Syndecan-4 contributes to endothelial tubulogenesis through interactions with two motifs inside the pro-angiogenic N-terminal domain of thrombospondin-1
Abstract
Thrombospondin-1 (TSP-1) is an extracellular matrix protein that modulates focal adhesion in mammalian cells and exhibits dual roles in angiogenesis. In a previous work, we showed that a recombinant 18 kDa protein encompassing the N-terminal residues 1-174 of human TSP-1 (TSP18) induced tubulogenesis of human umbilical vein endothelial cells and protected them from apoptosis. Our results indicated that these effects were possibly mediated by syndecan-4 proteoglycan, since binding of TSP18 to endothelial extracts was inhibited by anti-syndecan-4 antibody. Syndecan-4 is a heparan-sulfate proteoglycan that regulates cell–matrix interactions and is the only member of its family present in focal adhesions. In this report, we demonstrate that a monoclonal antibody against syndecan-4 blocks TSP18-induced tubulogenesis. Furthermore, through 2D adhesion and 3D angiogenic assays, we demonstrate that two sequences, TSP Hep I and II, retain the major pro-angiogenic activity of TSP18. These TSP-1 motifs also compete with the fibronectin Hep II domain for binding to syndecan-4 on endothelial cell surface, indicating that they may exert their effects by interfering with the recognition of fibronectin by syndecan-4. Additionally, TSP18 and its derived peptides activate the PKC-dependent Akt-PKB signaling pathway. Blockage of PKC activation prevented HUVEC spreading when seeded on TSP18 fragment, and on TSP Hep I and TSP Hep II peptides, but not on gelatin-coated substrates. Our results identify syndecan-4 as a novel receptor for the N-terminus of TSP-1 and suggest that TSP-1 N-terminal pro-angiogenic activity is linked to its capacity of interfering with syndecan-4 functions in the course of cell adhesion. J. Cell. Physiol. 214: 828–837, 2008. © 2007 Wiley-Liss, Inc.
Adult angiogenesis, the formation of new vessels either from pre-existing vascular structures or from the recruitment of bone marrow-derived endothelial precursors (Carmeliet, 2003), is highly dependent on the local levels of some major angiogenic growth factors, such as VEGFs, FGFs, and their cellular receptors (Cross and Claesson-Welsh, 2001). In fact, it has become clear in the last decade that cellular tubulogenesis results from the interplay of both genetic and microenvironment determinants. In particular, it is now recognized that the transient composition of the extracellular matrix (ECM) from a provisional environment (fibrin-rich) to a non-permissive state (basement membrane-rich) directs the differentiation of endothelial cells throughout vessel formation (Sanz and Alvarez-Vallina, 2003). This process also involves proteases responsible for the generation of bioactive fragments and neo-epitopes from ECM macromolecules (Roy et al., 2006).
Thrombospondin-1 (TSP-1) is a multifunctional glycoprotein which displays both adhesive and anti-adhesive properties for mammalian cells (Adams and Lawler, 2004). It was shown to exert a potent anti-angiogenic activity, both in vitro and in vivo, through its type I repeats and the pro-collagen domain (Tolsma et al., 1993; Iruela-Arispe et al., 1999). However, under certain conditions, TSP-1 can also promote angiogenesis. We and others have characterized pro-angiogenic effects, located at the N-terminal heparin-binding domain (HBD) of this glycoprotein (Chandrasekaran et al., 2000; Taraboletti et al., 2000; Ferrari do Outeiro-Bernstein et al., 2002). The HBD has been involved in the disassembly of focal contacts and stimulation of endothelial cell migration (Murphy-Ullrich et al., 1993; Vogel et al., 1993). Importantly, a few recent clinical studies suggest that the anti-angiogenic capacity of TSP-1 was decreased in metastatic deposits, as compared with less advanced primary tumors (Sutton et al., 2005) and that its expression by tumor cells was associated with the presence of venous invasion and with VEGF expression (Poon et al., 2004). Moreover, it was shown that some human cancers can bypass the angioinhibitory effects of TSP-1, by an intricate mechanism, which seems to involve the local balance of VEGF and TSP-1-activated transforming growth factor-β (TGF-β) (Filleur et al., 2001; Fontana et al., 2005). These conflicting data raise questions about the clinical significance of TSP-1 expression at sites of angiogenesis for different tumor sets. Thus, clearly molecular mechanisms need to be further identified.
We have previously shown that a recombinant 18 kDa HBD (TSP18), derived from the structure of the endothelial TSP-1, exerts a pro-angiogenic activity on endothelial cells growing on fibrin matrices (Ferrari do Outeiro-Bernstein et al., 2002). In addition, we showed by ELISA that monoclonal antibodies against the ectodomain of syndecan-4 prevented binding of TSP18 to endothelial cell extracts up to 70% indicating that the pro-angiogenic effect of the TSP18 fragment was probably due to the binding to syndecan-4.
The HS chains of syndecans, a proteoglycan family with four members (syndecans 1-, 2-, 3-, and 4), allow interactions with a large number of proteins, mainly heparin-binding growth factors, such as FGFs, VEGFs, TGF-β, midkine, and pleiotrophin (Fears and Woods, 2006). ECM proteins carrying HBDs, such as fibronectin (FN) and laminin, are also recognized by the HS chains of syndecans (Woods et al., 2000; Suzuki et al., 2003). In addition to using HS/CS chains in the recognition of extracellular ligands, syndecan-4 core protein can directly engage in protein–protein interactions (McFall and Rapraeger, 1997). These authors have demonstrated that a non-glycosylated recombinant fragment corresponding to the extracellular domain of syndecan-4, as well as a native shed syndecan-4, can bind to the surface of endothelial cells and fibroblasts and also support cell adhesion when used as a substrate. A recent study (Whiteford and Couchman, 2006) demonstrated that a conserved NXIP motif is required for cell adhesion that seems to be dependent on β1 integrins. Among the four members of the syndecan family, syndecan-4 is the only one involved in the formation of FN-induced focal adhesions, in cooperation with β1-integrin receptors (Woods and Couchman, 1994). Accordingly, the cytoplasmic V (variable) domain of the core protein of syndecan-4, but not of the other syndecans, interacts with important focal contact components, such as protein-kinase Cα (PKCα), syndesmos, and α-actinin (Oh et al., 1997; Baciu et al., 2000; Greene et al., 2003), thus influencing cell spreading and actin cytoskeletal organization.
In the present work, we characterized the peptide motifs within the 18 kDa N-terminal domain of TSP-1 (TSP18) bearing the pro-angiogenic activity described in our previous work (Ferrari do Outeiro-Bernstein et al., 2002). Using cell adhesion and three-dimensional in vitro angiogenesis assays, we demonstrated that two sequences that contain affinity for GAGs (TSP Hep I, aa 17–35 and TSP Hep II, aa 78–94) retain the major pro-angiogenic activity of TSP18. Competitive binding assays indicated that the two TSP-1 motifs could exert their effects by interfering with the recognition of FN by cell surface syndecan-4. Moreover, using a specific monoclonal antibody against syndecan-4 we showed that syndecan-4 is the receptor involved in the tubulogenic effect induced by the TSP-1 N-terminal domain. These data may contribute to a better understanding of the role of cell adhesion in angiogenesis, and corroborate reports showing that syndecan-4-null mice exhibit defective angiogenesis and repair responses (Echtermeyer et al., 2001).
Material and Methods
Peptides and proteins
Peptides derived from the N-terminus of TSP-1 (see Table 1) were synthesized in the Department of Biophysics at UNIFESP (Federal University of São Paulo, Escola Paulista de Medicina), using an automated bench-top simultaneous multiple solid-phase peptide synthesizer (PSSM 8 System; Shimadzu, Tokyo, Japan). In particular, we synthesized the three peptides (A1 or TSP Hep I, A2 or TSP Hep II, and A4) that are known to bind to GAGs (Murphy-Ullrich et al., 1993). We also synthesized two peptides (S/TSP Hep I and S/TSP Hep II) that suffered substitutions in amino acids essential for GAG binding, and two other peptides bearing heparin-independent adhesion motifs (A3 and A5). The peptides were purified by HPLC and monitored by their absorbance at 220 nm. The molecular masses were determined by MALDI-TOF MS and/or by sequencing using protein sequencer PPSQ-23 (Shimadzu). Phorbol 12-myristate 13-acetate (PMA) and porcine skin gelatin (GEL) were from Sigma (St. Louis, MO). FN adhesion-promoting peptide (FN Hep II-derived peptide, WQPPRARI, from the C-terminal domain of FN), which binds syndecan-4 (Woods et al., 1993) was purchased from Sigma. Monoclonal antibody 150.9, raised against a synthetic peptide from the extracellular N-terminus domain of syndecan-4, was produced by the Hybridoma Core Facility of the Multipurpose Arthritis and Musculoskeletal Diseases Center at UAB (Longley et al., 1999). TSP18, a recombinant heparin-binding fragment comprising amino acid residues 1–174 from the N-terminus of TSP-1, was expressed in E. coli strain A4255− and purified from inclusion bodies by chromatography on DEAE-sepharose, CM-sepharose, and heparin-sepharose as previously described (Legrand et al., 1994).
| Name | Amino acid residues | Amino acid sequence | Characteristics |
|---|---|---|---|
| A1 (TSP Hep I) | aa 17–35 | E L T G A A R K G S G R R L V K G P D | GAG-binding site |
| S/TSP Hep I | Substituted aa 17–35 | E L T G A A R K G S G N Q L V K G P D | Substitution of two amino acids |
| A2 (TSP Hep II) | aa 78–94 | M K K T R G T L L A L E R K D H S | GAG-binding site |
| S/TSP Hep II | Substituted aa 78–94 | M Q N T R G T L L A L A R K D H S | Substitution of three amino acids |
| A3 | aa 117–135 | G K Q H V V S V E E A L L A T G Q W K | |
| A4 | aa 170–189 | T R D L A S I A R L R I A K G G V N D N | GAG-binding site |
| A5 | aa 60–77 | V D A V R T E K G F L L L A S L R Q |
Cell culture
Human umbilical vein endothelial cells (HUVECs) were obtained by the treatment of umbilical veins with a 0.1% collagenase IV solution (Sigma) as described by Jaffe et al., 1973. Except for fetal calf serum (FCS, Cultilab, Campinas, Brazil) and medium 199/HEPES modification (M199, Sigma) all culture medium supplies were from Invitrogen do Brasil (São Paulo, SP). Primary cells were seeded into 25 cm2 bottles (Corning, Cambridge, NY) coated with porcine skin gelatin (GEL), and grown in M199 supplemented with 2 mM L-glutamine, 2.5 µg/ml amphotericin B, 100 µg/ml penicillin, 100 µg/ml gentamycin, and 20% FCS. Cells were maintained at 37°C in humidified 5% CO2 atmosphere until they reached confluence. At that time, cells were detached by brief treatment with a trypsin-EDTA solution, washed once by centrifugation, resuspended in M199 containing 0.1% bovine serum albumin (BSA) and immediately used in the experiments described below.
Adhesion assays
Ninety-six-well microtiter plates were coated with FN adhesion-promoting peptide (FN Hep II peptide), TSP18, or the different TSP-1 N-terminus-derived peptides (Table 1) (6 µg/cm2) in PBS, overnight at 4°C, and saturated for 90 min with M199 containing 0.1% BSA. Cells (1.5 × 105 cells/cm2) were seeded onto the coated plates and allowed to adhere for 1–2.5 h. Some experiments were carried out in the presence of either 10 µM cycloheximide or 100 nM cytochalasin E in order to prevent the endogenous synthesis of proteins or cell uptake of soluble peptides, respectively. To quantify cell adhesion, wells were rinsed thoroughly with M199 to remove unbound cells and the adherent cells were detected by incubating with M199 containing 1 mg/ml MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) for 2 h at 37°C. The solution was removed and replaced by 200 µl of isopropyl alcohol and absorbance was read at 595 nm. The cell number was calculated based on a standard curve made by seeding 3.5 × 103 to 2 × 105 HUVECs/cm2. In competition assays, cells were seeded in the presence of 10, 50, or 100 µM of the different peptides derived from the N-terminus of TSP-1 and quantification of adhered cells was made as described above.
In vitro fibrin gel assay
Fibrin gels used for three-dimensional cultures were formed by overnight polymerization of 3.0 mg/ml fibrinogen (Sigma) solutions with 1.0 IU/ml thrombin in PBS (pH 7.4) at 37°C. In some conditions, synthetic peptides derived from TSP-1 N-terminus (Table 1) were included in the gels at 20 µg/ml. After equilibration in M199 supplemented with 2% FCS for 2 h, HUVECs (1.0 × 105 cells/cm2) were seeded in M199 containing 2% FCS and incubated at 37°C in a humidified 5% atmosphere. Cultures were observed at different times, in order to follow modifications in endothelial cell tubulogenesis. In this model, cells initially migrate and organize themselves in the monolayer, resulting in the opening of cell-free areas within the first 24 h of assay. After nearly 3 days, the portions of intact monolayers become scarse, and cells around the open areas align to form structures that involve the participation of 5, 6, or more cells showing translucent cleft along their axis (lumen formation). The number of open areas generated by the reorganization of cell monolayers (in the first 24 h of assay) or the number of tube-like structures containing at least six cellular bodies (for longer periods, up to 72 h of assay) was quantified, as described previously (Ferrari do Outeiro-Bernstein et al., 2002). Conditions were run in duplicates and the experiments were performed at least three times.
In vitro basement membrane gel assay
Basement membrane gels used for three-dimensional assays were formed by the polymerization of Matrigel (11.46 mg/ml; Becton Dickinson Labware, Bedford, MA) for 30 min at 37°C. Before polymerization, either 20 µg/ml TSP18 fragment or 10 µg/ml of synthetic peptides from the N-terminus of TSP-1 were included in the gels. Cells (1.0 × 105 cells/cm2) were seeded in M199 containing 2% FCS and incubated at 37°C in a humidified 5% CO2 atmosphere. Short-term cultures (6 h, at a maximum) were observed at different times in order to follow modifications in endothelial cell phenotype. Endothelial “sprouts”, defined as the extensions of cell membrane arising from individual cell bodies, or from groups of cell bodies usually connecting neighboring cells, were detectable after 2 h and were quantified, by counting six high-power fields (100× magnification) in each condition, run in duplicate. The experiments were performed at least three times (n = 3). Controls using substituted peptides on GAG-binding consensus motifs were run in parallel.
PKC-inhibition adhesion assays
Adhesion assays were performed in 24-well plates coated with GEL, TSP18, and the peptides TSP Hep I and TSP Hep II, as described above. HUVECs were seeded in the presence of 10 nM Ro 318220 (bisindolylmaleimide-IX, Biomol International, Plymouth Meeting, PA), a selective inhibitor of protein kinase C (PKC). As control, cells were seeded in the presence of vehicle (dimethyl sulfoxide, DMSO). Four different fields per well were randomly selected and the degree of spreading was analyzed. Each condition was done in triplicate. The experiment was repeated twice.
Detection of Akt/PKB activation
Six-well cell culture plates were coated with TSP18 or synthetic peptides from the N-terminus of TSP-1 (10 µg/cm2) in PBS (pH 7.4) overnight at 4°C. The plates were blocked by incubation with M199 supplemented with 1% BSA for 1 h at 37°C. HUVECs (1.6 × 105 cells/cm2) were seeded onto the coated plates in serum-free M199 containing 0.1% BSA. After 90 min at 37°C in a 5% CO2 atmosphere HUVEC monolayers were incubated or not with another selective PKC inhibitor, 10 nM bisindolylmaleimide-I (BIM, Calbiochem, CA) or with Ro 318220 for 30 min at 37°C. Controls were performed using cell suspensions incubated with or without PKC inhibitors and/or the PKC activator PMA (30 nM, Calbiochem, CA) for 15 min after BIM addition. Cells were lysed in 20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1mM EGTA, 1% Triton X-100, 2.5 sodium pyrophosphate, 1 mM β-glycerolphosphate, 1mM Na3VO4, 40 µM leupeptin, and 1 mM phenylmethylsulfonyl fluoride. The protein content in the cell extracts was determined by the method of Coomassie Plus (Invitrogen do Brasil). Akt was immunoprecipitated from cell lysates using a polyclonal anti-Akt polyclonal antibody (1:50, Cell Signaling Technology, Danvers, MA) coupled to protein A/G-agarose (Santa Cruz Biotechnology, Santa Cruz, CA). Akt was analyzed by Western blotting. Equal amounts of proteins from each sample were subjected to 10% SDS–PAGE and transferred to Immobilon PVDF membranes (Millipore, São Paulo, SP). The membranes were incubated with anti-phospho Akt (Ser 473) or anti-Akt polyclonal antibody (Cell Signaling Technology). The bands, detected by using the ECL-enhanced chemiluminescence kit from GE Healthcare Latin America (São Paulo, SP), were quantified by densitometry using Scion Image Software (Scion Co., Frederick, MD).
Statistical analysis
The results are presented as mean of three different experiments made in triplicate and analyzed by non-paired Student's t-test using the Microsoft ExcelTM software statistical tool or by ANOVA followed by Bonferroni's t-test. Values of P < 0.05 were considered statistically significant.
Results
Identification of the major motifs of TSP18 exhibiting pro-angiogenic activity
The N-terminal domain of TSP-1 bears several cell-binding domains, which mediate the adhesion of different cell types, in both GAG-dependent and independent manners, as described by others (Murphy-Ullrich et al., 1993; Clezardin et al., 1997). In order to ascertain which regions inside TSP-1 N-terminal domain are responsible for the adhesion of endothelial cells, we performed adhesion assays using the different immobilized synthetic peptides described in Table 1. The panel shows five peptides (preliminarily named A1, A2, and A4, GAG-dependent; and A3 and A5, GAG-independent) previously described as adhesion motifs for endothelial or epithelial cells (Murphy-Ullrich et al., 1993; Clezardin et al., 1997). Peptides A1, A2, A3, and A5 are fully contained in the primary sequence of TSP18 (amino acids 1–174), whereas most of A4 amino acids are not present in the primary structure of this fragment. The peptides were immobilized on 96-well microtiter plates and endothelial cells were allowed to adhere on protein supports for 2.5 h, in the presence of cycloheximide at 10 µM to prevent endothelial protein synthesis, thus ensuring that the adhesion displayed was entirely due to the protein used as support for cell adhesion. As shown in Figure 1A, although peptides A1, A2, and A4 promoted the highest adhesion among the five sequences tested, all synthetic peptides were able to support HUVEC attachment, as compared to control BSA.
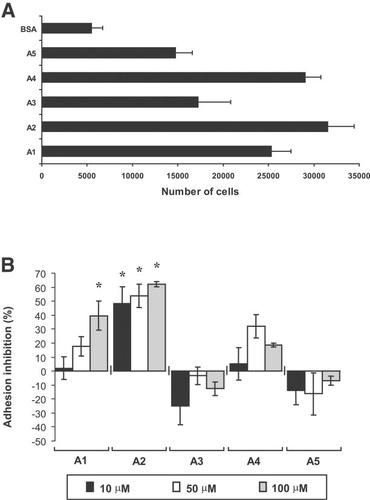
A: Adhesion of HUVECs to different peptides derived from the N-terminus of TSP-1. Cells (1.5 × 105 cells/cm2) were seeded onto plates coated with 6 µg/cm2 of different peptides (Table 1) derived from the N-terminal domain of TSP-1 and blocked with 0.1% BSA. Experiments were carried out in the presence of 10 µM cycloheximide and, after 2.5 h, wells were rinsed and the attached cells quantified as described in the section “Materials and Methods”; (B) Inhibition of HUVECs adhesion to TSP18-coated wells by different soluble peptides of TSP-1. HUVECs (1.5 × 105 cells/cm2) were seeded onto plates coated with 6 µg/cm2 of TSP18 in the presence of 10, 50, or 100 µM soluble TSP-1 peptides and 100 nM of cytochalasin E. Adhering cells were detected as above. The percentage of inhibition of adhesion was calculated based on the number of cells adhered on wells coated with TSP18 with no soluble proteins added; P < 0.02, as compared to control.
Nonetheless, as the N-terminal region of TSP-1 is globular, some of these sequences could be cryptic and not available for cell attachment on the 18 kDa recombinant TSP18. Thus, we designed another set of experiments, where HUVECs were seeded on plates coated with TSP18 in the presence of 10, 50, or 100 µM soluble synthetic peptides. The percentage of inhibition of HUVEC adhesion to TSP18 was calculated by comparing the adhesion obtained in the presence versus absence of soluble peptides. Figure 1B shows that peptides A3, A4, and A5 did not significantly inhibit HUVEC adhesion to TSP18 at any of the concentrations used. On the other hand, peptide A2 significantly inhibited HUVEC adhesion to TSP18, starting at 10 µM with up to 65% inhibition at 100 µM. Soluble peptide A1 was also able to significantly inhibit HUVEC adhesion to TSP18, but only at 100 µM concentration (40% inhibition). These data suggest that the sequences A2 and A1 might be the major recognition motifs involved in the effects described for TSP18 fragment as an inducer of endothelial tubulogenesis.
To test this hypothesis, we performed experiments where different peptides from TSP-1 were included in fibrin gels prior to its polymerization (Ferrari do Outeiro-Bernstein et al., 2002) so as to look at their pro-angiogenic activity, as measured by their ability to induce tube-like structures in HUVECs. Peptide A3 completely failed to induce tube formation whereas peptides A1 and A2 strongly induced angiogenic differentiation. Although to a lesser extent, peptides A4 and A5 also promoted significant formation of tubular structures (Fig. 2).
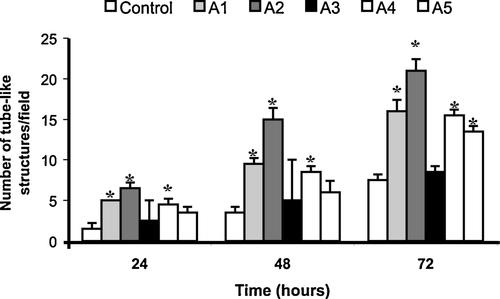
Effect of TSP-1 N-terminal peptides on HUVEC differentiation in fibrin angiogenesis assay. Each of five peptides (A1 [TSP Hep I], A2 [TSP Hep II], A3, A4, and A5) derived from the N-terminal domain of TSP-1 was mixed to fibrinogen (3 mg/ml) polymerizing gels at the final concentration of 20 µg/ml. HUVECs (1.0 × 105 cells/cm2) were seeded in M199 containing 2% FCS. Endothelial differentiation was determined at different days by counting the differentiated tube or cord-like structures in six high-power fields/well, as described in the section “Materials and Methods”. Each condition was done in duplicate and the experiment was repeated three times; P < 0.05, as compared to control.
We have previously shown that the induction of tube formation by TSP18 was disrupted by heparin (Ferrari do Outeiro-Bernstein et al., 2002). Since peptide A5 does not bear consensus motifs for GAG binding and the highly positively charged residues of A4 peptide—[177] RLRIAKGGVNDN [188], responsible for binding to heparin (Clezardin et al., 1997) are not contained in the primary structure of TSP18 fragment, we decided to focus our further experiments on the role of A1 and A2 peptides. These peptides will be referred from now on as TSP Hep I and TSP Hep II, respectively, as previously proposed (Murphy-Ullrich et al., 1993).
The pro-angiogenic activity of TSP18 fragment is inhibited by an anti-syndecan-4 antibody
In order to confirm whether the pro-angiogenic activity of TSP18 was mediated by the engagement of syndecan-4, we performed in vitro angiogenesis assays in the presence of a monoclonal antibody directed against the ectodomain of this cell surface HSPG. Since the fibrin-based assay, usually measured in days, was not suitable for tubulogenesis assays in the presence of antibodies that would be rapidly cleared from cell surfaces, we used a Matrigel angiogenesis assay. In doing so, we have observed that TSP18 fragment also exerts its pro-angiogenic activity in a basement membrane-rich microenvironment. Figure 3A and B shows that the formation of sprouts from endothelial cell bodies, induced by TSP18 fragment, was fully inhibited by an anti-syndecan-4 monoclonal antibody. This antibody had no effect on the tubulogenic activity induced by Matrigel matrices where no TSP18 was added prior to matrix polymerization, demonstrating that the effect of anti-syndecan-4 antibody in sprouting inhibition was specifically related to the N-terminus of TSP-1. In addition, an irrelevant antibody did not have any effect on endothelial tubulogenesis in either presence or absence of TSP18. These results demonstrate that syndecan-4 is the endothelial cell surface receptor responsible for TSP18-induced angiogenic differentiation.
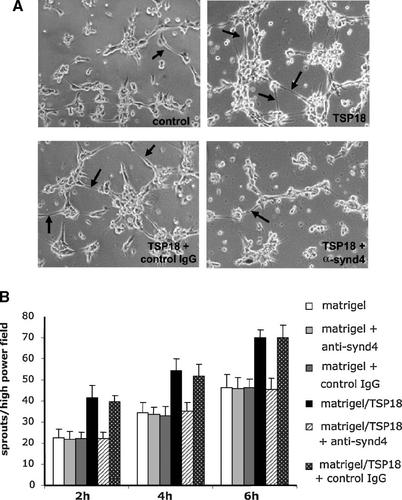
A monoclonal antibody against the ectodomain of the core protein of syndecan-4 inhibits TSP18-induced capillary-like differentiation. A: Morphological aspect of HUVECs (1.0 × 105 cells/cm2) in short-term Matrigel differentiation assays (6 h). Cells were seeded on Matrigel containing 20 µg/ml TSP18 in the presence of 15 µg/ml of an anti-syndecan-4 MAb or a purified non-immune mouse IgG. Endothelial sprouts are indicated by the arrows. B: The number of sprouts emerging from either individual cell bodies or from cell clusters were counted at 2, 4, and 6 h after seeding, in six high-power fields were counted per well, with each condition run in duplicate. The experiment was repeated three times. P < 0.05, as compared to the control (Matrigel alone, without the addition of TSP18 or antibodies). Magnification: 100×.
The effects of peptides TSP Hep I and II in the fibrin angiogenesis assay were also reproducible with HUVECs seeded on Matrigel (Fig. 4A,B). The replacement of TSP Hep I or TSP Hep II by the substituted S/TSP Hep I and S/TSP Hep II peptides (see Table 1), which were modified in order to disrupt their GAG-binding consensus motifs, resulted in the total abrogation of their pro-angiogenic activity.
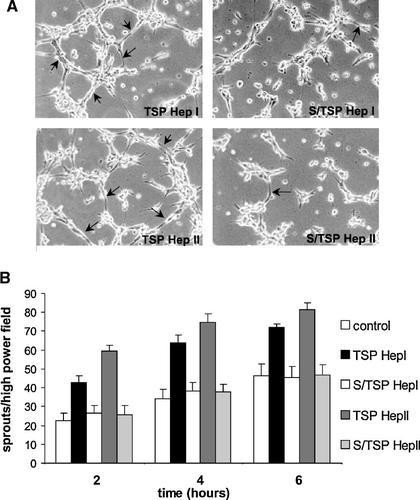
Substitution of amino acids in GAG-binding consensus motifs of TSP Hep I and TSP Hep II peptides disrupts endothelial sprouting in the Matrigel assay. A: HUVECs (1.0 × 105 cells/cm2) morphological aspect on Matrigel matrices containing 10 µg/ml of either TSP Hep I or TSP Hep II, or their substituted counterparts S/TSP Hep I or S/TSP Hep II, after 6 h. B: The number of sprouts (arrows), emerging from either individual cell bodies or from cell clusters, was counted at 2, 4, and 6 h after seeding, in six high-power fields, as described for Figures 2 and 3; P < 0.05, as compared to the control (Matrigel alone). Magnification: 100×.
The pro-angiogenic peptides TSP Hep I and TSP Hep II compete with fibronectin for endothelial cell binding
Several studies have shown that the adhesion-inducing activity of the ECM protein FN involves both heparin- and integrin-dependent interactions (Pytela et al., 1985; Woods et al., 2000). Syndecan-4 drives focal adhesion formation through binding to the high-affinity C-terminal HBD of FN (FN HepII). Thus, we decided to verify whether the pro-angiogenic peptides TSP Hep I and TSP Hep II interfere with the binding capacity of endothelial syndecan-4 to FN Hep II peptide. We performed cell adhesion assays where HUVECs were seeded on wells coated with a FN Hep II-derived peptide (FN pept) described to bind syndecan-4 and induce focal adhesion formation, both in the absence and the presence of soluble TSP Hep I or TSP Hep II. Since it has been described that the N-terminal HBD of TSP-1 is essential for TSP-1 uptake and endocytosis (Murphy-Ullrich and Mosher, 1987), we performed these experiments in the presence of cytochalasin in order to prevent the clearance of soluble peptides. As shown in Figure 5, both peptides led to significant dose-dependent 50% decrease in HUVEC binding to FN Hep II-derived peptide (FN pept). The effect was specific, since the addition of equimolar amounts of the substituted peptides, (S/TSP Hep I or S/TSP Hep II), did not modify the adhesion of HUVECs on FN Hep II-coated wells. These observations strongly suggest that the pro-angiogenic activity of the N-terminal domain of TSP-1 is linked to its capacity of interfering with syndecan-4 functions in the course of cell adhesion.
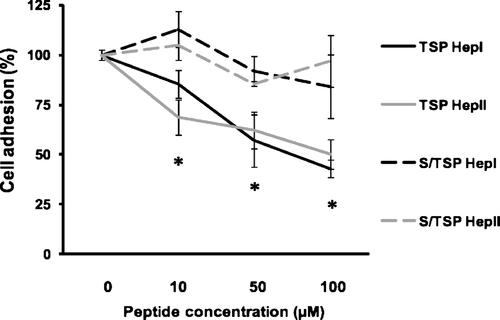
Adhesion of HUVECs to a syndecan-4-binding site derived from the C-terminal domain of fibronectin is inhibited by TSP Hep I and TSP Hep II. HUVECs were seeded on plates coated with a peptide derived from FN Hep II (FN pept) at 6 µg/cm2 in the presence of increasing doses of soluble TSP-1-derived peptides or their substituted counterparts (10, 50, and 100 µM) and cytochalasin E (100 nM). After 1 h, wells were rinsed and attached cells were quantified as described in the section “Materials and Methods”. Percentage of cell adhesion was calculated based on the number of cells attached to wells coated with FN pept where no soluble proteins were added (P < 0.01).
Blockade of protein-kinase C leads to inhibition of HUVEC spreading to the pro-angiogenic fragment TSP18
A hallmark of the engagement of syndecan-4 in cell adhesion events is its ability to promote the activation of protein-kinase C-α (PKC-α) (Oh et al., 1997). PKC is also activated during cell spreading (Vuori and Ruoslahti, 1993). Moreover, syndecan-4 was shown to provide additional interactions which are essential to the formation of integrin-mediated focal adhesions (Woods et al., 2000). To further examine whether the interaction of HUVECs with pro-angiogenic motifs of TSP-1 N-terminus involved PKC-mediated signaling, we analyzed adhesion and spreading of endothelial cells seeded on TSP18 fragment. As shown in Figure 6 (part A), the treatment of HUVEC cultures, at the moment of seeding, with 10 nM Ro 318220, a broad spectrum selective inhibitor of PKC isoforms, resulted in a significant increase in the number of non-spread cells on TSP18 (94 ± 13% of round cells, Fig. 6B), as compared with untreated cells seeded on the same protein (52.7 ± 8.6% of round cells, Fig. 6B). On gelatin-coated surfaces, the relative ratio of spread and round endothelial cells was not significantly affected by the addition of Ro 318220 (Fig. 6A,B). Cell spreading on peptides TSP Hep I and TSP Hep II was also inhibited by Ro 318220 (from 14.7 ± 1.4% to 3.9% for TSP Hep I, and from 47.4 ± 8.4% to 10.8% for TSP Hep II—not shown). Comparable results were obtained for all supports (TSP18, TSP Hep I, and TSP Hep II) when BIM, another PKC selective inhibitor, was used in the adhesion assays (not shown).
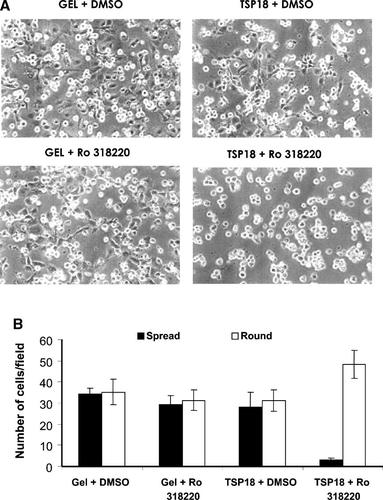
Adhesion and spreading of HUVECs to TSP18 or gelatin in the presence of a selective PKC inhibitor. Cells were seeded on plates coated with either TSP18 fragment or gelatin (GEL) in the presence of the selective PKC inhibitor RO 318220 (10 nM) or the vehicle (DMSO). A: Morphological aspect of the cultures seeded on TSP18 or GEL, 2 h after seeding. B: Quantification of cell shape phenotypes (spread/round) adhering on both substrates in the presence and absence of PKC inhibitor, 2 h after seeding; (P < 0.01). Magnification: 100×.
These results indicate that the receptor(s) for TSP18 on HUVECs elicits PKC-dependent signaling response, which is compatible with the idea of a major participation of syndecan-4 in the intracellular cell signaling in response to the N-terminal domain of TSP-1.
PKC activation by the pro-angiogenic peptides TSP Hep I and TSP Hep II induces PKB/Akt signaling pathway
We have previously shown that the viability status of HUVECs was maintained in the course of the angiogenic differentiation promoted by TSP18 fragment included in fibrin matrices (Ferrari do Outeiro-Bernstein et al., 2002). PKC was shown to activate PI3-kinase, which in turn activates Akt/protein-kinase B (PKB) (Ilan et al., 1998). To study the further consequences of PKC activation following endothelial cell adhesion on pro-angiogenic motifs of TSP18, we have examined the downstream activation of PKB/Akt pathway. Figure 7 shows that the adhesion of HUVECs to immobilized TSP18, TSP Hep I, and TSP Hep II elicits a strong increase in Akt phosphorylation. Moreover, Akt activation was dependent on PKC activity, since the inhibition of this kinase with the selective inhibitor BIM (10 nM) resulted in approximately 2-, 4-, and 2.5-fold decrease in Akt phosphorylation levels, for cells adhering on TSP18-, TSP Hep I-, and TSP Hep II-coated supports, respectively. Comparable results were observed when Ro 318220 (10 nM) was used in the Akt signaling assay (data not shown).
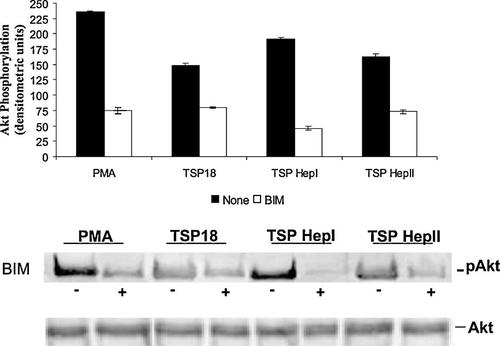
Detection of Akt/PKB activation on endothelial cells seeded on different TSP-1 N-terminal peptides. HUVECs (1.6 × 105 cells/cm2) were seeded onto plates coated with different adhesive supports, in serum-free M199 containing 0.1% BSA. After 90 min, monolayers were preincubated or not with the PKC inhibitor (BIM-10nM) for 30 min. After cell lysis, Akt/PKB was immunoprecipitated and equal amount of proteins were subjected to 10% SDS–PAGE. After electrotransfering onto membranes, the separated proteins were probed with a monoclonal anti-phospho Akt (Ser 473). Blots were also probed with a polyclonal anti-Akt antibody to ensure that equal amounts of total Akt were loaded. The phosphorylated Akt band (pAkt, 60 kDa) was quantified by densitometry using Scion Image Software. Controls were performed using cell suspensions incubated with or without BIM and/or the PKC activator PMA (30 nM) (P < 0.05).
We also observed that when cells adhering for 90 min to TSP Hep I or TSP Hep II-coated supports were incubated with PKC inhibitors (BIM or Ro 318220) for 30 min—that is, the same condition of the Akt signaling assay—pre-spread cells were significantly induced to adopt a round phenotype (approximately 40% of reversion, as compared to untreated cultures—not shown). Since Akt phosphorylation was strongly inhibited in these conditions, this observation suggests that Akt phosphorylation participates in the modulation of endothelial spreading.
Taken together, our observations provide evidence that syndecan-4 is a major mediator of the pro-angiogenic and possibly pro-survival activities of TSP18 N-terminal fragment of TSP-1 in vitro. These effects involve two specific sequences, TSP Hep I and TSP Hep II that have the ability to inhibit the interactions between syndecan-4 and FN.
Discussion
The N-terminal HBD of TSP-1 has been shown to contain several adhesive sequences that can exert pro-angiogenic activities, whose mechanisms remain to be elucidated (Chandrasekaran et al., 2000; Taraboletti et al., 2000; Ferrari do Outeiro-Bernstein et al., 2002).
In a previous work, we showed that heparin-dependent interactions of HUVECs with a TSP18 N-terminal fragment (amino acids 1–174) of TSP-1 resulted in induction of tubulogenesis, possibly through the engagement of cell surface syndecan-4 (Ferrari do Outeiro-Bernstein et al., 2002). In this work, we show that two adhesive sequences, known as TSP Hep I (amino acids 17–35, ELTGAARKGSGRRLVKGPD) and TSP Hep II (amino acids 78–94, MKKTRGTLLALERKDHS), both bearing high-affinity GAG-binding sites (Clezardin et al., 1997), exert a similar pro-angiogenic effect as TSP18. These peptides also inhibited the adhesion of HUVECs to TSP18, indicating that these are the main sequences used by HUVECs to interact with the N-terminal domain of TSP-1. In fact, the study of the crystal structure of TSP-1 N-terminal domain (amino acids 1–240) and its complex with a synthetic pentameric heparin (Tan et al., 2006) has shown one major positively charged region as the main responsible for TSP-1 interaction with heparin. Additionally, the positively charged residues at the TSP Hep I (R29, K32) and TSP Hep II (K80, K81) regions locate along with residues R42, R77, and K106 and despite the fact that these residues are well separated on the primary sequence, the authors described that they congregate to form a patch. Thus, these heparin-binding motifs might be accessible in native N-terminal fragments.
Other peptides also exhibited pro-angiogenic activities: the peptide A4 (amino acids 170–189) induced a tubulogenic response, but we excluded this peptide from subsequent analysis, since most of its sequence is not contained in the primary structure of TSP18 and it did not significantly inhibit endothelial binding to TSP18. Another peptide, A5 (amino acids 60–77), exerted a pro-angiogenic effect, but only after 72 h. This peptide did not inhibit binding of HUVECs to TSP18. Nonetheless, we cannot discard possible cooperation between Hep I, Hep II, and A5, since the amino acid residue R77 is described as one of the key residues present at the positively charged patch that interacts with heparin (Tan et al., 2006). The importance of the heparin-affinity motif in the activities of TSP Hep I and TSP Hep II was stressed by the fact that the substitution of essential amino acid residues inside these GAG-binding sequences resulted in the blockage of their pro-angiogenic effects.
TSP18-induced tubulogenesis was fully abolished by an anti-syndecan-4 monoclonal antibody. This effect was specifically related to the N-terminal of TSP-1, since the same antibody had no effect on endothelial tubulogenesis when no TSP18 was added to Matrigel. Recently, a role for syndecan-2 in the regulation of angiogenesis of microvascular endothelial cells has been proposed (Fears et al., 2006). However, the authors neither address the role of intact cell surface syndecans nor the role of TSP-1 in their angiogenic assays.
It has been shown that the binding of syndecan-4 to a C-terminal HBD of FN, known as FN Hep II motif, has a crucial role in the formation of focal adhesions (Woods and Couchman, 1994) in cooperation with RGD-dependent integrins. We found that TSP Hep I and TSP Hep II peptides inhibited the adhesion of HUVECs to the Hep II domain of FN, suggesting that their pro-angiogenic activity could be related to their ability to directly bind to cell surface syndecan-4, thus interfering with FN recognition by endothelial cells. It has been suggested that the interference of another matricellular protein, tenascin-C, with syndecan-4 functions in cell adhesion by acting as a direct ligand to the C-terminal Hep II domain of FN (Huang et al., 2001). In the case of Hep I and Hep II peptides of TSP-1, we found no evidence that they interacted with the FN Hep II domain in this study (our unpublished results). TSP-1 is in fact thought to interact with the N-terminal domain of FN and with non-heparin-binding motifs on C-terminal domain (Homandberg and Kramer-Bjerke, 1987; Zafar et al., 1992).
Syndecan-4 engagement and multimerization on cell surface promotes the activation of protein-kinase Cα (PKCα) (Oh et al., 1997), and the activation of PKC was shown to precede cell spreading on FN (Vuori and Ruoslahti, 1993). Thus, we investigated whether the adhesion and spreading of HUVECs on TSP18-coated supports, characterized in our previous work (Ferrari do Outeiro-Bernstein et al., 2002), was dependent on PKC activation. We found that selective inhibitors of PKC isoforms prevented endothelial spreading on TSP18, without affecting cell adhesion. Also, during tubulogenesis in HUVECs, the activation of PKC can lead to the activation of phosphatidylinositide-3′-OH kinase (PI3K), which has Akt/PKB as a downstream target (Ilan et al., 1998). Akt/PKB is recognized as a key modulator of cell survival, mainly by inactivating the mitochondrial-dependent cell death machinery (Kennedy et al., 1999). In our previous work, we showed that TSP18 promoted endothelial cell survival (Ferrari do Outeiro-Bernstein et al., 2002). In the present study, we clearly showed that the adhesion of HUVECs to TSP18, TSP Hep I, and TSP Hep II led to the PKC-dependent activation of Akt/PKB survival pathway, which also seemed relevant for maintenance of cell spreading. Our data reinforce the idea that an intermediate state of adhesion supports cell survival (Murphy-Ullrich, 2001).
The consistent work of Murphy-Ullrich's group, who described the role of the HBD of TSP-1 in focal adhesion disassembly (Murphy-Ullrich et al., 1993), implicates the participation of CRT/LDL receptor-related protein (LRP) co-complex in the recognition of the TSP Hep I motif (Orr et al., 2003). It is possible that the functional site(s) for CRT/LRP binding on TSP Hep I motif is (are) slightly different from those identified for syndecan-4 in this study, since the amino acid substitutions resulting in inactive peptides used in Orr's study are different from the substitutions we have performed. However, we cannot exclude that the stimulation of PI3-kinase signaling by CRT/LRP complex (Goicoechea et al., 2000) may contribute to the activation of Akt/PKB that we have observed in our assays. In the present work, we have not addressed the role of several β1 integrins that recognize specific sequences within the HBD of TSP-1 (reviewed by Elzie and Murphy-Ullrich, 2004) and could contribute, together with syndecan-4, to the modulation of endothelial cell adhesion and angiogenesis.
Despite the accumulating evidence that the N-terminal HBD of TSP-1 and smaller derived peptides, represent a molecular arsenal for providing a stimulating pro-angiogenic microenvironment for endothelial cells, the physiological relevance of these observations remains to be fully demonstrated. Specifically, the HBD of TSP-1 is rapidly cleaved in vitro by several proteases relevant to the vascular compartment (Morandi et al., 1994; Bonnefoy and Legrand, 2000) and its processing by a disintegrin and metalloprotease with thrombosspondin motifs (ADAMTS-1) in vivo was recently shown under situations of tissue repair (Lee et al., 2006). It is conceivable that the stroma of wounded, inflammatory, or invasive/metastatic tumoral tissues in which an increased expression of TSP-1 was found (Poon et al., 2004; Sutton et al., 2005) would also contain such fragments.
In conclusion, the confirmation that syndecan-4 participates in angiogenesis may open new perspectives, especially for the understanding of events related to wound repair and tissue remodeling. In this regard, it has been shown that mice lacking syndecan-4 have significant delayed wound healing and impaired angiogenesis in the granulation tissue (Echtermeyer et al., 2001). Since the reactive stroma of solid tumors have been increasingly compared to the inflammatory microenvironment, it seems crucial to determine the local balance of factors which would favor the predominance of anti- or pro-angiogenic activities of TSP-1.
Acknowledgements
We are most grateful to Marcos de Oliveira Temperini for the excellent technical assistance in cell culture maintenance, and to the following organizations, for their support to this research: The National Council for Scientific and Technological Development (CNPq, Brazil), the Foundation for the Support of Research of the State of Rio de Janeiro (FAPERJ), the Foundation for the Support of the State of São Paulo (FAPESP), the CAPES (Ministry for Education, Brazil)/COFECUB (France) Cooperation Agreement, and the PRONEX/FAPERJ Program (Brazilian Ministry for Science and Technology); and INSERM (Institut National de la Santé et de la Recherche Médicale, France).




