PTHrP (107–139) in these cells. In fact, VEGFR-2 transactivation by various PTHrP-unrelated factors has been demonstrated to be independent of MMPs in endothelial cells (Tanimoto et al., 2002; Zhang et al., 2003; Fujita et al., 2006; Petreaca et al., 2007). Putative candidate mechanisms for this transactivation might include intracellular calcium transients—which occur in PTHrP (107–139)-stimulated osteoblastic cells (Valín et al., 2001)—and src kinases (Tanimoto et al., 2002; Fujita et al., 2006; Petreaca et al., 2007). Additional studies are needed to elucidate the putative role of any of these pathways in VEGFR-2 activation by this C-terminal domain of PTHrP in human osteoblastic cells.
VEGFR-2 signaling, which has been extensively studied in endothelial cells following VEGF stimulation, activates both PI3K and ERK pathways related to several biological responses including an increase in cell viability (Gerber et al., 1998; Tanimoto et al., 2002; Zhang et al., 2003; Santos and Dias, 2004; Fujita et al., 2006; Mathews and Berk, 2008). Our data demonstrate that activation of these pathways is crucial for the pro-survival effect of PTHrP (107–139) in human osteoblastic cells. Interestingly, PI3K and ERK signaling appear to be mediators for VEGFR-2 gene induction by transient exposure to this PTHrP peptide for 24 h in these cells (de Gortázar et al., 2006). Collectively, these findings suggest that PTHrP (107–139) through these intracellular pathways might exert a complex control on VEGFR-2 expression and activation as a positive feedback mechanism to stimulate human osteoblastic cell survival.
Runx2 activation appears to be a key event coordinating various transduction signals to modulate osteoblast function (Franceschi et al., 2003). Specifically, a recent in vitro study in mouse osteoblastic cells suggests that this activation plays a pivotal role in modulating osteoblast survival (Bellido et al., 2003). Our present findings, together with recent studies (de Gortázar et al., 2006), demonstrate that activation of this transcription factor occurs in human osteoblastic cells upon stimulation with PTHrP (107–139), and this can be prevented by a VEGFR-2 inhibitor, and also by ERK and PI3K inhibitors in these cells. Moreover, the important role of Runx2 on osteoblast survival was emphasized by the present observation that a dnRunx2 construct abrogated the pro-survival effect of PTHrP (107–139) in human osteoblastic cells. It has also been suggested that Runx2 might induce the transcription of anti-apoptotic genes, namely Bcl-2, a major modulator of osteoblast apoptosis (Bellido et al., 2003; Pantschenko et al., 2005). Supporting this notion, we found here that the PTHrP (107–139)-induced increase of both Bcl-2 protein expression and the Bcl-2/Bax ratio associated with its protective effect on human osteoblastic cell survival was abolished by the dnRunx2 construct.
In conclusion, our data indicate the pivotal role of VEGFR-2 in the pro-survival effect of the C-terminal domain of PTHrP in human osteoblastic cells. To our knowledge, the present study is the first one demonstrating that VEGFR-2 transactivation may also occur in cells other than endothelial cells. These in vitro findings provide new evidence supporting the potential anabolic action of PTHrP (107–139) in bone.
Acknowledgements
We are indebted to N. Vilaboa, Ph.D. (Laboratorio de Metabolismo Óseo, Hospital La Paz, Madrid) for providing hOB cell cultures. We thank M.L. Villanueva-Peñacarrillo, Ph.D. (Department of Metabolismo, Nutrición y Hormonas, Fundación Jiménez Díaz) for her support in binding studies. We also thank M.S. Davis for proofreading the manuscript. V.A., J.A.A. and IA are fellows of Fundación Conchita Rábago. A.R. de G., Ph.D., was a fellow of Fundación Conchita Rábago, and she is currently a Faculty member at the Medicine School (San Pablo-CEU University). V.A. was the recipient of a travel stipend from Sociedad Española de Investigaciones Óseas y Metabolismo Mineral (SEIOMM) to present portions of this study at the 28th. Annual Meeting of ASBMR in Philadelphia (2006), and of a Young Investigator Award from SEIOMM. M.V.A.-A. is the recipient of a research contract from Instituto de Salud Carlos III.




