In vitro activated human T lymphocytes very efficiently attach to allogenic multipotent mesenchymal stromal cells and transmigrate under them
Abstract
The regulatory effect of human multipotent mesenchymal stromal cells (MSC) on allogenic T lymphocytes is extremely powerful and of important clinical relevance, but the mechanisms underlying this process are not fully elucidated. We report here that T lymphocytes activated with a sub-mitogenic stimulus such as phytohemaglutinin alone (PHA), or with mitogenic stimuli such as PHA + interleukin-2 (P-IL2), or immobilized anti-CD3 + anti-CD28 mAb (a3-28), tightly bound allogenic MSC and transmigrated within 4 h under them, where they remained for approximately 60 h. Allogenic MSC induced T cell proliferation in cultures containing sub-mitogenic PHA concentrations, and inhibited the mitogenic effect of P-IL2 or a3-28. Anti-γ-IFN mAb or L-tryptophan complementation partially restored proliferation in P-IL2 and a3-28 cultures, whereby γ-IFN-synthesizing CD3+ cells were detectable. MSC-lymphocyte contact hindrance using transwells abrogated proliferation in PHA cultures, restored it integrally in P-IL2 cultures, and partially in a3-28 cultures. These data suggest that MSC-induced T lymphocyte regulation results from the combination of various processes. Allogenic cell–cell contact, as demonstrated by the PHA co-cultures is per se stimulatory, whereas γ-IFN synthesized by activated T lymphocytes, which activates indolamine 2,3-dioxygenase in MSC, and L-tryptophan depletion, which is induced by this enzyme, are inhibitory. Transmigration is nevertheless pivotal for the establishment of the inhibition by these mediators because it targets lymphocytes under the stroma in small extracellular spaces surrounded by MSC, where L-tryptophan is efficiently destroyed, leading to T lymphocyte proliferation arrest. In conclusion lymphocyte transmigration under allogenic MSC potentiates the inhibitory effect of soluble mediators generated by these cells. J. Cell. Physiol. 214: 588–594, 2008. © 2007 Wiley-Liss, Inc.
Multipotent mesenchymal stromal cells (MSC) have originally been identified by their ability to differentiate into many mesenchymal lineages (Friedenstein, 1961; Pittenger et al., 1999; Horwitz et al., 2005), a property that makes them promising tools for cellular therapies (Le Blanc and Ringden, 2005). However their most amazing property is their ability to inhibit allogenic T lymphocyte proliferation, as recently illustrated by clinical trials demonstrating that acute graft-versus-host disease (GVHD) subsequent to allogenic hematopoietic stem cell (HSC) grafting could be overruled by the infusion in the leukemic patient of MSC provided by a third party donor (Ringden et al., 2006).
Though it is now firmly established that MSC inhibition of T lymphocytes is mediated via a MHC-independent process (Le Blanc et al., 2003; Tse et al., 2003) the molecular mediators controlling such an inhibition are not fully identified yet. Soluble factors seem to be prominently involved in MSC-induced inhibition in humans, and increasing evidence indicates that γ-IFN produced by activated T lymphocytes is involved in the process. By contrast, the identification of other factors remains controversial (Di Nicola et al., 2002; Tse et al., 2003; Meisel et al., 2004; Le Blanc et al., 2004a; Krampera et al., 2005, 2006; Plumas et al., 2005). In addition the extensive regulatory property of MSC may induce a generalized blockade of T lymphocytes. Mice models indeed suggest that some tumors, which are spontaneously eliminated by the immune system can escape control if implanted in animals simultaneously with MSC (Djouad et al., 2003). Altogether these observations indicate that a better understanding of MSC-T lymphocyte interaction is required to ensure patient safety and therapeutic efficiency.
In this study we exclusively used unirradiated human MSC amplified in a medium devoid of animal serum (Doucet et al., 2005; Muller et al., 2006), and exposed purified CD3+ human T lymphocytes to three “standard” stimulations including: (i) phytohemaglutinin alone (PHA), which mimics a mild inflammatory situation that primes T lymphocytes (Modiano et al., 1999) but does not allow their proliferation (Lipsky et al., 1976); (ii) PHA plus interleukin-2 (P-IL2), which mimics an active, but non-specific inflammatory environment permissive to T lymphocyte proliferation; and (iii) microbeads coated with anti-CD3 plus anti-CD28 mAb (a3-28), which should mimic the situation whereby a T cell is maximally stimulated by a fully mature antigen presenting cell loaded with the cognate peptide. These investigations showed that the regulatory effect of MSC upon allogenic T lymphocyte proliferation resulted from the combination of several processes comprising cell contact and soluble mediators. Soluble factors mediated T cell growth arrest, whereas cell–cell interactions initially delivered a stimulatory signal to T lymphocytes. However in situations of intense T lymphocyte stimulation, cellular interactions were associated with growth arrest. This is consistent with the hypothesis that in this latter situation cell contact provides to activated T cells the anchor required for their transmigration into spaces under the MSC that become inhibitory due to their depletion in L-tryptophan (tryp) provoked by the γ-IFN activated indolamine 2,3-dioxygenase (IDO) in MSC.
Materials and Methods
Cytokines, chemicals and monoclonal antibodies
Dulbecco's modified Eagle medium (DMEM), Iscove's modified Dulbecco's medium (IMDM), penicillin–streptomycin and trypsin–EDTA solution were from Gibco BRL, Paisley, UK. Interleukin-2 was from Roche Pharma, Reinach, Swizerland; PHA was from Sigma–Aldrich, Buchs, Switzerland. Five (and 6)-carboxy fluorescein diacetate, succinimyl ester (CFSE) was from Molecular Probes Europe, Leiden, The Nederlands. FAZER PE enhancing kit was from Miltenyi Biotech, Bergisch Gladbach, Germany.
FITC-labeled antibodies
Anti-CD45 (clone J33 mIgG1) was from Immunotech, Marseille, France; anti-HLA-ABC (clone G46-2.6 mIgG1) from BD Biosciences, San Diego, CA; anti-CD105 (clone 8 E11, mIgM) from Diaclone Research, Besançon, France; anti-CD80 (clone 2D10.4, mIgG1) from e-Biosciences, San Diego, CA.
RPE-labeled antibodies
Anti-CD3 (clone UCHT-1, mIgG1), and anti-CD14 (clone Tuk4, mIgG2a) were from DakoCytomation Glostrup, Denmark; anti-CD34 (clone 8G12 mIgG1), and anti-CD11b (clone ICRF44, mIgG1) from BD Biosciences; anti CD86 (clone BU63 mIgG1) from Ancell Corp., Bayport, MN.
Biotin-labeled antibodies
Anti-γ-IFN (clone B27) was from Serotec, Oxford, UK; anti-CD90 (clone 5E10 mIgG1), and anti-HLA-DR (clone B8.12.2 mIgG2b) from Immunotech; anti-CD117 (clone 104D2 mIgG1) and anti-CD19 (clone SJ25-C1, mIgG1) from Caltag, Burlingame, CA. Monoclonal isotype controls: FITC, PE, and biotin-labeled mIgG1, mIgG2a, mIgG2b, and mIgM were from Dakocytomation. Alexa fluor488-labeled anti-FITC (mIgG fraction) was from Molecular probes; functional grade, blocking anti-γ-IFN (clone NIB-42, mIgG1) and its control were from e-Bioscience.
MSC purification, amplification and in vitro differentiation
Femoral heads and condyles were collected during total hip or knee replacements, according to the guidelines of the local ethics committee, and prepared and amplified as previously reported (Suva et al., 2004) with the exception that cells were cultured in medium complemented with 5% human platelet supernatant (HPS) (Doucet et al., 2005) instead of 10% foetal calf serum (FCS) plus recombinant platelet-derived growth factor. After 3–4 weeks, cell phenotype was analyzed by flow cytometry and cells were tested for multilineage differentiation (Jaiswal et al., 1997; Mackay et al., 1998; Pittenger et al., 1999) and for regulatory effect on T lymphocytes (see below).
T lymphocytes purification and co-cultures with allogenic MSC
CD3+ T lymphocytes were obtained from peripheral blood of healthy donors after Ficoll-Paque gradient and negative selection using the Dynal T cell isolation kit (Dynal Biotech ASA, Oslo, Norway), following the manufacturer's instructions. Immunobead-purified T lymphocytes were labeled with 80 nM of CFSE for 10 min at 37°C in Hank's balanced salt solution (Lyons and Parish, 1994) prior to co-culture with allogenic MSC. Higher concentrations of CFSE did not increase the cell staining and were found cytotoxic in our hands. MSC were seeded at 5 × 104 cells in 24 well plates in 500 µl of IMDM supplemented with 5% HPS 24–48 h before the assays. Assays were run in fresh IMDM containing 10% FCS with 5 × 104 allogenic lymphocytes per well. Co-cultures were complemented or not with PHA 5 µg/ml (PHA), PHA 10 µg/ml plus IL-2 100 U/ml (P-IL2) or with beads coated with a3-28 (Dynal Biotech) in a ratio of 0.5 bead per T lymphocyte originally seeded. When transwells were used (Costar, Corning incorporated, Corning, NY, pore size 0.4 µm) over the 24-well plates, total culture volume was extended to 700 µl. Culturing T lymphocytes in transwells in absence of MSC did not alter the proliferation induced by a3-28 or P-IL2 (not shown). When used, blocking anti-γ-IFN mAb and its control were adjusted to 10 µg/ml. Cell proliferation was assessed after 5 days of co-culture if not specified otherwise. T lymphocytes were recovered by gentle pipetting of the culture supernatants. When recovered at an earlier time (i.e., 4–48 h) T lymphocytes were obtained after trypsination of the adherent layer with a solution of 0.05% of trypsin in EDTA, for 1 min at 37°C. When intracellular cytokine determination by flow cytometry was performed, 5 µg/ml of Brefeldin A were added 4 h before the termination of the cultures.
Flow cytometry analysis
Cell surface labeling
Fifty thousands cells were incubated at 4°C for 15 min in 50 µl of a solution of phosphate-buffered saline, 2% FCS, 0.02% sodium azide containing 20% of human serum AB+ and further processed as reported (Suva et al., 2004), with the exception of the CD105 staining that required a further stage of amplification using the Alexa fluor488-labeled anti-FITC. The appropriate isotype control comprising such a secondary reagent was used in this case. At the end of the staining procedure cells were suspended in phosphate-buffered saline, 2% FCS, 0.02% sodium azide and 10 µg/ml of 7-amino-actinomycin D to exclude dead and apoptotic cells from the analysis (Schmid et al., 1994). Analyses were run on a FACScan or FACScalibur analyzer from BD Biosciences for three and four color determination, respectively.
Intracellular detection of γ-IFN
Cultured cells were processed using the FIX-PERM kit of Pharmingen, according to the supplier's instruction. Cells were simultaneously labeled with biotin-labeled anti-γ-IFN plus streptavidin-PE and FITC-labeled anti-CD45 mAb to exclude eventual contaminating MSC from the analyses.
Video recording
Co-cultures of allogenic MSC and T lymphocytes were performed in 35 mm Petri dishes. P-IL2 was added or not at the onset of the cultures and cells were monitored for 48 h at 37°C with a Zeiss Axiovert S100TV microscope using a 10× plan-Neofluar 0.3 NA objective (Carl Zeiss AG, Feldbach, Switzerland) coupled with a cooled, 12 bits TE/CCD interlined Coolsnap HQ camera (Photometrics, Ropper Scientific, Trenton, NJ). Images were captured every 5 min and analysis was performed with the Metamorph 6.3 software (Molecular Devices Corp., Downington, PA).
Confocal laser scanning microscopy
Freshly purified T cells were labeled with CFSE as above, and further stained with FITC-labeled anti-CD45 mAb. Lymphocytes were then incubated overnight in 35 mm Petri dishes over allogenic MSC in presence of P-IL2. The next day, cultures were gently washed and stained with biotin-labeled anti-CD90 mAb plus PE-labeled strepavidin, and this signal was further enhanced using the FAZER PE-enhancing kit following the manufacturer's instructions. Cell cultures were equilibrated in PBS containing 2% FCS and maintained on ice until analysis. Z-stack confocal images acquisition was performed directly in the Petri dish using an upright microscope (Axiolmager ZI, Carl Zeiss AG, Feldbach, Switzerland) equipped with a Zeiss LSM 510 Meta confocal scanhead (Carl Zeiss AG). Fluorescence images were collected by dipping a 63× 0.95 NA Zeiss Achroplan water immersion objective into the PBS buffer. The two fluorophores were sequentially excited using the 488 nm line of the 30 mW multi-lines argon laser and the 561 nm line of the 15 mW DPSS laser respectively, and each fluorescence emission was detected in two different channels (BP 505-550 and LP 575 emission filters, pinholes size set to 184 and 172 µm, respectively, with optical sections of 0.26 µm × 0.26 µm × 0.84 µm resolution for each channel). Altogether this methodology allowed generating 21 adjacent sections following the z-axis comprising the totality of the cell layer thickness.
Statistical analysis
Means and SDs are shown. Statistical significance was calculated using Mann–Whitney non-parametric rank test.
Results
MSC amplified in vitro with human platelet supernatant (HPS) inhibit or induce CD3+ allogenic T lymphocyte proliferation
Human MSC amplified in IMDM with 5% HPS exhibited a typical fibroblastic-like morphology. FACS analyses (mean ± SD of five experiments) showed that they were CD90+ (100 ± 0.5%), CD105+ (83 ± 7%), HLA-ABC+ (98 ± 1%), HLA-DRlow or negative (13 ± 24%), and negative for CD117, CD34, CD45, CD11b, CD19, CD14, CD80, and CD86. Moreover, when induced with appropriate media, HPS-amplified MSC differentiated into adipocytes, osteoblasts, and chondrocytes (Fig. 1a,b).
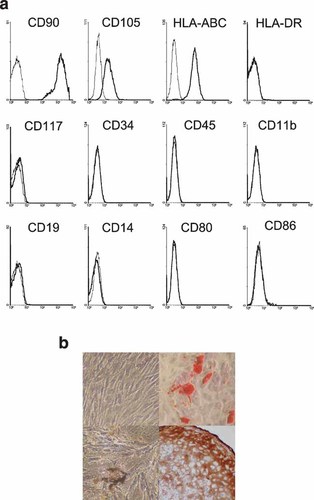
Phenotype and in vitro differentiation of MSC amplified in human platelet supernatant. a: FACS analysis of relevant markers of MSC amplified in 5% HPS. One experiment representative of 5 is shown. b: In vitro differentiation of MSC. Upper left part: amplified, undifferentiated human MSC exhibiting the typical fibroblast-like morphology; upper right part: adipocytic differentiation; lower left part: osteoblastic differentiation; lower right part: chondrocytic differentiation.
Human peripheral blood CD3+ T lymphocytes (95 ± 4.5% purity, mean ± SD, n = 8) from healthy blood donors stimulated for 5 days with 5 µg/ml of PHA did not significantly proliferate compared to unstimulated cells whereas the majority of CD3+ lymphocytes stimulated with P-IL2 or a3-28 did so (Fig. 2a). Whereas allogenic MSC did not initiate the proliferation of unstimulated T cells, they significantly induced that of PHA-primed lymphocytes and partially prevented the mitogenic effect of a3-28 or P-IL2 (P < 0.01, Fig. 2a).
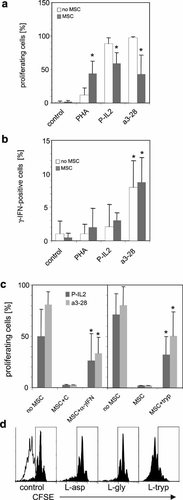
Modulation of T lymphocyte proliferation by allogenic MSC amplified in vitro with HPS. a: CD3+ human T lymphocytes labeled with CFSE were incubated ± allogenic MSC either as such (control) or in presence of PHA, P-IL2, or a3-28 for 5 days. Retrospective proliferation was assessed by FACS. Mean percentages of proliferating cells ± SD of six values for PHA and 15 values for control, P-IL2 and a3-28 are shown. * Significantly different compared to cultures in absence of MSC (P < 0.01). b: CD3+ T lymphocytes were incubated ± allogenic MSC either as such (control) or in presence of PHA, P-IL2, or a3-28, and γ-IFN-synthesizing cell frequencies were assessed 20 h later by flow cytometry. Mean percentages ± SD of γ-IFN-synthesizing cell frequencies are shown. * Significantly different compared to non-stimulated T cells (P < 0.01; n equals 6, 4, 5, and 7 for no stimulation (control), PHA, P-IL2 and a3-28, respectively). c: Left part: Mean values ± SD of CD3+ T cell proliferation obtained after stimulation with PIL-2 or a3-28, ±MSC, with control mAb (C), or anti-γ-IFN mAb (α–γIFN). * Significantly different compared to control and cultures without MSC (P < 0.032, n = 5 for P-IL2, and P < 0.01, n = 8 for a3-28). The frequency of proliferating cells was set arbitrarily to approximately 2% in the control culture of each independent experiment. This gate was then used to measure the percentage of proliferating cells in other conditions. Right part: Mean values ± SD of lymphocyte proliferation with P-IL2 or a3-28 ± allogenic MSC, in a regular medium or in medium complemented with 200 µM of L-tryptophan (tryp). Proliferation threshold was set as above. * Significantly different compared to control and cultures without MSC (P < 0.056, n = 4, for P-IL2, and P < 0.009, n = 8 for a3-28). d: Specificity of L-tryptophan desinhibition. CFSE profiles obtained after cultures with P-IL2 in absence (white profile) or presence (black solid profiles) of allogenic MSC, in cultures supplemented or not with 200 µM of L-asp, L-gly, or L-tryp.
These observations suggest that allogenic MSC amplified with HPS do not initiate, in the absence of exogenous stimulus, the proliferation of quiescent T cells but deliver a signal that is co-stimulatory when the initial exogenous stimulus is sub-mitogenic, and an inhibitory signal when the exogenous stimulus is mitogenic.
γ-IFN is synthesized by T lymphocytes in presence of allogenic MSC and is involved in the proliferation arrest
The frequency of γ-IFN-synthesizing CD3+ T lymphocytes was determined after 20 h of co-cultures with allogenic MSC. In absence of stimulus as well as in presence of PHA, γ-IFN+ cells were not detectable (0.5 ± 0.6%, and 2 ± 2.8% mean ± SD of six and four experiments, respectively). In co-cultures stimulated with P-IL2, γ-IFN-producing cells represented 3.0 ± 1.1% (n = 5, Fig. 2b), whereas with a3-28, they represented 8.7 ± 3.7% (n = 7, P < 0.01, significantly higher compared to all other conditions, see Fig. 2b). Moreover the frequency of γ-IFN+ cells was not significantly affected by the presence of MSC.
The addition of a blocking anti-γ-IFN monoclonal antibody in cultures on day 0 and 3 allowed a partial recovery of the proliferative activity of stimulated T lymphocytes. This effect was more efficient in cultures stimulated with a3-28 than in cultures stimulated with P-IL2 (P < 0.01 and P < 0.03, respectively, Fig. 2c, left part). Excess of L-tryptophan (200 µM) which, by contrast to other IDO inhibitors, spared the MSC layer, significantly restored, in the presence of MSC, mitogen-induced CD3+ T lymphocytes proliferation (Fig. 2c, right part). Tryp-induced desinhibition was specific because the excess of other amino acids such as L-asparagine or L-glycine did not prevent MSC-driven T lymphocyte proliferation inhibition (Fig. 2d).
Upon activation lymphocytes rapidly bind MSC and transmigrate under the stroma
Phase-contrast microscopy revealed that in stimulated co-cultures exclusively, dark cobblestone-like cells appeared after 4 h and remained visible for 24–60 h (Fig. 3a, left and middle part). These cells resembled to hematopoietic stem cells that tightly associate with, and often transmigrate under a hematopoiesis-supporting stroma (Ploemacher et al., 1989) (Fig. 3a, right part). Differential harvesting of the supernatant and adherent cell fractions after 4 h of co-culture showed that in absence of stimulus, T lymphocytes resided in the supernatant, whereas in the stimulated cultures they were exclusively rescued from the adherent trypsinized fraction (Fig. 3b). After 24 h of culture, this situation remained, as evidenced by the recovery of a CD3+ population exhibiting a small forward and side scatter from the trypsinized fraction (Fig. 3c). This tight, early adherence of T lymphocytes to MSC was reversible, since T lymphocyte harvesting after 5 days of culture as reported in Materials and Methods did not require trypsination.
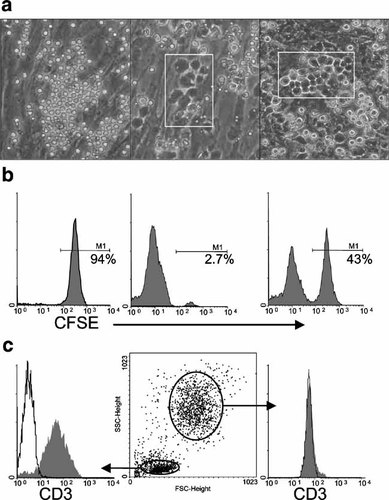
Activated T lymphocytes tightly bind MSC and modify their morphology. CD3+ T lymphocytes were cultured over allogenic MSC. a: Micrographs obtained with a phase contrast inverted microscope after 8 h of co-culture without stimulus (left), or with a3-28 (middle). CD34+ hematopoietic stem cells after 5 weeks of culture over the MS-5 stroma are shown for comparison (right, VK, unpublished). White rectangles emphasize the location of dark, cobblestone-like cells. These data are from one experiment representative of 5. Original magnification 100×. b,c: Differential harvesting of the co-cultures. b: Localization of CSFE-labeled T lymphocytes after 4 h of co-culture. Left part: no stimulus, supernatant fraction; central part: no stimulus, adherent fraction obtained after trypsination; right part: P-IL2 stimulation, adherent fraction. The threshold of CFSE signal was set according to CD3+CFSE+ T lymphocytes from the same batch cultured in absence of MSC. c: CD3 expression in the adherent phase of a co-culture stimulated for 24 h with P-IL2. Left part: gate on cells of small forward and side scatter (lymphocytes). Gray profile is CD3, open profile is isotype control. Medium part: gates used for this analysis. Right part: same analysis as before but gated on high FSC/SSC events (i.e., MSC). CD3 profile and isotypic control are superimposable. These data are from one experiment representative of 5.
Video recording of co-cultures during the first 48 h showed that unstimulated lymphocytes were extremely motile and remained spherical, whereas T lymphocytes stimulated with P-IL2 moved slowly over MSC, modified their morphology and rapidly migrated through the stromal layer (Fig. 4a,b, and movies of the first 4 h provided as supplementary data). Finally, confocal laser scanning microscopy indeed confirmed that activated T lymphocytes had migrated under the MSC after overnight incubation and were trapped between the MSC layer and the bottom of the culture vessel (Fig. 4c).
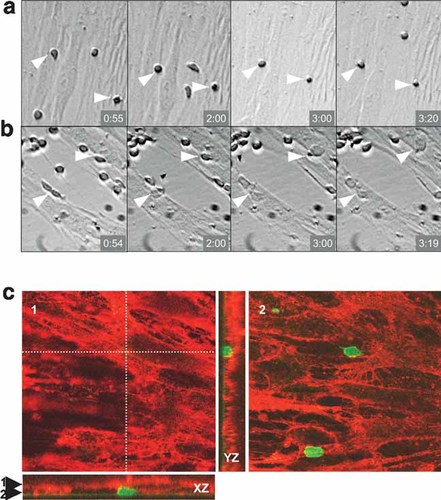
Activated T lymphocytes transmigrate under the stroma. a,b: CD3+ T lymphocytes were seeded over allogenic MSC and pictures were recorded every 5 min. Time elapsed since the initiation of the recording is depicted on the lower right corner of each slide. a: Culture in absence of stimulus. Arrowheads point at the only two cells that remained in the field of the camera during the 4 h-recording process. b: Culture stimulated with P-IL2. Arrowheads point at T lymphocytes which transmigrated under the MSC layer within approximately 3 h. Original magnification 100×. c: Confocal laser scanning microscopy. (1, 2) horizontal and (XZ, YZ) vertical views showing an example of a transmigrating T cells (green) positioned below the MSC stroma (red). The localization of the horizontal views (1: top view and 2: bottom view) is indicated by the arrowheads on the XZ cross-section. XZ and YZ localizations are indicated by the orthogonal dotted lines. In the top view (1), only the MSC are visible, whereas in the bottom view (2) T cells, which are positioned below MSC, can be seen. Original magnification 630×.
Collectively these data show that upon activation T lymphocytes bind MSC very rapidly and reversibly move under the stromal layer.
MSC-T lymphocyte cellular interaction is implicated in the modulation of T lymphocyte proliferation
MSC-T lymphocyte interactions were further investigated using two chamber cultures. MSC were seeded on the bottom of the wells and T lymphocytes were laid in the transwells above. In co-cultures with PHA, transwells inhibited T cell proliferation (Fig. 5a, upper parts and b). In P-IL2 cultures cell contact prevention restored T lymphocyte proliferation to a level that was statistically identical to that of cultures in absence of MSC. In a3-28 stimulated cultures, transwells significantly restored cell proliferation, but to a value that was lower compared to that observed in absence of MSC (Fig. 5a, median and lower parts and b).
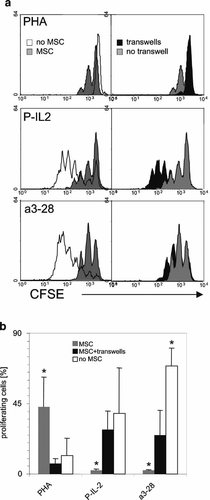
Effect of MSC-T cell contact upon lymphocyte proliferation. CFSE+T lymphocytes were stimulated or not in presence or absence of allogenic MSC, with or without transwells and proliferation was assessed after 5 days. a: Left parts: CFSE profiles of T lymphocytes stimulated alone (empty profiles) or in presence of MSC (gray profiles) with PHA 5 µg/ml (upper parts), with P-IL2 (mid parts) and with a3-28 (lower parts). Right parts: culture with MSC exclusively. CFSE profiles of cultures with and without transwells are in black and gray, respectively. One experiment representative of 6 is shown. b: Mean values ± SD (n = 6) of CD3+ T cell proliferation obtained after stimulation with PHA, a3-28 and P-IL2 in absence or presence of transwells (0.4 µm pore diameter). *P < 0.009 compared with the co-cultures performed with transwells.
These data suggest that depending on the stimulus, MSC-T lymphocyte contact is associated with a stimulatory or an inhibitory signal delivered by MSC to T lymphocytes.
Discussion
The amplification of adherent cells derived from femoral heads and condyles, with HPS, in conditions devoid of bovine protein, generated cells exhibiting a surface phenotype and multilineage differentiative properties showing that they were bona fide MSC. As far as their immunomodulatory properties were concerned, we observed contrasting effects depending upon the stimulus used to activate T lymphocytes. MSC did not deliver a mitogenic signal to purified, unstimulated T cells. By contrast, in the presence of PHA alone, which does not by itself induce T lymphocyte proliferation (Lipsky et al., 1976), allogenic MSC were co-stimulatory. When providing a fully mitogenic stimulus such as P-IL2 or a3-28, HPS-derived MSC efficiently prevented T cell proliferation, an observation in agreement with other studies (Di Nicola et al., 2002; Le Blanc et al., 2003; Tse et al., 2003; Krampera et al., 2005).
Our data suggest that a positive correlation exists between γ-IFN-producing T lymphocyte frequency and the inhibitory effect of MSC on T cells. In cultures with PHA alone, where γ-IFN+ cells were quasi-undetectable, MSC promoted proliferation; in cultures with P-IL2 where γ-IFN+ cell frequency was intermediate, inhibition was moderate, and with a3-28 where γ-IFN+ cell frequency was the highest, inhibition was the most extensive. Moreover as suggested by the significant restoration of proliferation produced by tryp complementation it is most likely that activated MSC mediate T lymphocyte inhibition in a large proportion via tryp depletion (Meisel et al., 2004; Plumas et al., 2005; Krampera et al., 2006).
We report for the first time that activated T lymphocytes, irrespective of the stimulus and in marked contrast to unstimulated cells, very efficiently attach to MSC, transmigrate under them, and remain trapped for up to 60 h before reverse-transmigrating into the supernatant. Transwell experiments which block both cellular contact and transmigration showed that these processes promoted lymphocyte proliferation in cultures with PHA. By contrast, and in apparent opposition with the above data, transwells induced the integral restoration of lymphocyte proliferation in cultures with P-IL2, as if no soluble mediator was involved in the inhibition. Furthermore, transwells did not fully restore proliferation in a3-28 co-cultures.
We may tentatively explain the contrasting effects of allogenic MSC on T lymphocytes as follows: (1) Cultures with PHA: As PHA by itself primes T lymphocytes but does not allow their transit in S phase (Modiano et al., 1999), no proliferation occurs in absence of accessory cells. In co-cultures with allogenic MSC, PHA-activated T lymphocytes firmly bind these cells and transmigrate under them. Allogenic cell contact may provide the secondary signal required for the proliferation of PHA-primed T lymphocytes in this situation, as no γ-IFN is synthesized and consequently the inhibitory action of IDO is turned off. Our preliminary experiments (not shown) showing that PHA-induced proliferation decreases on the mean to 79% of it original value when 500 U/ml of recombinant γ-IFN are added to the MSC 24 h before the addition of the T lymphocytes are consistent with this contention. (2) Cultures with PHA + IL-2: γ-IFN secretion is sizeable but rather low, inducing in co-cultures a moderate activation of IDO within the cytoplasm of MSC. This generates a local depletion in tryp around MSC that is inhibitory only under the stroma where tryp is efficiently destroyed because extracellular spaces are small and surrounded by MSC. Upon transmigration under MSC, T lymphocytes get trapped in these tryp-depleted areas that impair the stimulation initially provided by P-IL2 and allogenic contact. Upon reverse-transmigration, when lymphocytes return to a permissive environment they remain inhibited because tryp depletion induces a long-term blockade of T lymphocytes in late G1 (Munn et al., 1999). (3) Cultures with anti-CD3 plus anti CD28-mAb: In co-cultures with a3-28 T lymphocytes are maximally stimulated and secrete amounts of γ-IFN that are sufficient to induce in turn, via an intense activation of IDO in MSC, a generalized tryp depletion. Consequently both the spaces under the stroma and the main supernatant of a3-28 cultures become inhibitory. When T lymphocytes are prevented from transmigrating under the stroma by the transwells their proliferation remain restricted because tryp levels in the supernatant are, by contrast to P-IL2 cultures, sufficiently decreased to induce a significant inhibition in transwells as well.
The nature of the molecule(s) involved in this early cellular interaction is not elucidated yet. Majumdar et al. (2003) reported that V-CAM-1/VLA-4 interaction was involved in the binding of T lymphocytes to MSC. However we were not able to inhibit the appearance of cobblestone-like cells in the activated cultures by adding a blocking anti-α4-integrin mAb (not shown). This apparent discrepancy may be related to the technical differences between the assays. Majumdar et al. washed off unbound T lymphocytes and assessed cell interactions after 1 h of incubation already. By contrast, we did not intent to disrupt the process of cell–cell interaction as the final readout of our assay was the observation of the appearance of cobblestone-like cells. It may well be that in this situation the eventual inhibition of V-CAM-1/VLA-4 may be hidden by other molecules also involved in the cell contact. The I-CAM/LFA-1 interaction may indeed also be involved because we observed a delay of cobblestone-like cell appearance in cultures complemented with anti-LFA-1 mAb. This phenomenon did not have an impact on cell proliferation inhibition (not shown).
Whether T lymphocyte transmigration through a mesenchymatous stroma also happens in vivo and is involved in the regulatory effect of MSC upon T lymphocytes remains to be determined. Our preliminary experiments show that in co-cultures of autologous cells, lymphocytes also generate cobblestone-like cells upon activation, suggesting that such a process and possibly transmigration as well are not restricted to an artificial allogenic setting. Looking at physiological situations, IDO-related tryp depletion is implicated in the acceptance of the semi-allogenic fetus in pregnant mammals, but whether or not migration of lymphocytes through a stroma is required to ensure maternal tolerance toward the fetus has not been addressed (Baban et al., 2004). In experimental animal models, donor MSC are found in the lungs, in the spleen and the intestine (Ringden et al., 2006). In human patients, infused MSC detection is very difficult. DNA originating from the MSC donor has been detected in the colon of leukemic patients suffering from GVHD after allogenic HSC transplantation (Ringden et al., 2006), suggesting that the grafted MSC can home to sites where the disease is active and may inhibit such a process in situ. However, the eventual generation in the recipient of a MSC-derived, inhibitory stroma has not been observed so far. By contrast, tumors are frequently surrounded by a stroma containing mesenchymatous elements that may act as a barrier to antigen presentation (Blankenstein, 2005; Yu et al., 2006). Moreover IDO activity is constitutive in many primary tumors (Uyttenhove et al., 2003), thus suggesting that MSC forming the peritumoral stroma may comprise tryp-depleted spaces that can inhibit tumor-specific cytotoxic T cells.
In conclusion, this study showed that T lymphocytes, upon activation, rapidly bound allogenic MSC, and transmigrated under them. This process results either in lymphocyte proliferation when the initial stimulus provided is sub-mitogenic and γ-IFN secretion by T lymphocytes is absent or low, or in lymphocyte growth inhibition when the initial stimulus is mitogenic by itself and γ-IFN production by T cells is elevated. These in vitro observations are in agreement with contrasting in vivo data showing that allogenic MSC on the one hand promote inflammation in murine experimental auto-immune models (Djouad et al., 2005), but on the other hand inhibit very efficiently T lymphocytes in highly inflammatory situations as illustrated in leukemic patients suffering form severe GVHD (Le Blanc et al., 2004b). Such a 2-sided effect of allogenic MSC on T lymphocyte proliferation will require further investigations before using MSC as therapeutic tools in routine clinical protocols.
Acknowledgements
We would like to thank Nicolette Brouwers, Joan Stalder, and Carine Grannavel for excellent technical assistance. Special thanks to Dr. Richard Stern for editorial advice.




