Cell surface protein detection with immunogold labelling in ESEM: Optimisation of the method and semi-quantitative analysis
Abstract
In this work we used a combination of immunogold labelling (IGL) and environmental scanning electron microscopy (ESEM) to detect the presence of a protein on the cell surface. To achieve this purpose we chose as experimental system 3T3 Swiss Albino Mouse Fibroblasts and galectin-3. This protein, whose sub-cellular distribution is still under discussion, is involved in a large number of cell physiological and pathological processes. IGL technique has been utilised by many authors in combination with SEM and TEM to obtain the identification/localisation of receptors and antigens, both in cells and tissues. ESEM represents an important tool in biomedical research, since it does not require any severe processing of the sample, lowering the risk of generating artefacts and interfere with IGL procedure. The absence of metal coating could yield further advantages for our purpose as the labelling detection is based on the atomic number difference between Nanogold spheres and the biological material. Using the gaseous secondary electron detector (GSED) compositional contrast is easily revealed by the backscattered electrons component of the signal. In spite of this fact, only few published papers present a combination of ESEM and IGL. Hereby we present our method, optimised to improve the intensity and the specificity of the labelling signal, in order to obtain a semi-quantitative evaluation of the labelling signal. J. Cell. Physiol. 214: 769–776, 2008. © 2007 Wiley-Liss, Inc.
The development of immunochemical techniques is one of the emerging fields in biological and medical sciences. The introduction of reliable immunological methods for producing good quality monoclonal and polyclonal antibodies and for coupling proteins with gold particles to develop immunological probes, allows researchers to investigate a wide range of antigens in cells and tissues.
Gold particles may be conjugated to primary antibodies for one-step identification of antigens but are more usually employed as secondary antibody labels.
Colloidal gold labelling techniques were first introduced by Faulk and Taylor (1979) when they absorbed anti-salmonella rabbit gamma globulins to gold particles for one-step identification and localisation of salmonella antigens (Faulk and Taylor, 1979).
In some instances the identification of cellular components with immunogold labelling (IGL) by SEM was restricted due to difficulties in resolving the particles and charging of non-sputtered specimens under the electron beam (Hoyer et al., 1979). Improved resolving power of SEM instruments, the use of backscattered electron (BSE) imaging and deposition of cells directly onto metal stubs with carbon coating have greatly improved this situation and the method is now considered to be extremely sensitive and specific, giving rapid analysis of protein distribution over wide areas of cells and tissue structures (De Harven et al., 1984; Williams et al., 2001). Gold probes for electron microscopy are available in size ranging from 1 to 40 nm (Namork and Heier, 1989).
The silver enhancement reaction offers the possibility to stain the antigen with little particles, and then to enlarge their diameter to more easily detectable size. In presence of silver ions and a reducing agent, such as hydroquinone, gold particles act as catalysts to reduce silver ions to metallic silver, that is deposited onto the gold. The main advantage of silver enhancement procedure is the possibility of SEM observation at relative low magnification and evaluation of marker localisation in different cell surface areas (Hayat, 1989; Namork, 1991).
The introduction of environmental scanning electron microscope (ESEM), working in gaseous atmosphere, represented a new perspective for many researchers in biological field. Environmental scanning electron microscopy (ESEM) is a relatively new technique that makes it possible to examine practically any material, including biological tissues, wet or dry, insulating or conducting, because it allows the introduction of a gaseous environment in the specimen chamber. The choice of tissue processing procedures is vital for efficient detection and quantification of target antigen in cells by IGL electron microscopy.
Many steps from conventional SEM preparation protocols, although attempting to preserve sample ultrastructure, also affect the native antibody binding activity of the antigen under study (Bendayan, 1995) and can interact negatively with the immunolabelling process.
Many monoclonal antibodies work well only against fresh antigens but they recognise still those mildly fixed (Polok and Van Noorden, 1986). Glutaraldehyde can often cause antigens collapse, whilst all aldehyde fixatives produce charged residuals that are responsible both for antibody electrostatic interactions and silver precipitation. On the other hand, osmication and all kinds of dehydration can cause loss of gold nanoparticles.
Although a great number of biomedical samples have been analysed using ESEM, in many works the authors have reported their difficulties in dealing with wet samples (Martinez-Alvarez et al., 2000; Mestres et al., 2003) and still used one or more steps from conventional SEM sample preparation protocols. In our experimental work on cell cultures and tissues we obtained high-resolution ESEM images on natural samples that underwent only a slight fixation (Muscariello et al., 2005; Rosso et al., 2006).
In ESEM, a gaseous detection device enables images to be obtained in secondary (SE) mode. This signal is mixed with the BSE image to provide strong material and morphological contrast. As there is no need for conductive coating of biological material; immunophenotypic characterisation of cells can be achieved by staining with colloidal gold reagents (conjugated antibodies or streptavidin-gold) with subsequent silver enhancement.
Secondary electron (SE, generated by inelastic collisions) imaging is the main method for examining structural characteristics of specimens, while BSEs (generated by elastic collisions) imaging offers less resolution power but reveals compositional differences. Because of the non-conductive nature of biological specimens, ESEM image of colloidal gold stained sample offers a very strong contrast signal due to SE-BSE mixed components, thus making it very simple to identify the immunopositive cells in the microarchitecture of the tissue (McMenamin et al., 2002).
In spite of this, there are still few published studies in which ESEM-IGL was used.
McMenamin et al. (2002) used immunolabelling and ESEM to study the tunica vesciculosa lens (TVL) of the rat eye. In particular they demonstrated that the cells surrounding the developing lens, postulated to play a role in regression of the TVL, have the morphologic and immunophenotypic characteristics (ED1+, ED2+, MHC class II-, and CD11b/18-) of resident tissue macrophages (hyalocytes).
The aim of this work is to optimise a method that employees the combination of ESEM and IGL technique to the high-resolution identification/localisation of specific proteins on the cell surface. Furthermore we want to test the reliability and the possible advantages of a semi-quantitative analysis for the interpretation of labelling patterns, that is for the determination of staining specificity level changes due to conjugate probe concentration.
This kind of approach has been followed previously in a good number of works with TEM (Momayezi et al., 2000; Ramandeep et al., 2001; Soltys et al., 2001), rarely in SEM (Tomczok et al., 1994) but never in ESEM.
In this work we want to achieve a full exploitation of ESEM advantages, the possibility of imaging the sample after minimum processing, keeping it highly hydrated.
We decided to focus on galectin-3, a protein currently under study in our laboratory.
The galectins are a growing family of β-galactoside-binding proteins, widely distributed in metazoan organisms. The galectins are cytosolic proteins. However, there is abundant evidence for their secretion from the cytosol via non-classical pathways or translocation to the nucleus or the other cellular compartments (Hughes, 1997, 1999). They have been shown to play roles in many biological events, such as embryogenesis, adhesion and proliferation of cells, apoptosis, mRNA splicing, bacterial colonisation and modulation of the immune response (Liu et al., 2002; Rabinovich et al., 2002).
Moreover, galectins play a key role in various pathological states, including autoimmune diseases, allergic reactions, inflammation, tumour spreading, atherosclerosis and diabetic complications (Perillo et al., 1998; Wada and Makino, 2001).
Each of the 14 known galectins exhibits a specific pattern of expression in various cells and tissues. Galectin-3 has been detected in activated macrophages, eosinophils, neutrophils, mast cells, the epithelium of the gastrointestinal and respiratory tracts, the kidneys and some sensory neurons (Hughes, 1997, 1999).
Although galectin-3 is predominantly located in the cytoplasm, it has also been detected in the nucleus, on the cell surface or in the extracellular environment, suggesting a multifunctionality of this molecule. Its extracellular location on the cell surface and in the extracellular milieu indicates its participation in cell–cell and cell–matrix adhesion (Krezslak and Lipinska, 2004).
In order to demonstrate the specificity of the labelling, based on literature data, we chose 3T3 Swiss Albino mouse fibroblasts as a galectin-3 expressing cell line (Moutsatsos et al., 1987; Hubert et al., 1995). D16 mouse mesoangioblast cells where considered as possible negative control.
A work by Moutsatsos et al. (1986) reported the presence of galectin-3 on the cell surface of 3T3 fibroblasts. A recent article from Guévremont et al. (2004) demonstrated an increased surface expression of galectin-3 in human chondrocytes from OA cartilage.
In our work ESEM immunolabelling will be also supported by biochemical evaluation, immunofluorescence and flow cytometry detection of galectin-3.
Materials and Methods
Chemicals
The following reagents and kits were used for biological tests: anti-galectin-3 rabbit polyclonal IgG (200 µg/ml; Santa Cruz Biotechnology, Santa Cruz, CA) antibody and goat anti-rabbit IgG-HRP conjugated (400 µg/ml; Santa Cruz Biotechnology); goat anti-mouse IgG-Alexa fluor 488 conjugated (2 mg/ml; Molecular Probes, Eugene, OR); PE-conjugated Mouse anti-human galectin-3 and PE-conjugated Mouse IgG1, Kappa Isotype control by BD Biosciences Pharmingen (San Diego, CA), 7-aminoactinomycin D by Molecular Probes.
Nanogold®—anti-Rabbit IgG (80 µg/ml; Nanoprobes, Yaphank, NY); Immun-Star HRP substrate kit (Bio-Rad, Milan, Italy); protease inhibitor cocktail (Sigma, Milan, Italy); LI Silver Enhancement Kit (Molecular Probes); 10 mm diameter polyethylene terephthalate (PET; Goodfellow, Huntington, UK) circular chips were employed as cell substrate for ESEM and fluorescent microscopy experiments.
Cell culture
Stock cultures of Swiss albino mouse 3T3 cells were maintained in Dulbecco's modified Eagle's medium (DMEM), supplemented with 10% foetal bovine serum and 1 mM sodium pyruvate in humidified 5% CO2, 95% air atmosphere at 37°C.
D16 mouse mesoangioblast cells derived from dorsal aorta of mouse embryo (Minasi et al., 2002) were maintained in DMEM, supplemented with 10% foetal bovine serum and 1 mM sodium pyruvate in humidified 5% CO2, 95% air atmosphere at 37°C.
For experimental purposes, Swiss 3T3 and D16 mouse mesoangioblast cells were plated on 10 mm diameter PET dishes at 1 × 104 cells/dish in DMEM containing 1 mM sodium pyruvate, 10% foetal bovine serum and used after 1 day of seeding.
Biochemical evaluation of galectin-3
For Biochemical evaluation of galectin-3 both cell types, 3T3 and D16 were harvested with a scraper in ice and lysated in 0.2 ml of ice-cold RIPA buffer with 50 mM HEPES, pH 7.4, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 150 mM NaCl, 10% glycerol, 1.5 mM MgCl2, 1 mM EGTA, 1 mM sodium orthovanadate, 10 mM sodium pyrophosphate, 100 mM NaF, and 1 mM phenylmethylsulfonyl fluoride and protease inhibitor cocktail from Sigma as suggested by the supplier.
Lysates were clarified by centrifugation at 15,000 rpm for10 min and the pellets discarded. After centrifugation, supernatants were transferred to fresh tubes, then to protein assay (Bio-Rad). Successively, samples were normalised for protein concentration and precipitated in trichloroacetic acid (TCA) 30% (4°C, 15 min). TCA precipitates were rinsed three times with ethanol and resuspended in sample buffer (200 mM Tris-HCl, pH 6.8, 1 mM EDTA, 6% SDS, 4% 2-mercaptoethanol, 10% glycerol) boiled 5 min and resolved by 12% SDS–PAGE.
After SDS–PAGE, proteins were transferred to polyvinylidene difluoride (PVDF) membranes. Membranes were blocked for 1 h with 5% non-fat dried milk in Tris buffer saline + Tween-20 0.05% pH 7.2 (TBST) and incubated for 2 h at 4°C with anti-galectin-3 polyclonal antibody (1/200 diluted). The membranes were rinsed three times with TBS–0.05% Tween-20, incubated with secondary antibodies (horseradish peroxidase-conjugated goat anti-rabbit IgG antibody) diluted 1:5,000 in phosphate buffer solution (PBS), for 1 h at room temperature. After rinsing three times with TBS Tween-20 0.05%, the immunoreactive bands were visualised with enhanced chemiluminescence detection reagents (ECL, Bio-Rad, Hercules, CA). Protein signals on PVDF membranes were assessed with the ChemiDoc™ XRS imaging densitometer (Bio-Rad, CA), using the Quantity One software program (Bio-Rad, CA).
In order to verify the presence of Gal-3 in the cell membrane of 3T3 Swiss albino Mouse Fibroblasts, we used a procedure for the cell membrane isolation (Guévremont et al., 2004).
Briefly, 3T3 Swiss albino were recovered with a cell scraper in 50 mM Tris-HCl, 150 mM NaCl, pH 7.5 buffer.
Cells were resuspended in ice-cold RIPA buffer with 50 mM HEPES, pH 7.4, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 150 mM NaCl, 10% glycerol, 1.5 mM MgCl2, 1 mM EGTA, 1 mM sodium orthovanadate, 10 mM sodium pyrophosphate, 100 mM NaF, and 1 mM phenylmethylsulfonyl fluoride and protease inhibitor cocktail from Sigma as suggested by the supplier, and submitted to freeze-thaw cycle, sonicated at 40 V (Branson 1510, Bransonic, Danbury, CT) for 20 sec, and then centrifuged at 200g. Pellets containing nuclei were discarded while supernatants were centrifuged at 100,000g. After this step, pellets contained membrane enriched fractions, while supernatants were composed of cytosol. Pellets were then resuspended in 0.2 ml of ice-cold RIPA buffer. Both pellets and supernatants were submitted to Western blot procedure for Gal-3 determination as described before. Each experiment was performed in duplicate.
Immunofluorescence and flow cytometry detection of galectin-3
Swiss 3T3 and D16 cells seeded at 1 × 104 cell/disc were grown for 1 day on PET discs, then rinsed with PBS and fixed for 5 min with pure methanol precooled at −20°C, permeabilised with 0.1% Triton X-100 in PBS for 10 min, and blocked for 20 min in 1% bovine serum albumin (BSA) in PBS. After three washes in PBS (5 min each), immunofluorescence staining was carried out incubating samples with anti-galectin-3 antibody (1 µg/ml) for 1 h. After three washes in PBS (5 min each), 488 Alexa fluor-conjugated goat anti-rabbit IgG antibody (1/2,000 diluted) was added for 1 h. After three washings in PBS, samples were mounted on glass coverslip and observed in fluorescence using a Nikon Eclipse Te 2000-s immunofluorescence microscope. Images were collected with a 40× (Nikon LWD Plan) lens.
For a better visualisation of membrane galectin-3 staining in 3T3 Swiss albino mouse fibroblasts, laser confocal images were obtained using a Nikon Confocal Microscope C1 equipped with a EZ-C1 Software for data acquisition and analysis. Labelling procedure was performed as described before, but to assure the non-permeabilisation, cell fixation was carried out with a 4% paraformaldehyde solution in PBS for 10 min and Triton X-100 was avoided.
For flow cytometry detection of galectin-3 in 3T3 Swiss albino mouse fibroblasts, cells were briefly trypsinised, resuspended 4 × 105/mL for sample, rinsed in PBS, blocked for 30 min in PBS containing 0.1% BSA on ice and incubated with phycoerythrin (PE) conjugated anti-galectin-3 antibody (dilution 1/50) for 30 min on ice. Non-immune serum was used at the same concentration and served as control. After centrifugation (1,200 rpm) and three times washing in PBS (5 min each) cells were resuspended in PBS, then samples were read in a Becton Dickinson FACS Calibur (equipped with ModFit LT software). A cell non-permeable stain, 7-aminoactinomycin D (7-AAD) was added 5 min before reading to exclude permeabilised cells from the analysis, as it penetrates into the cells only when the membrane is disrupted.
Galectin-3 Immunogold labelling
- (1)
Swiss 3T3 and D16 cells seeded at 1 × 104 cell/disc were grown for 1 day on PET discs.
- (2)
Cells were rinsed twice with PBS (preheated at 37°C) and fixed for 3 min with pure methanol precooled at −20°C.
- (3)
Samples were washed twice with TBST for 5 min.
- (4)
Blocking with 1% normal serum and 5% non-fat dried Milk TBST was carried out for 40 min.
- (5)
Samples were washed twice with TBST for 5 min.
- (6)
Incubation with anti-galectin-3 rabbit polyclonal antibody, at variable dilutions (according to manufacturer prescriptions from 1:40 upwards) in TBST, was carried out for 1 h at room temperature.
- (7)
Samples were washed three times with TBST for 5 min.
- (8)
Incubation with anti-rabbit Nanogold conjugate antibody, at variable dilutions (according to manufacturer prescriptions from 1:50 upwards) in 5% non-fat dried milk TBST, was carried out for 1 h at room temperature.
- (9)
Samples were washed three times with TBST for 10 min.
- (10)
Samples were washed three times with distilled H2O for 10 min.
- (11)
Silver Enhancement reaction was carried out according to manufacturer prescriptions, in the recommended range different temperature and time conditions were assayed to obtain a cleaner signal. In this respect the reaction was stopped with 1% acetic acid.
- (12)
Samples were washed three times with distilled H2O for 10 min.
This protocol was adjusted on 3T3; different primary and secondary antibody concentrations were assayed in combination to define the optimum conditions. First step was to identify, for constant Nanogold conjugate concentration, the highest anti-galectin-3 antibody dilution producing high density labelling on the cell surface. As this method allows us to detect only the signal generated by the Nanogold reagent, improper primary interactions must be verified via external controls, such as galectin-3 lacking cells. Then Nanogold probe right dilution had to be determined first, samples processed omitting primary antibody served as internal controls for specificity. As additional purpose semi-quantitative evaluation of labelling intensity was performed both in treated and control samples, in order to test its suitability to describe the variation of probe binding specificity.
The adjusted protocol was then applied also to D16 cells, to definitively prove the reliability of the method. Each experiment was repeated at least three times.
Environmental scanning electron microscopy
Sample was imaged in ESEM mode at 15 kV, using Peltier Stage and gaseous secondary electron detector (GSED). The chamber parameters were settled to 5°C temperature and 3.60 Torr pressure.
Experiments were carried out in triplicate, for each secondary antibody dilution a database of both low and high magnification images was recorded.
No Critical Point Dryer or alcohol dehydration was carried out before ESEM.
Image analysis
Nanoparticles size and number evaluation was performed with XT Image Analysys Software (S.I.S F.E.I.). Nanoparticles size was calculated using the ‘arbitrary distance’ measuring option. Nanoparticles count was performed using the ‘touch-count’ option on the database images, choosing 10 randomly selected cell surface areas of 5 × 5 µm for each experiment. To achieve a better count acquisition we drew a 1 µm2 mesh grid on each selected area. High magnification images (12,000×, working distance 7.5 mm) were used as they allowed to distinguish label nanoparticles from precipitates, both for size and shape.
Lower magnification images were used to define the distribution of the label on single cells (3,000–6,000×) or all over sample areas.
Results
Biochemical evaluation of galectin-3
In Figure 1 we show a typical result from Western blot procedure.
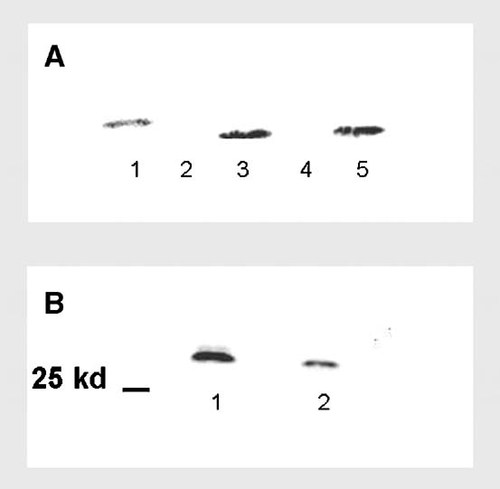
Western blotting analysis of galectin-3 showing results from two different experiments. a: Lane 1 = standard, lanes 2 and 4 = D16 cell lysate, lanes 3 and 5 = 3T3 cell lysate. b: Lane 1 = membrane enriched fraction, lane 2 = whole cell lysate.
In the first experiment (Fig. 1a) we compared Gal-3 expression in 3T3 fibroblasts and D16 cells. Only 3T3 cells showed the proteic band (about 30 kDa) of galectin-3 protein while the signal was absent in D16 cells.
These data demonstrated that 3T3 Swiss albino mouse fibroblasts express galectin-3 protein, while D16 cells could be used as negative control (galectin-3 absence).
Further biochemical analyses on fractionated cell lysate (Fig. 1b), showed that this protein is localised both in the cytosol and on the cell surface of 3T3 fibroblasts. In particular the higher signal intensity of the membrane enriched fraction demonstrated the presence of Gal-3 at cell membrane level.
Immunofluorescence and flow cytometry detection of galectin-3
Figure 2 shows fluorescence pictures of 3T3 Swiss albino mouse fibroblasts and D16 mesoangioblast mouse cells. As depicted in part A the fluorescence signal of galectin-3 was located in cytoplasm, nucleus and probably cell membrane. The signal was more intense in the middle part of the cells, where the nuclei are located. On the contrary, part B shows the absence of galectin-3 fluorescence in D16 cells, as the observed signal was comparable with background.
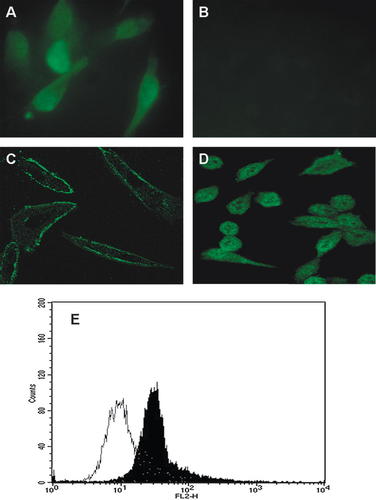
Immunofluorescence and flow cytometry detection of galectin-3 in Swiss albino mouse fibroblasts. Part A: Fibroblasts fluorescence image, permeabilised cells, magnification 40×. Part B: D16 mouse mesoangioblast cells fluorescence image, permeabilised cells, magnification 40×. Part C: fibroblasts laser confocal image, non-permeabilised cells, magnification 40×. Part D: fibroblasts laser confocal image, permeabilised cells, magnification 40×. Part E: flow cytometry, non-permeabilised cells immunostained with a PE-conjugate monoclonal anti-galectin-3 (filled histogram) or with non-immune serum (open histogram).
In order to demonstrate the presence of membrane galectin-3, we performed a confocal laser observation. Clearly, as showed in part C of Figure 2, non-permeabilised fibroblasts were labelled on the edges, while permeabilised cells (part D) showed a stronger signal spread over the whole section, excepted some small round zones, that probably correspond to nucleoli. This data indicate that in 3T3 cells, galectin-3 is located on the cell surface, as well as in the cytoplasm and in the nucleus. Further evidence was provided by FACS analysis.
Results showed that only 7% of total cells had a disrupted membrane and were then excluded from the analysis. As showed in Figure 2 (part E) we observed a shift of the PE signal in presence of anti-galectin-3 antibody compared with the cells treated with non-immune serum.
Galectin-3 immunolabelling in ESEM
Galectin-3 stain was detected with considerable intensity on the surface of 3T3 fibroblasts.
ESEM allowed to image the label particles and the cell surface with its morphological details in conditions of high hydration, 3.60 Torr at 5°C corresponding to 60% relative humidity.
The labelling was evident and clearly identifiable in the contest of the surrounding cell surface, thanks to the mixed SE-BSE signal detected by the GSED.
Methanol fixation was very effective, as it restricted antigen collapse and, at the same time, avoided the accumulation of electrical charge throughout the sample typical of aldheyde treatment.
As silver enhancement reaction optimal conditions (15 min at 6°C) were taken those that led to the formation of round shaped, easily detectable particles with average diameter = 58.4 nm and, at the same time, produced less precipitates. Occasional particles above 100 nm were considered self-nucleation precipitates and thus not taken in count in the semi-quantitative analysis. Longer reaction times increased the diameter of the particles, reducing the needed magnification, but raised self-nucleation interactions between silver ions.
Dilution 1:200 for primary antibody was chosen, because it resulted to give still an intense staining compared with lower dilutions, while proceeding further we observed an evident signal weakening.
The concentration of secondary antibody strongly affected the number of particles observed in the samples. In order to find the optimal concentration of the secondary antibody, we carried out experiments using different dilutions of the gold conjugate. Dilutions less than 1/200 resulted in a extremely abundant particle deposition, so this was fixed as lower threshold. The optimal dilution was 1/600 as it represented the lower dilution which resulted in aspecific interactions removal, keeping still high the informational content of the signal.
Figure 3 shows ESEM-IGL results on 3T3 Swiss Albino Mouse Fibroblasts at different secondary antibody dilutions, 1/200 (parts A and B) and 1/600 (parts C and D), B and D are the labelled samples, A and C are the controls.
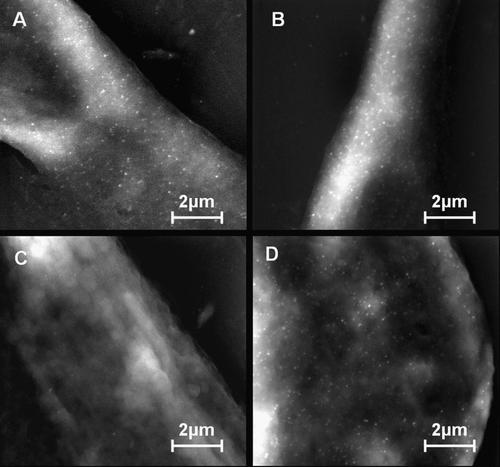
Immunogold labelling of galectin-3 on 3T3 Swiss albino mouse fibroblasts with 1/200 diluted primary antibody at different conjugate dilutions. A,B: 1–200; (C,D) 1–600 (A–C are controls without primary anti-galectin-3 antibody).
Galectin-3 distribution was widespread and homogeneous all over the 3T3 cells surface; particles were also detected in the deposited extracellular matrix, but not on the PET substrate.
Semi-quantitative analysis was used to study the relation between labelling specificity and probe concentration. Figure 4 shows the histogram reporting the number of gold particles observed in the samples and in the controls (using secondary antibody only) at three different antibody dilutions (1/200, 1/400, 1/600).
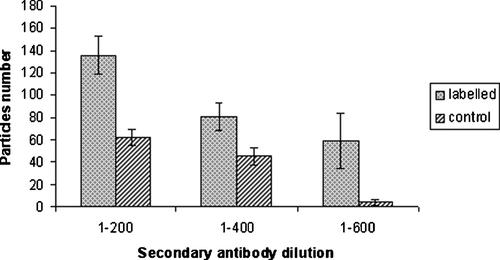
Semi-quantitative analysis of galectin-3 staining particles on the cell surface of 3T3 Swiss albino mouse fibroblasts. Different Nanogold conjugate dilutions were tested with constant primary antibody dilution (1/200).
Going from 1/200 to 1/600 the number of particles decreased both in labelled samples and in controls.
In the labelled sample series there is a nearly proportional correlation between the number of particles decrease and the secondary antibody dilution factor. However the number of particles observed in the controls, for 1/200 and 1/400 dilutions, is more constant compared to the labelled samples, while for 1/600 it considerably falls down.
Therefore increasing dilution up to 1/600, the difference between labelled and control samples became huge. Using this concentration we are sure that the observed label particles originate only from specific interactions with primary antibody. Moreover Figure 4 shows the standard deviation (SD) calculated for all sample data groups. Higher values were obtained in the labelled samples, compared to the corresponding controls, the higher SD value was recorded for 1–600 labelled samples. When we compare the number of particles observed for the last two antibody dilutions (1/400 and 1/600), the differences between the labelled samples are not significant.
The labelling experiment on galectin-3 negative D16 cells, was performed using the same protocol, with 1/200 primary and 1/600 diluted secondary antibody. No gold particle formation was observed on the cell surface nor in the extracellular matrix, validating the method and demonstrating the highly specific nature of primary antibody interaction with the target antigen and the Nanogold conjugate.
ESEM images of labelled D16 cells have not been reported, because they only show a ‘naked’ cell surface, just as the control in Figure 3 part C.
Conclusive experiments were performed to test if, increasing primary antibody concentration we could achieve an higher labelling yield.
Figure 5 shows the results from different combinations, also resuming 1/200 result from the previous experiment. Primary antibody 1/150 dilution actually resulted in particle number increase compared to 1/200 sample; but, as both exhibit high SD values, the difference was not significant. The same concentration did still not generate any particle on D16 cells, that served as external control, so it could be used in alternative to 1/200. Dilution 1/100 gave a significatively higher labelling signal, but on D16 some particles were observed. Finally primary antibody dilution above 1–200 resulted in signal weakening, as predicted.
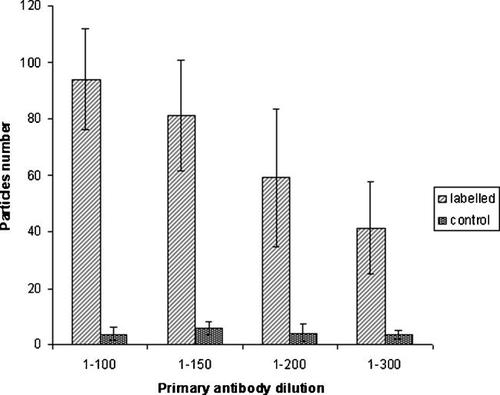
Semi-quantitative analysis of galectin-3 staining particles on the cell surface of 3T3 Swiss albino mouse fibroblasts. Different primary antibody dilutions were tested with constant Nanogold conjugate dilution (1/600).
Discussion
The expression of galectin-3 in 3T3 fibroblasts and its absence in D16 cells were demonstrated unanimously by Western blotting, fluorescence microscopy, flow cytometry and ESEM-IGL. In particular, all the obtained results matched in demonstrating the expression of this protein on the cell surface.
In our previous experimental results we achieved the best ESEM imaging conditions around 70% relative humidity (r.h.) for cell monolayers (Muscariello et al., 2005). In this work we demonstrated that cell integrity and immunophenotypic characteristics are still preserved under conditions of lower r.h.
Thermodynamic and kinetic arguments show that humidity under 100% corresponds to metastable states suitable for stabilizing hydrated biological tissues and cells. This argument has been extensively discussed by Stokes (2003) in a brilliant review about the physical principles involved in ESEM technology. Real aqueous phases, such as those found in the interiors of mammalian cells, are neither dilute nor ideal: macromolecules such as proteins and polysaccharides interact strongly with water molecules (Ellis, 2001). Tai and Tang (2001) empirically noted that, in biological samples, lowering of the equilibrium vapour pressure at humidities of ca. 90% occurs. Additional lowering of the chamber pressure (20–25%) could be introduced due to kinetic properties of the physiological solutions, thus specimens tolerate slowly dehydrating conditions in a finite time (Stokes, 2003).
In ESEM images of 3T3 fibroblasts, galectin-3 showed a quite uniform distribution all over the cell surface, while the fluorescence stain resulted to be more intense on the nuclear zone.
Laser confocal imaging gave us more precise informations on the sub-cellular distribution of this protein.
The presence of galectin-3 on the cell surface, as well as in the cytoplasm and nucleus, was confirmed.
The cell surface localised galectin-3 seems to represent only a smaller part of the total. Nuclear fluorescence was evident, being excluded from dark zones that probably correspond to nucleoli.
It has been shown that in 3T3 mouse fibroblasts, the nuclear versus cytoplasmic distribution of this protein depended on the proliferation state of the analysed cells. In quiescent cultures of fibroblasts, galectin-3 was predominantly cytoplasmic; however, proliferating cultures of the same cells showed intense nuclear staining for this protein (Moutsatsos et al., 1987; Hubert et al., 1995).
Owing to its affinity for polylactosamine glycans, galectin-3 binds to glycosylated extracellular matrix components, including laminin, fibronectin, tenascin and Mac-2 binding protein (Woo et al., 1990; Probstmeier et al., 1995). Some cell-surface adhesion molecules, like integrins, are also ligands for galectin-3. Preliminary evidence suggests that galectins, through binding to the extracellular domains of one or both sub-units of an integrin, may positively or negatively modulate integrin activation, and affect binding with extracellular ligands (Hughes, 2001). Despite to the specific binding of galectin-3 to glycoconjugates, there are still controversies whether galectins facilitate or inhibit cell adhesion.
On the whole the role of this protein in determining the cell fate seems to be highly dependent on the tissue and on the metabolic state (Dumic et al., 2006).
The semi-quantitative analysis results demonstrate the dramatic effect of secondary antibody concentration on the immunogold staining process. The lower SD values calculated for control samples, indicate that the observed staining pattern is due to a non-specific interaction background formation and that it is not affected by the real features of the cells.
On the contrary the specific stain signal shows much more differences, as the expression of galectin-3 actually depends on the developing cell cycle stage.
Indeed the differences become more evident, and the SD values higher, when the antibody dilution causes an almost complete background lowering. This confirms that removing the background, the focus of this work, it is a main importance issue, even if very often underestimated.
Our experience confirms that background formation could occur mainly for two reasons, incorrect ion interactions during silver enhancement and non-specific binding of the conjugate antibody.
The primary antibody concentration had less dramatic effect on the background formation, except for very high concentrations, but it affected clearly the labelling yield.
Some authors have pointed out before that in immunogold localisation of antigen via electron microscopy, advance knowledge regarding the presence of antigen, as well as verification of primary antibody/conjugate activity, is highly desirable (Kaur et al., 2002). Based on many scientific opinions and on our own experience, ESEM represents a very important tool in cell biology. We suggest that it also provides an ideal context for IGL procedures. The absence of complex sample preparation protocols reduces greatly the risk of antigen loss and background diffusion, the lack of metal coating allows to identify and characterise the staining particles with great accuracy. We achieved this using only the GSED, but maybe the use of a BSE detector, still in ESEM modality, could bring a great improvement of the images quality.
In conclusion we present a reliable and quick method for identification/localisation of galectin-3 on the cell surface, that allows to preserve unaltered the immunophenotypic characteristics, and thus could be advantageous also for other antigens with similar sub-cellular localisation.
Furthermore the semi-quantitative analysis could be used to relate different levels of antigen expression on the cell surface in relation with different physiological stages.




