Extracellular nucleotides regulate CCL20 release from human primary airway epithelial cells, monocytes and monocyte-derived dendritic cells
Abstract
Extracellular nucleotides regulate ion transport and mucociliary clearance in human airway epithelial cells (HAECs) via the activation of P2 receptors, especially P2Y2. Therefore, P2Y2 receptor agonists represent potential pharmacotherapeutic agents to treat cystic fibrosis (CF). Nucleotides also modulate inflammatory properties of immune cells like dendritic cells (DCs), which play an important role in mucosal immunity. Using DNA-microarray experiments, quantitative RT-PCR and cytokine measurements, we show here that UTP up-regulated ∼2- to 3-fold the antimicrobial chemokine CCL20 expression and release in primary HAECs cultured on permeable supports at an air–liquid interface (ALI). Both P2Y2 (ATPγS, UTP, INS365) and P2Y6 (UDP, INS48823) agonists increased CCL20 release. UTP-induced CCL20 release was insensitive to NF-κB pathway inhibitors but sensitive to inhibitors of ERK1/2 and p38/MAPK pathways. Furthermore, UTP had no effect on interleukin-(IL)-8 release and reduced the release of both CCL20 and IL-8 induced by TNF-α and LPS. Accordingly, UTP reduced the capacity of basolateral supernatants of HAECs treated with TNF-α or LPS to induce the chemoattraction of both CD4+ T lymphocytes and neutrophils. In addition, we show that, in monocyte-derived DCs, ATPγS, and UDP but not UTP/INS365-stimulated CCL20 release. Likewise, UDP but not ATPγS was also able to increase CCL20 release from monocytes. Pharmacological experiments suggested an involvement of P2Y11 or P2Y6 receptors through NF-κB, ERK1/2, and p38/MAPK pathways. Altogether, our data demonstrate that nucleotides may modulate chemokine release and leukocyte recruitment in inflamed airways by acting on both epithelial and immune cells. Our results could be relevant for further clinical investigations in CF. J. Cell. Physiol. 211: 716–727, 2007. © 2007 Wiley-Liss, Inc.
Extracellular nucleotides are important signaling molecules released by several cell types, including epithelial and immune cells, which exert diverse autocrine and paracrine biological effects by acting on multiple nucleotide-gated ion channel P2X and G-protein coupled P2Y receptors expressed in different cell types (see for reviews: el-Moatassim et al., 1992; Ralevic and Burnstock, 1998; Lazarowski and Boucher, 2001; Abbracchio et al., 2003; Leipziger, 2003). Extracellular nucleotides are known to play a crucial role in regulating ion transport, airway surface hydration, and mucociliary clearance by activating P2Y and adenosine receptors (Lazarowski et al., 2004; Picher et al., 2004). One ongoing area of research suggests that P2Y receptor activation may have therapeutic value in cystic fibrosis (CF), one of the most common lethal autosomal recessive genetic diseases characterized by viscous, underhydrated mucus that obstructs the airways of the patients (Parr et al., 1994; Davis et al., 1996; Kellerman et al., 2002; Marcet and Boeynaems, 2006). Aerosolized nucleotides, by acting on P2Y2 receptors, might be used as therapeutic agents in CF to bypass the cAMP-regulated Cl− defect, by stimulating Ca2+-activated Cl− secretion, thus promoting hydration of airway secretions and thereby improving the mucociliary clearance (Knowles et al., 1991; Mason et al., 1991; Clarke et al., 1994; Bennett et al., 1996; Morse et al., 2001; Marcet and Boeynaems, 2006). Pyrimidines (UTP and UTP analogs) seem preferable to ATP in CF therapy, because they are not degraded into adenosine which induces bronchoconstriction and possibly airway inflammation and pulmonary fibrosis (Chunn et al., 2006). Phase I studies have shown that the use of aerosolized P2Y2 receptor agonists (UTP, INS365, INS37217) is safe and improves mucociliary clearance, and in recent Phase II trials in CF patients INS37217 improved lung function as assessed by forced expiratory flow (Knowles et al., 1991; Bennett et al., 1996; Olivier et al., 1996; Noone et al., 1999, 2001; Sabater et al., 1999; Yerxa et al., 2002; see for review: Marcet and Boeynaems, 2006).
The function of the airway epithelium goes beyond the operation of the mucociliary clearance. Airway epithelial cells (AECs) play also a key role in innate and adaptative immunity (Stick and Holt, 2003). Epithelial cells, in the airways as elsewhere, are indeed a source of inflammatory mediators like antimicrobial peptides and various cytokines (Bals and Hiemstra, 2004). Antimicrobial peptides like defensins play a key role in mucosal defense: in the lung, the human beta defensin (HBD)-1 is secreted constitutively, whereas HBD-2 is up-regulated in case of inflammation (Singh et al., 1998). In response to inflammatory cytokines, AECs secretes also the chemokine CCL20 (Starner et al., 2003; see for review: Stick and Holt, 2003) also called macrophage inflammatory protein-3α (MIP-3α), Exodus-1 or LARC (see for review: Schutyser et al., 2003). CCL20 released in airways has a dual action: it attracts immune cells but it also behaves as a defensin molecule (Reibman et al., 2003; Starner et al., 2003). AECs could stimulate T lymphocyte activation and proliferation in response to antigenic, allogeneic, and anti-CD3 stimulation (Oei et al., 2004) and promote dendritic or polymorphonuclear cells migration (Thorley et al., 2005, Mul et al., 2000). Dendritic cells (DCs) are able to open the tight junctions between adjacent epithelial cells and take up bacteria directly from the lumen; furthermore epithelial cell-derived factors have been shown to modulate the function of DCs (Rimoldi et al., 2005). These mechanisms are likely to play an important role in CF which is associated with bacterial infection of the airways (particularly with Pseudomonas aeruginosa) and an exaggerated inflammatory response, characterized by influx of neutrophils and high concentrations of IL-8 in bronchoalveolar lavage fluid (Balough et al., 1995; Khan et al., 1995; Davis et al., 1996).
Extracellular nucleotides have an immunomodulatory role involving several P2Y receptors. In particular ATP induces a semi-maturation state of human DCs, characterized by up-regulation of co-stimulatory molecules, inhibition of IL-12 production, and potentiation of IL-10 (Schnurr et al., 2000; Wilkin et al., 2001, 2002; la Sala et al., 2001). More recently, ATP was shown to stimulate the secretion of thrombospondin-1 and to potentiate indoleamine 2,3-dioxygenase activity, two factors that could be involved in immune tolerance (Marteau et al., 2005). In addition, treatment of DCs with ATP modifies their repertoire of chemokines and chemokine receptors (la Sala et al., 2002). In particular ATP strongly reduced the capacity of DCs to attract monocytes and immature DCs by inhibiting their release of MCP-1 and MIP-1α (Horckmans et al., 2006).
In the present study, we investigated whether the P2Y2 agonist UTP exerts an immunomodulatory effect on primary human airway epithelial cell (HAECs). We show that UTP induces CCL20 release from HAECs, but inhibits the release of CCL20 and IL-8 induced by TNF-α or LPS as well as the resulting neutrophils and CD4+ T cells chemotaxis. On the other hand we show for the first time that human monocytes and monocyte-derived DCs stimulated by ATPγS or UDP release CCL20. This dual effect may enhance airways defense against pathogens and at the same time protect against excessive inflammation.
MATERIALS AND METHODS
Reagents
ATP, ATPγS, ADP, ADPβS, UTP, UDP, UDP-glucose, suramin, LPS, PD98059, SB203580 were purchased from Sigma (Bornem, Belgium). Caffeic acid phenethyl ester (CAPE) was purchased from Biomol International, LP (Exeter, United Kingdom). INS365 (Up4U, P1, P4-di(uridine 5′-) tetraphosphate) and INS48823 (2′,3′(phenylacetaldehyde acetal)Up3U) were a gift from Dr. B. Yerxa (Inspire, Durham, NC). Recombinant human IL-8 and CCL20 were purchased from R&D Systems (Abingdon, United Kingdom). Human TNF-α was a gift from Dr. B. Robaye (ULB, IRIBHM, Belgium).
Subjects/tissue samples
Inferior turbinates were collected from 18 non-CF patients and one CF patient (homozygous deltaF508) who required surgical turbinectomy for nasal obstruction or septoplasty (Dr. S. Hassid, ORL Department, Erasme Hospital). Asthmatic or allergic patients were excluded from the study. All procedures were approved by the Ethics Committee of the School of Medicine of the Université Libre de Bruxelles.
Isolation and culture of human nasal epithelial cells (HNECs)
The primary cultures of human epithelial cells derived from nasal mucosa of inferior turbinates were performed by a method adapted from Wu et al. (1985). After excision, the nasal mucosa of inferior turbinates was immediately immersed in HBSS (Ca2+/Mg2+-free; Invitrogen, Merelbeke, Belgium)-HEPES (25 mM, Gibco product, Invitrogen, Merelbeke, Belgium) culture medium containing 100 U/ml penicillin (Gibco, Invitrogen), 100 µg/ml streptomycin (Gibco, Invitrogen), 100 µg/ml of gentamicin sulfate (Gibco, Invitrogen), and 2.5 µg/ml amphotericin B (Gibco, Invitrogen). After repeated washings with cold HBSS-HEPES medium, tissue was cut off in 1–3 mm large specimens using sterile scalpels and then digested with 0.1% Pronase (Sigma) at 4°C overnight under continuous rotation. After incubation, fetal calf serum (FCS) was added to a concentration of 10%, and nasal epithelial cells were detached from the stroma by gentle agitation. The cell suspensions were filtered through 100 µm cell strainer nylon filter (Falcon, Becton Dickinson, Franklin Lakes, NJ), centrifuged at 150g for 10 min at 4°C, and the pellet was resuspended in 10% FCS-HBSS-HEPES medium and centrifuged again. The second cell pellets were then suspended in the serum-free Dulbecco's modified Eagle's (DMEM)/F12 (1:1) medium (Invitrogen) containing insulin (5 µg/ml; Sigma), apo-transferrin (5 µg/ml; Sigma), epidermal growth factor (10 ng/ml; VWR International, Leuven, Belgium), epinephrine (5.5 µM, Sigma), hydroxycortisone (0.4 µg/ml; Sigma), 3,3′,5-triiodothyronine (2 × 10−9 M, Sigma), endothelial cell growth supplement (4 µg/ml, Sigma), retinoic acid (300 nM, Sigma), amphotericin B (2.5 µg/ml), streptomycin (100 µg/ml), penicillin (100 U/ml) gentamicin sulfate (50 µg/ml), and L-glutamine (2 mM).
The cells were plated (106/cm2) on collagen-coated Transwell® permeable supports (6.5 mm diameter; 0.4 µm pore size; ELSCOLAB, Belgium) and incubated in a humidified atmosphere of 5% CO2 at 37°C. The culture medium was changed daily. Monolayer cell confluence was achieved after 7–10 days of culture. Morphological observation using a phase contrast microscope showed that HNECs consisted primarily of epithelial cells. The epithelial nature of the cultured cells was confirmed using immunocytochemistry with cytokeratin immunofluorescence labeling (Cytokeratin Cocktail (Ab-4) monoclonal, Oncogene Research Products, EMD Biosciences, Inc., Darmstadt, Germany), showing 95% of positive cells. Once HNECs reached confluence, they were cultured in an air–liquid interface (ALI) for 2 weeks. During the air–liquid culture, EGF was removed from the culture medium and retinoic acid was added at the final concentration of 50 nM. Tested agents were added in general on the apical side for the last 24 h in 300 µl of culture media. Samples (300 µl of apical medium and 2 ml of basolateral medium) were aliquoted and stored at −80°C before analysis. Experiments were conducted on cultures of transepithelial electrical resistance >300 ohm/cm2.
Microarray experiments
HNECs cultured at an ALI were apically stimulated by UTP (300 µM, Sigma) for 3 h. The stimulation was stopped by addition of TRIZOL® reagent (Life Technologies, Groningen, Netherlands) directly on the cells. Total RNAs were then purified on a Qiagen RNeasy kit column (Westburg, Leusden, Netherlands) according to the manufacturer's instructions. Microarray experiments were then made at the VIB microarray facility of K.U.L.-Leuven using slides containing 21,000 human sequences as fully described at www.microarrays.be/service.htm. Arrays were scanned at 532 and 635 nm using a Generation III scanner (Amersham BioSciences, Diegem, Belgium). Image analysis was performed with ArrayVision (Imaging Research, Inc., Ontario, Canada).
Reverse transcription polymerase chain reaction experiments
Total RNA was isolated as described above and reverse transcribed using random hexamers and Superscript-II Reverse Transcriptase according to manufacturer's instructions (Invitrogen). To rule out any genomic DNA contamination, total RNA was incubated with RNase-free DNase (Invitrogen) and experiments were also performed without reverse transcriptase (RT) to control for the cDNA origin of the amplified fragment. To amplify target cDNAs, a set of specific primers for the monoexonic P2Y2, P2Y4, and P2Y6 genes receptors and the P2Y11 receptor were designed and synthesized (Eurogentec, Seraing, Belgium): P2Y2 sense, 5′-GCTTCAACGAGGACTTCAAG-3′ and P2Y2 antisense primer, 5′-CACGCTGATGCAGGTGAGGA-3′; P2Y4 sense, 5′-TGGCATTGTCAGACACCTTGTATGTG-3′ and P2Y4 antisense, 5′-AAGCAGACAGCAAAGACAGTCAGCAC-3′; P2Y6 sense 5′-CCCTGCTGGCCTGCTACTGTCTCCTG-3′ and P2Y6 antisense, 5′-CTAATTCTCCGCATGGTTTGGGGTTGG-3′; P2Y11 sense, 5′-CCCCCGCTGGCCGCCTACCTCTATCC-3′ and P2Y11 antisense, CGCAGCCCAACCCCGCCAGCACCAG-3′. PCR experiments were carried out using primers (500 pM each) with 2.5 U of the Taq DNA polymerase (Invitrogen) in 1.5 mM MgCl2. The PCR amplification conditions were: 94°C for 5 min, 1 cycle; 94°C for 1 min, 50°C for 1 min, 72°C for 1 min, 35 cycles; 72°C for 7 min, 1 cycle. PCR products were analyzed using 1.5% agarose gel electrophoresis and ethidium bromide coloration. As a negative control, PCR was carried out as before, except that water was added instead of DNA template.
Real-time RT-PCR expression analysis
One microgram of total RNA was isolated and reverse transcribed as described above. Diluted cDNA (5 µl) was analyzed using 2× SYBR Green PCR Master Mix (Applied Biosystems, Lennik, Belgium) by a 7500 Fast Real-Time PCR System and 7500 Fast software (Applied Biosystems) following the manufacturer's protocol. Gene-specific primers were designed according to sequences to cover the conserved peptide sequence region and, when it is possible, in interexonic gene sequences. PCR primers used were: human CCL20 forward: CTGGCTGCTTTGATGTCAGT; human CCL20 reverse: CGTGTGAAGCCCACAATAAA; human IκB-α forward: CTTCAGATGCTGCCAGAGAGT; human IκB-α reverse: GCCTCCAAACACACAGTCATC; human β-actin forward: AGAAAATCTGGCACCACACC; human β-actin reverse: GGGGTGTTGAAGGTCTCAAA. The PCR were conducted in 96-well optical reaction plates. Briefly, each well contained a 25-µl reaction mixture that contained 12.5 µl of SYBR Green PCR Master mix, 1 µl of each forward and reverse primer, 5.5 µl of water and 5 µl of cDNA samples. The SYBR Green dye was measured at 530 nm during the extension phase. The threshold cycle (Ct) value reflects the cycle number at which the fluorescence generated within a reaction crosses a given threshold. The Ct value assigned to each well thus reflects the point during the reaction at which a sufficient number of amplicons have been calculated. The relative mRNA amount in each sample was calculated based on its Ct in comparison to the Ct of the two most stable housekeeping genes chosen by the software geNorm® from a set of nine tested candidate reference genes. From this, a gene expression normalization factor can be calculated for each sample based on the geometric mean of a defined number of reference genes. The purity of amplified product was determined as a single peak of dissociation curve. Real-time PCR were conducted in duplicate for each sample from 2 to 4 different donors, and the mean value was calculated. This procedure was performed in at least two independent experiments. The results were calculated as follows (2(Ct of CCL20 − Ct of housekeeping gene)) × (normalization factor) and expressed in arbitrary units. A mean of values obtained in each experiment performed in different donors were calculated and expressed as a ratio of stimulated values/control values.
Inositol phosphate measurements
HNECs were seeded (4 × 106 per well) on 6-well dishes and labeled for 24 h with 10 µCi/ml [myo-D-2-3H]inositol in DMEM/F12 (1:1) medium containing 5% FCS, antibiotics and amphotericin. Cells were incubated for 2 h in Krebs–Ringer–HEPES (KRH) buffer (124 mM NaCl, 5 mM KCl, 1.25 mM MgSO4, 1.45 mM CaCl2, 1.25 mM KH2PO4, 25 mM HEPES, pH 7.4, and 8 mM D-glucose). The cells were then incubated in presence of the tested compounds (nucleotides, from Sigma or INS365, a gift from Inspire) together with LiCl (10 mM) for 20 min. The incubation was stopped by the addition of 1 ml of an ice-cold 3% perchloric acid solution. Total inositol phosphates (IPs) were extracted and separated on Dowex columns as previously described (Pirotton et al., 1991). The radioactivities of the different IP fractions (IP1, IP2, IP3, IP4) were added and normalized to that in phosphatidylinositol.
Cytokine quantification by ELISA
Cytokine concentrations were measured by ELISA (CCL20 and SLPI: R&D Systems; IL-8 and IL-10: Flexia Biosource, Nivelles, Belgium) according to manufacturer's instructions. Apical and basolateral secretions were recovered by directly collecting the apical (300 µl) and basolateral (2 ml) culture media. After a brief centrifugation these collected media were stored at −80°C before ELISA analysis. ELISA experiments were run in triplicate to ensure reproducibility.
Isolation of human neutrophils
Heparinized human venous blood was obtained from healthy volunteers and mixed with an equal volume of 3% dextran T500 in saline. Erythrocytes were allowed to sediment for 20 min, and the leukocyte-rich plasma was then centrifuged on Ficoll-Paque at 400g for 45 min. The pellet was collected and the contaminating erythrocytes were removed by hypotonic lysis. Isolated neutrophils were resuspended at 106 cells/ml in Hanks' balanced salt solution (HBSS) containing glucose (10 mM). Flow cytometric and videomicroscopy analyses revealed an homogeneous population of polymorphonuclear cells. Cell viability was checked by the Trypan blue exclusion method and was routinely found to be 95%.
Isolation of CD4+ T cells
CD4+ T cells were isolated from buffy coats of healthy volunteers using a CD4+ cell isolation kit II (Miltenyi Biotech, Bergisch, Gladbach, Germany) that depletes non-CD4+ cells (CD8, CD14, CD16, CD19, CD36, CD56, CD123, TCR γ/δ, and glycophorin A) by an immunomagnetic separation. This method gives more than 95% of CD4+ T cells. T cells were then incubated for 24 h at 37°C with complete medium in absence or in presence of agonists.
Preparation of human monocytes and monocyte-derived dendritic cells
Human monocytes and immature DCs were derived from adherent peripheral blood monocytes of normal donors as previously described (Romani et al., 1994). In brief, PBMCs were isolated from leukocytes-enriched buffy coats by standard density gradient centrifugation using Lymphoprep solution from Nycomed (Oslo, Norway), resuspended in complete medium and 2.5 × 108 PBMC were allowed to adhere during 2 h at 37°C at 5% CO2 in air in 75-cm2 cell culture flasks. Non-adherent cells were removed and rinsed-adherent cells were cultured in 15 ml of FCS-containing medium to obtain an homogenous population of monocytes (95% of CD14 positive cells). To obtain DCs, monocytes were supplemented with 800 U/ml of GM-CSF and 500 U/ml of IL-4. GM-CSF and IL-4 were added twice a week. The purity of each cell preparation was evaluated by checking expression of CD1a, HLA-DR, CD14, CD83, and CD3 (90–95% of the DCs were CD1a and HLA-DR positive). The medium for monocytes or DCs culture was RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum (Hyclone, Logan, UT), 25 mM HEPES buffer, 2 mM L-glutamine, 1 mM sodium pyruvate, 50 mg/ml gentamicin, all purchased from Gibco BRL (Ghent, Belgium), and 5 × 10−5 M β-mercaptoethanol (Merck, Darmstadt, Germany).
Flow cytometric analysis
Cell staining was performed using FITC-conjugated anti-human HLA-DR, CD83 and CD3 and PE-conjugated anti-human CD1a and CD14, supplied by PharMingen (San Diego, CA). Staining was made on 2 × 105 cells in 100 µl of PBS with 0.1% sodium azide for 30 min in the dark at 4°C. After washing with 2 ml of PBS, samples were analyzed on a FACSort® (Becton Dickinson). Data were analyzed and presented using Cellquest software (Becton Dickinson); the number of gated events was at least 10,000.
Chemotaxis assay
Migration assays for neutrophils and CD4+ lymphocytes were performed using 96-wells (Millipore®, Brussels, Belgium) chambers separated by a polycarbonate filter with a pore size of 5-µm. The lower chamber was filled with 130 µl of supernatant of basolateral HNECs plus 0.1% FCS. Neutrophils or CD4+ T cells (105/well) were placed into the upper chamber and incubated at 37°C for 2 h. After this period, migrated cells were quantified using the luminescence ATP detection assay system (ATPlite™, PerkinElmer, Zaventem, Belgium) according to the manufacturer's instructions. Briefly, migrated cells in the lower chamber were lyzed, substrate solution (luciferase and D-luciferin) was added and the production of light caused by the reaction of cellular ATP with added luciferase and D-luciferin was measured using the microplate luminometer LB96V (EG&G Berthold Technologies, Vilvoorde, Belgium). Luminescence values were converted in number of migrated cells using an internal linearized standard curve of neutrophils or CD4+ T cells performed for each experiment.
RESULTS
P2Y receptors responsive to uridine nucleotides are functionally expressed in primary human nasal epithelial cells (HNECs)
We first tested mRNA expression of uridine nucleotide-sensitive P2Y receptors in HNECs. As shown in Figure 1A, PCR products were amplified from cDNAs (n = 2 donors) of HNECs cultured in ALI (see Methods Section) with P2Y2 (331-bp), P2Y6 (455-bp) but not P2Y4 or P2Y11 primers. In addition, HNECs responded to uridine nucleotide stimulations (UTP and the UTP derivative INS365, 100 µM, n = 3 donors) by activating the phosphoinositide signaling pathway (Fig. 1B).
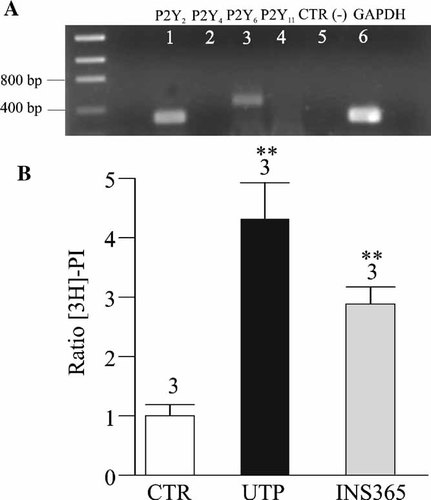
Uridine nucleotides P2Y receptor expression in HNECs. A: The extraction of total RNA and the amplification by RT-PCR were performed as described in Methods Section. P2Y2, P2Y4, P2Y6, and P2Y11 sets of primers were used to detect nucleotide P2Y receptors expressed in HNECs. Amplification products were obtained with P2Y2 (331-bp, lane 1) and P2Y6 (455-bp, lane 3) but not with P2Y4 (lane 2) or P2Y11 (lane 4) sets of primers. GAPDH primers were used as positive control (lane 6). As described in Methods Section, negative controls were carried out for RT with all sets of primers (not shown) and for PCR experiments (lane 5). B: Effects of UTP and INS365 on the IP accumulation in HNECs. The cells were incubated with UTP or INS365 (100 µM) or without agonists (CTR) for 20 min. The data represent the mean ± SE; n = 3, (**P < 0.01).
UTP-regulated expression of CCL20 mRNA in HNECs
Since P2Y2 agonists are potential therapeutic agents in CF, we focused our investigation on the immunomodulatory properties of UTP on HNECs. Using microarray technology, we determined the effect of UTP (300 µM, 3 h) on gene expression in HNECs cultured in ALI. A few weak regulations were observed in response to UTP but were not reproduced with a second preparation of HNECs except for CCL20 which was up-regulated more than twofold in both experiments. We further focused our study on CCL20 which displays a major interest in the context of airway inflammation. First, we conducted real-time RT-PCR analysis for CCL20 expression in UTP-treated and TNF-α-treated samples. As shown in Figure 2A, CCL20 transcripts were elevated 2.4 ± 0.25-fold by UTP 300 µM after 3 h (n = 4), and they had returned to the basal level after 24 h. On comparison, CCL20 transcripts were enhanced 7 ± 0.3-fold by TNF-α after 3 h (n = 2) and returned to the basal level after 24 h of stimulation. One possible mechanism of chemokine regulation involves mRNA stability as investigated in several works for CCL20 mRNA regulation (Scapini et al., 2002; Kao et al., 2005). To elucidate whether UTP could alter stability of CCL20 mRNA, cultures treated with or without UTP or TNF-α for 3 h were exposed to the RNA synthesis inhibitor, actinomycin D (Fig. 2B). The ratio of the relative mRNA abundance of CCL20 and hIκB-α transcripts, well-known to be short-lived, was calculated from two independent experiments, normalized with the stable housekeeping gene β-actin and expressed as percent. These experiments showed that 3 h following the addition of actinomycin D (2 µg/ml), the level of UTP (3 h)-induced CCL20 transcripts was reduced by 49 ± 9%. On comparison the TNF-α-induced transcripts were also reduced by 52 ± 3% in the same conditions. As control, we showed that the hIκB-α transcripts were much less stable than β-actin or CCL20 and were dramatically reduced by actinomycin D treatment (Fig. 2B). These data indicate that CCL20 mRNA stability was similar between UTP- and TNF-α-induced responses.
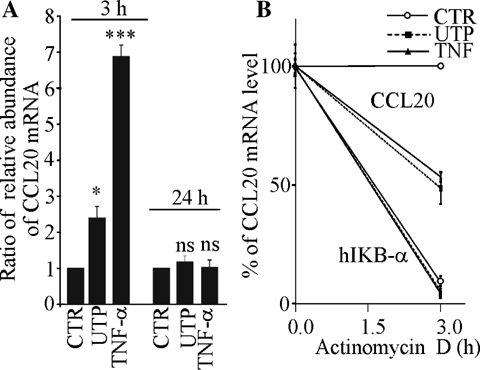
Effects of UTP on CCL20 transcripts in HNECs. A: UTP up-regulated CCL20 expression in HNECs. HNECs were treated with or without apical UTP (300 µM). After 3 or 24 h of stimulation, these cultures were harvested for RNA purification. Real-time RT-PCR analysis was conducted for nine housekeeping genes (the two most stable ones were chosen by GeNorm software) and CCL20 in these RNA samples. Relative level of CCL20 mRNA after normalization with the two most stable genes in each RNA sample was expressed as ratio between stimulated and unstimulated conditions. Values are mean ± SE of duplicates of four independent experiments. Student's t-test was performed using Prism software (GraphPad, CA) (*P < 0.05; ***P < 0.001). B: Characterization of CCL20 mRNA stability after addition of a transcription inhibitor, actinomycin D. HNECs were treated with or without apical UTP (300 µM) or TNF-α (30 ng/ml). After 3 h of stimulation, these cultures were harvested for RNA purification at point 0 h in absence of actinomycin D treatment, and actinomycin D (2 µg/ml) was added to another part of these cultures which were harvested for RNA at 3 h after addition of actinomycin D. Real-time RT-PCR analysis was conducted for β-actin, CCL20, and hIκB-α in these RNA samples. Relative level of CCL20 mRNA after normalization with β-actin in each RNA sample was converted in ratio between stimulated and unstimulated conditions. Results are expressed as 100% of starting level (without actinomycin D) for each condition. Values are mean ± SE of duplicates of two independent experiments.
UTP regulated the release of CCL20 from HNECs
We quantified CCL20 levels (ELISA, Quantikine kit, R&D) in supernatants from untreated and UTP-treated primary HNECs cultured in ALI. After 24 h of incubation we observed a constitutive release of CCL20 that was higher on the apical than on the basolateral side. The mean quantities of CCL20 constitutively secreted apically (n = 11 donors) and basolaterally (n = 10 donors) were 23 ± 5.6 and 8 ± 2.3 ng/mg of proteins, respectively. Since the apical and basolateral volumes are 300 µl and 2 ml respectively, the mean experimental concentrations of apical and basolateral CCL20 obtained were 1.92 ± 0.46 and 0.11 ± 0.03 ng/ml, respectively. The apically secreted protein is physiologically concentrated in the small volume of the airway surface liquid (ASL). Because the depth of ASL in cultures grown at the ALI is ∼20 µm (Jayaraman et al., 2001) and the surface area of the epithelial sheet is 4.6 cm2, the apically secreted CCL20 would be physiologically diluted in 9.4 µl of ASL, resulting in an estimated apical concentration of 61 ± 14 ng/ml (n = 11). Because the real basolateral volume of distribution is unknown, the in vivo concentration of CCL20 in the subepithelial space cannot be precisely estimated. Then, we investigated the effects of apical stimulation by UTP on CCL20 release by HNECs. Figure 3A,B shows that apical UTP (300 µM, 24 h) increased significantly the apical CCL20 release by 3.2 ± 1.8-fold to 72.4 ± 40 ng/mg of proteins (giving an experimental concentration of 6.1 ± 3.3 ng/ml or a theoretical apical concentration of 192.2 ± 108 ng/ml, n = 11, P < 0.01) and the basolateral release by 2.85 ± 1.3-fold to 22.8 ± 10.4 ng/mg of proteins (giving an experimental concentration of 0.31 ± 0.14 ng/ml, n = 10, P < 0.01). As control, basolateral application of UTP failed to provoke any effect on CCL20 level (data not shown). The effect of UTP on the apical and basolateral release of CCL20 was mimicked by another P2Y2 agonist, ATPγS (100 µM, 24 h) and by UDP (300 µM, 24 h), a product of UTP degradation by ectonucleotidases and agonist of the P2Y6 receptor (Fig. 3C). It was also reproduced by the dinucleotide derivatives INS365 (Up4U, 100 µM, 24 h) and INS48823 (a Up3U derivative, 100 µM, 24 h) that have a prolonged half-life and are agonists of P2Y2 and P2Y6 receptors, respectively (Pendergast et al., 2001) (Fig. 3B,C). Finally, since P2Y2 agonists may have therapeutic value in CF, we tested the effects of UTP on CCL20 release in HNECs isolated from one CF patient. UTP (300 µM, 24 h) was also able to double the apical and basolateral CCL20 release in CF HNECs (data not shown).
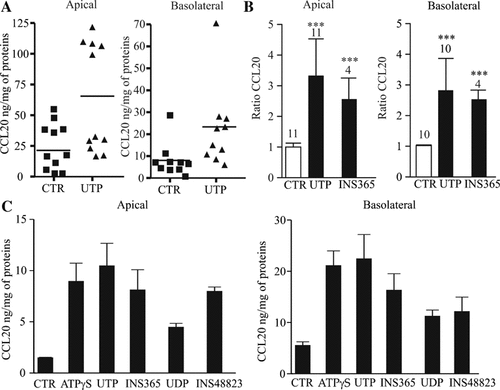
UTP-induced CCL20 secretion by HNECs. A: HNECs were apically stimulated with UTP (300 µM, 24 h) and CCL20 level in apical and basolateral compartments was measured as described in Methods Section and expressed as ng of CCL20 per mg of proteins. Results obtained for different donors were reported in graph. Horizontal bars represent mean levels of CCL20 for each condition. B: CCL20 levels were expressed as ratios between UTP-stimulated (n = 11), INS365-stimulated (n = 4) and control (unstimulated) CCL20 levels measured in different donors. Non-parametric and paired test of Wilcoxon and Student's t-test were performed using Prism software (GraphPad, CA) (***P < 0.001). C: HNECs were apically stimulated for 24 h with ATPγS, UTP, INS365, UDP, and INS48823 at 100 µM and CCL20 level on apical and basolateral sides was measured and expressed as ng of CCL20 per mg of proteins. Results obtained in triplicate were reported in graph and are representative of two independent experiments.
UTP-prevented TNF-α- and LPS-induced CCL20 release from HNECs
In airway obstructive diseases like CF, airway epithelium is often submitted to inflammatory and infectious environments. Thus, we tested the effects of pro-inflammatory stimuli (TNF-α and LPS) on CCL20 release. Apical application of LPS alone (100 ng/ml, 24 h) failed to evoke a rise in apical CCL20 secretion whereas it stimulated by threefold the basolateral release of CCL20 (P < 0.01) (Fig. 4A,B). The apical CCL20 increase induced in response to apical application of UTP was not significantly different in the presence or in the absence of apical LPS (Fig. 4A,B; P > 0.05). On the other hand, apical application of TNF-α (30 ng/ml, 24 h) increased the apical release of CCL20 by 3.9 ± 1.6-fold (n = 4; P < 0.05) and the basolateral one by 4.1 ± 0.8-fold (n = 4, P < 0.001). Apical UTP (300 µM, 24 h) blocked both the TNF-α- and LPS-induced CCL20 release (n = 3–4; P < 0.05) (Fig. 4A,B) and in these conditions the level of CCL20 was not significantly different from the one obtained with UTP alone (Fig. 4A,B). This inhibitory effect of UTP on TNF-α-induced CCL20 release was also observed in CF HNECs (data not shown). Furthermore, using real-time PCR, we observed that CCL20 transcript level was similar between TNF-α and TNF-α plus UTP after 3 h of stimulation whereas TNF-α-induced CCL20 protein release was reduced by UTP after 3 h of stimulation (data not shown). Then, we found, using actinomycin D experiments as before, that UTP had no influence on the decay of the CCL20 transcripts induced by TNF-α (data not shown).
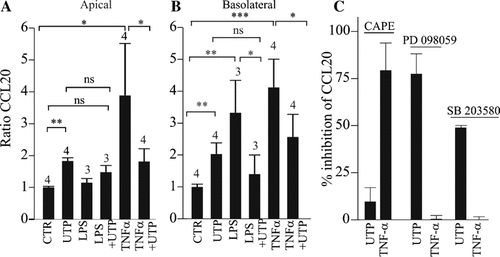
Inhibition by UTP of CCL20 release induced by pro-inflammatory stimuli in HNECs. HNECs were incubated 24 h with apical TNF-α (30 ng/ml) or LPS (100 ng/ml) with or without UTP (300 µM) and CCL20 levels in apical (A) and basolateral (B) sides were measured. CCL20 levels were expressed as ratio between stimulated and control (unstimulated) CCL20 levels measured from different donors as indicated upon bars. C: Effect of NF-κB, ERK1/2, and p38/MAP inhibitors on UTP-induced CCL20 release. HNECs were pre-treated with various inhibitors (CAPE, 100 µM; PD98059, 25 µM; SB203580, 25 µM) for 30 min, then further treated with UTP (300 µM), TNF-α (30 ng/ml), and TNF-α plus UTP always in presence of appropriate inhibitors. CCL20 levels were measured 24 h after the treatment. Values are mean ± SE of duplicates of two different donors and normalized with control and expressed as percent of inhibition of CCL20 responses for each agonist in presence of inhibitors. Student's t-test were performed using Prism software (GraphPad, CA) (ns, not significant; *P < 0.05; **P < 0.01; ***P < 0.001).
Effects of inhibitors of NF-κB, ERK1/2 and p38 pathways on UTP- and TNF-α-stimulated CCL20 release in HNECs
To elucidate the possible signaling pathways involved in UTP-enhanced CCL20 release, inhibitors of various signaling pathways were used (Fig. 4C). Recently, it has been shown that P2Y6 receptor activation in osteoclasts could induce NF-κB pathway activation (Korcok et al., 2005). Furthermore, CCL20 expression regulation has been shown to involve a JAK-independent but p42/44 extracellular signal responsive kinase (ERK1/2) and NF-kappaB-dependent signaling pathways (Kao et al., 2005). Then, we first tested the effects of the CAPE, an inhibitor of the activation of the NF-κB pathways (Natarajan et al., 1996), on the UTP- or TNF-α-induced CCL20 release in HNECs. As shown in Figure 4C, CAPE (100 µM, 24 h) blocked (∼80%) the TNF-α-induced CCL20 release but had no suppressive effect on UTP-induced CCL20 release. Since P2Y receptor activation in AECs and in monocytes could trigger ERK1/2 pathway (Santiago-Perez et al., 2001; Yang et al., 2004), we tested the effects of the ERK1/2 inhibitor PD98059 (25 µM, 24 h). Figure 4C shows that PD98059 strongly inhibited (79 ± 15%, n = 2) the release of CCL20 induced by UTP without affecting TNF-α response. Since it has been shown that P2Y receptors could activate p38 mitogen-activated protein kinase (MAPK) in neutrophils (Meshki et al., 2004), we also tested the p38 inhibitor SB203580. SB203580 (25 µM, 24 h) had no effect on TNF-α response but inhibited by 50% of the UTP response (Fig. 4C). The inhibitory effect of UTP on TNF-α-induced CCL20 release was still observed in the presence of PD98059 or SB203580 (data not shown) suggesting an involvement of other pathways than ERK1/2/p38/MAPK.
Effects of UTP stimulation on the release of other cytokines from HNECs
Several major inflammatory proteins such as IL-8 or IL-10 were not present on the tested microarray slides. We therefore analyzed their potential regulation by UTP using specific ELISAs. Our studies in primary cultures showed that HNECs secrete constitutively large amounts of IL-8. The mean IL-8 quantities secreted apically and basolaterally in absence of stimulus were 281 ± 56.4 and 1514 ± 394 ng/mg of proteins, respectively (n = 16). Apical stimulation by UTP (300 µM, 24 h) failed to provoke any significant increase in IL-8 secretion (296 ± 62 and 1,341 ± 218 ng/mg of proteins on apical and basolateral sides, respectively; P > 0.05; n = 16) (Fig. 5). We also tested the effects of UTP treatment on the secretion of the major anti-inflammatory cytokine IL-10. In our experiments, we failed to detect any variation of IL-10 level after apical stimulation of HNECs by UTP (300 µM, 24 h) (n = 3; data not shown). Finally, it has been reported that SLPI release, an elastase inhibitor which has anti-inflammatory and antimicrobial properties (Bals and Hiemstra, 2004), was promoted by nucleotides in a human tracheal epithelial cell line (Merten et al., 1996). We therefore tested whether UTP may induce SLPI secretion in human primary HNECs cultured in ALI. Although we observed a strong constitutive release of SLPI by HNECs on apical and basolateral sides (range of experimental concentrations from 28 to 160 ng/ml on apical against 10 to 60 ng/ml on basolateral side), we failed to detect any effect of UTP (n = 3, data not shown).
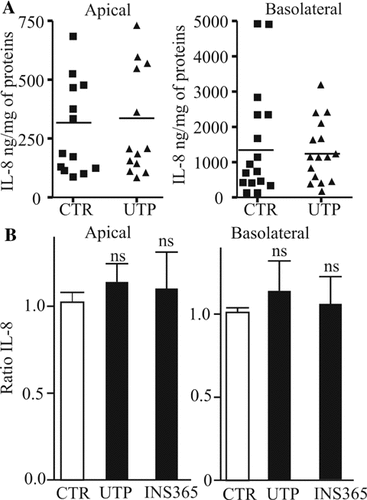
UTP effects on IL-8 secretion by HNECs. A: HNECs were apically stimulated with UTP (300 µM, 24 h) and IL-8 levels in apical and basolateral sides were measured and expressed as ng of IL-8 per mg of proteins. Results obtained for different donors were reported in graph. Horizontal bars represent mean levels of IL-8 for each condition. B: IL-8 levels were expressed as ratio between UTP-stimulated, INS365-stimulated, and control unstimulated IL-8 levels measured in different donors.
UTP prevented TNF-α- and LPS-induced IL-8 release from HNECs
IL-8 is an important pro-inflammatory cytokine in airways and plays a crucial role in many airway diseases like CF. Since we observed an inhibitory effect of UTP on TNF-α-induced CCL20 increase, we tested whether UTP could also affect the TNF-α-induced IL-8 release. TNF-α (30 ng/ml, for 24 h) increased by 2.8 ± 0.9-fold and 2.25 ± 0.3-fold respectively the apical (n = 4; P < 0.01) and basolateral (n = 4; P < 0.001) concentrations of IL-8 (Fig. 6A,B). As shown in Figure 6A,B, LPS (100 ng/ml, 24 h) failed to induce an apical release of IL-8 (n = 3; P > 0.05) whereas it increased by 2.3 ± 0.8-fold the basolateral IL-8 secretion (n = 3; P < 0.05). Figure 6A,B also show that UTP abolished the TNF-α- and LPS-induced IL-8 release (n = 3–4; P < 0.01). The same inhibitory effect of UTP was also observed in CF HNECs (data not shown).
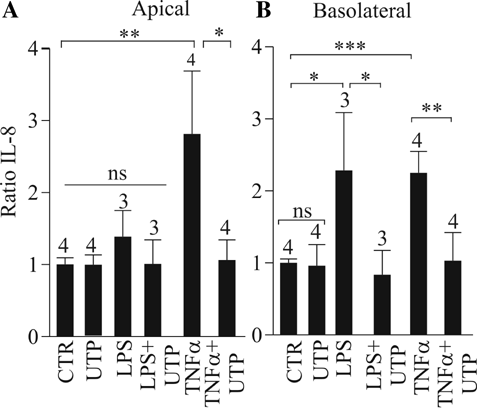
UTP inhibition of the basolateral IL-8 release induced by pro-inflammatory stimuli in HNECs. HNECs were incubated 24 h with apical TNF-α (30 ng/ml) or LPS (100 ng/ml) with or without UTP (300 µM) and IL-8 levels on apical (A) and basolateral (B) sides were measured. CCL20 levels were expressed as ratio between stimulated and control (unstimulated) CCL20 levels measured from different donors as indicated upon bars. Student's t-test were performed using Prism software (GraphPad, CA) (ns, not significant; *P < 0.05; **P < 0.01; ***P < 0.001).
Pre-treatment of HNECs with UTP inhibited the neutrophils and CD4+ T lymphocytes chemotaxis induced by inflammatory stimuli
Since IL-8 is the most potent neutrophil chemoattractant by acting on CXCR1 and CXCR2 receptors and CCL20 is known to attract CCR6-expressing immune cells, we tested the effect of UTP on the capacity of HNECs basolateral supernatants to induce neutrophils or CD4+ lymphocytes chemotaxis. Flow cytometric analysis revealed that neutrophils (n = 4) failed to express the CCR6 receptor at their cell surface whereas 60 ± 5% of CD4+ T lymphocytes (n = 4) expressed CCR6 receptors (data not shown). Figure 7 shows that, neutrophil or CD4+ T lymphocyte chemotaxis induced by basolateral supernatants of HNECs pre-treated with TNF-α (30 ng/ml, 24 h) or LPS (100 ng/ml) was abolished when UTP (300 µM, 24 h) was added together with TNF-α or LPS (n = 3; P < 0.01). Basolateral medium of HNECs apically stimulated with UTP for 24 h failed to promote a significant increase in the chemotaxis of neutrophils or CD4+ T lymphocytes (n = 4; P > 0.05). We observed the same inhibitory effect of UTP on neutrophil or CD4+ T lymphocytes chemotaxis with the basolateral supernatants of CF HNECs treated with TNF-α (data not shown).
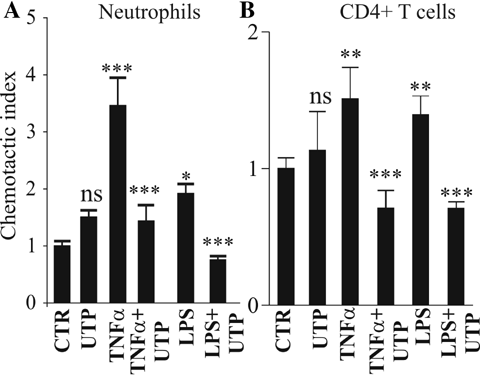
Inhibition by UTP of neutrophil and CD4+ T lymphocyte chemotaxis induced by basolateral supernatants of HNECs pre-treated with pro-inflammatory stimuli. HNECs were incubated 24 h with apical TNF-α (30 ng/ml) or LPS (100 ng/ml) with or without UTP (300 µM). Then, basolateral supernatants of HNECs were put in the lower chamber of a chemotaxis chamber and 105 neutrophils (A) or CD4+ T lymphocytes (B) were incubated 2 h in the upper chamber. Then, the number of migrated neutrophils or CD4+ T lymphocytes was calculated as described in Methods Section. Results were expressed as chemotactic index representing the ratio of migrated cells between stimulated and control conditions. Data are expressed as mean of ratio ± SE; n = 4. Student's t-test was performed using Prism software (GraphPad, CA) (*P < 0.05; **P < 0.01; ***P < 0.001).
Extracellular nucleotides induced CCL20 release from monocytes and DCs
DCs are immune cells playing a key role in mucosal immune response which can intimately interact with epithelial cells (Rimoldi et al., 2005). Therefore, it could be hypothesized that nucleotides released by epithelial cells may affect DC or monocyte functions by a paracrine pathway. As shown in Figure 8A, we observed that adenine triphosphate nucleotides (ATP, 300 µM and ATPγS, 100 µM) and UDP (300 µM) but neither UTP nor INS365 significantly (P < 0.001) stimulated CCL20 release from DCs. ATPγS was the most efficient nucleotide: 44 ± 13 pg/ml for control versus 631 ± 191 pg/ml for ATPγS (n = 10; P < 0.001) (Fig. 8A). Figure 8B shows that ATPγS-enhanced CCL20 release was dependent on the ATPγS concentration with a half-maximal effective concentration (EC50) value of 12.8 ± 2.8 µM. The ATPγS-induced CCL20 release was partially (∼50%) prevented by suramin, an antagonist of P2Y11 receptors and other P2 receptors (data not shown). In addition, ADP and the ADP derivative ADPβS failed to evoke any CCL20 increase (data not shown). Furthermore, UDP (609 ± 209 pg/ml, n = 7; P < 0.001) but neither UTP, nor INS365 (Fig. 8) nor UDP-glucose (not shown) promoted release of CCL20 from DCs. Figure 8C shows that, like UDP, the P2Y6 agonist INS48823 was able to increase the secretion of CCL20 in a concentration-dependent manner, with an EC50 value of 12.7 ± 4.4 µM. Figure 8D shows that LPS (100 ng/ml, 24 h) strongly increased the CCL20 release up to 50 times (1892 ± 674 pg/ml; n = 6). Finally, we tested whether nucleotides may alter the LPS-induced CCL20 release as previously observed in AECs. Our results showed that contrary to UDP which failed to have any effect, ATPγs and to a lesser extent ATP potentiated the LPS-induced CCL20 release (Fig. 8D).
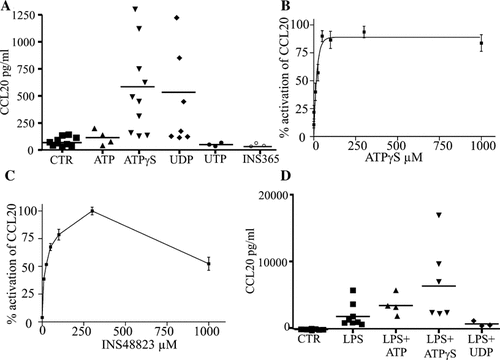
Regulation of CCL20 release by nucleotides in dendritic cells. A: CCL20 release was measured in supernatants of DCs treated for 24 h in the absence or presence of 100 ng/ml LPS with ATP 300 µM, ATPγS 100 µM, UDP 300 µM, UTP 300 µM, or INS365 100 µM. Results obtained for n = 4–10 donors were reported in graph. Horizontal bars represent mean levels of CCL20 for each condition. B,C: Concentration-dependent (1–1,000 µM) effects of a 24 h stimulation by ATPγS and INS48823 on the enhancement of CCL20 release in DCs. Results were expressed as mean ± SE of duplicates samples from n = 3 donors. D: CCL20 levels were expressed as ratio between LPS with or without nucleotides-stimulated and control unstimulated CCL20 levels, n = 4–10 as indicated upon the bars. Non-parametric and paired test of Wilcoxon and Student's t-test were performed using Prism software (GraphPad, CA) (see Results).
Effects of inhibitors of NF-κB, ERK1/2, and p38 pathways on nucleotide-stimulated CCL20 release in DCs
As previously done for HNECs, we tested the possible signaling pathways involved in ATPγS- and UDP-enhanced CCL20 release in DCs using specific inhibitors (Fig. 9). As shown in Figure 9, CAPE (50 µM, 24 h) blocked both ATPγS- and UDP-induced CCL20 release (respectively by 83 ± 4% and 95 ± 3%; n = 3), as well as the one induced by LPS (100 ng/ml, 24 h). Whereas PD98059 (25 µM, 24 h) blocked ATPγS response (70 ± 6%, n = 3), it inhibited only partially (30 ± 3.6%; n = 3) the UDP response. On the other hand, the p38/MAPK inhibitor SB203580 (25 µM, 24 h) reduced by 60–70% of the ATPγS, UDP, and LPS responses. These data suggest that in DCs ATPγS-enhanced CCL20 release involved NF-κB, ERK1/2 as well as p38/MAPK pathways while UDP-induced CCL20 increase mainly involved NF-κB and p38/MAPK pathways. Moreover, LPS-induced CCL20 release was mainly due to NF-κB and p38/MAPK pathways.
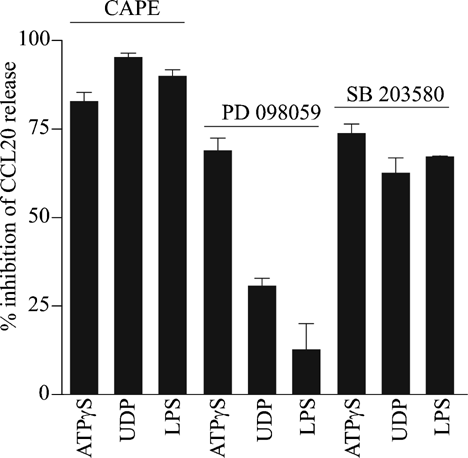
Effect of NF-κB, ERK1/2, and p38/MAPK inhibitors on nucleotides induced CCL20 release by DCs. DCs were pre-treated with various inhibitors (CAPE, 50 µM; PD98059, 25 µM; SB203580, 25 µM) for 30 min, then further treated with ATPγS (100 µM), UDP (300 µM), and LPS (100 ng/ml) always in presence of appropriate inhibitors. CCL20 levels were measured 24 h after the treatment. Values are mean ± SE of duplicates of three different donors and normalized with control and expressed as percent of inhibition of CCL20 responses for each agonist in presence of inhibitors.
UDP-induced CCL20 release from monocytes
Finally, we tested whether nucleotides may affect CCL20 release from other immune cells. Figure 10 shows that adenine nucleotides slightly increased CCL20 secretion from human monocytes while UDP, but not UTP, strongly up-regulated the CCL20 release from monocytes (n = 3). On the contrary, we failed to detect any CCL20 release in response to any nucleotide (100 µM, 24 h) in freshly isolated human neutrophils or T CD4+ lymphocytes (data not shown).
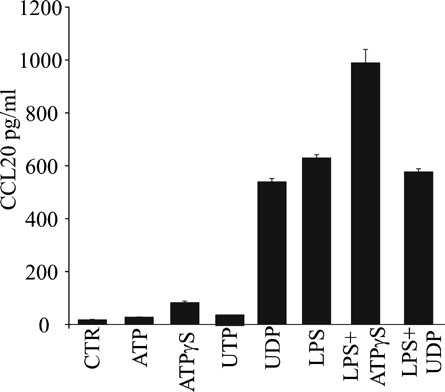
Regulation of CCL20 release by nucleotides in monocytes. Monocytes were treated for 24 h in the absence or the presence of 100 ng/ml LPS (as indicated on graph) with 300 µM ATP, 100 µM ATPγS, 300 µM UTP, or 300 µM UDP. CCL20 levels were measured as described in Methods Section. The displayed data are the means ± SD of triplicates and representatives of three independent experiments.
DISCUSSION
Numerous studies investigated the role of nucleotides in the regulation of Cl− transport and mucociliary clearance in epithelia (Knowles et al., 1991, Mason et al., 1991, Olivier et al., 1996; Paradiso et al., 2001; Marcet et al., 2003, 2004; Lazarowski et al., 2004, see for reviews: Leipziger, 2003; Marcet and Boeynaems, 2006; Palmer et al., 2006; Tarran et al., 2006). However, little is known about the immunomodulatory properties of nucleotides in airway epithelia. One could hypothesize that nucleotides released by epithelial cells could exert autocrine and paracrine effects on epithelial and immune cells. In the present study, we showed for the first time that nucleotides modulate the release of the chemokines CCL20 and IL-8 from AECs and thereby could alter immune cells recruitment in airways. Since aerosolized nucleotides (INS365, INS37217) are currently in clinical trials for CF treatment, our findings may have both physiological and pharmacotherapeutic relevance.
Nucleotides modulate CCL20 and IL-8 release from HNECs
Previous works have shown that the airway epithelium released the chemokine CCL20 in response to various inflammatory mediators (Reibman et al., 2003; Starner et al., 2003; Kao et al., 2005). In the present work, we first observed that HNECs constitutively secreted CCL20. Then, we demonstrated that in basal conditions, UTP up-regulated CCL20 expression and release by normal HNECs as well as by CF HNECs. CCL20 mRNA level was increased by UTP after 3 h of stimulation and returned to basal level within 24 h. Following actinomycin D treatment, the rate of decay of CCL20 transcripts induced by UTP and TNF-α was similar. These data indicate that UTP-induced CCL20 mRNA increase was not due to stabilization of CCL20 mRNA but rather involved transcriptional activation.
Since UTP may be hydrolyzed in UDP by ectonucleotidases and subsequently activate P2Y6 receptors, we tested INS365, a P2Y2 and P2Y4 agonist with prolonged half-life and used in clinical trials in CF (Pendergast et al., 2001), and ATPγS (a slowly hydrolysable P2Y2 and P2Y11 agonist). Both agonists reproduced the effect of UTP on CCL20 release, suggesting an involvement of the P2Y2 receptor, according to the absence of P2Y4 and P2Y11 mRNAs in our HNECs cultures. Furthermore, we show that both UDP and INS48823 (a P2Y6 agonist with prolonged half-life) increased CCL20 release by HNECs indicating also an involvement of P2Y6 receptors. Taken together, our results indicate that activation of both P2Y2 and P2Y6 receptors led to an increase in CCL20 release by HNECs. Our inhibitors experiments suggest that P2Y2 and/or P2Y6 receptor activation induced CCL20 release through an ERK1/2/p38 based mechanism but not through NF-κB signaling pathways in HNECs. Since we still observed the inhibitory effect of UTP on TNF-α-induced CCL20 release in the presence of PD98059 or SB203580, that suggested that this inhibitory effect of UTP observed in pro-inflammatory conditions may involve other pathways than ERK1/2/p38/MAPK. In addition, we failed to detect any effect of UTP on CCL20 mRNA expression level or stability in TNF-α-stimulated cells after 3 h of stimulation. Our data rather suggest that the inhibitory effect of UTP on TNF-α-induced CCL20 release might depend on post-transcriptional mechanisms.
A previous work has shown that P2Y2 receptor activation evokes only a modest increase in IL-8 release from bronchial epithelial cells but potentiates the IL-8 release induced by a mucopurulent supernatant of CF patients (Ribeiro et al., 2005). Here, we show that mucosal UTP alone failed to evoke regulation of IL-8 secretion by HNECs. Furthermore, we show that, in pro-inflammatory conditions, mucosal UTP was capable of significantly preventing TNF-α or LPS-induced CCL20 and IL-8 releases. The difference of effects induced by UTP on IL-8 release in pro-inflammatory conditions between Ribeiro et al. (2005) and our data may come from the airway origin (upper airway in our study but lower airway for Ribeiro et al.), and the inflammatory conditions (TNF-α/LPS in our experiments but a pool of mucopurulent multi-infected lung materials of CF patients in Ribeiro's study). In CF, production of pro-inflammatory cytokines like IL-8 is up-regulated in AECs resulting in an elevated neutrophil infiltration in airways (Tabary et al., 1998). Our data suggest that in pro-inflammatory conditions, UTP may prevent an excessive inflammation by reducing IL-8 secretion by airway epithelium.
CCL20 is known as an antimicrobial chemokine
CCL20 is a bi-functional peptide which also exerts antimicrobial activity against a wide spectrum of mainly Gram-negative bacteria (Starner et al., 2003; Yang et al., 2003). Recombinant CCL20 at 0.3 µg/ml kills ∼90% f E. coli bacteria (Hoover et al., 2002; Yang et al., 2003). In our experiments, HNECs generated in response to UTP about 0.5 ng of CCL20 per well (∼20 ng/mg of total proteins) in ASL, resulting in a theoretical mean apical concentration of ∼0.35 µg/ml. According to Hoover et al. (2002), our data suggest that CCL20 level induced by nucleotides might be high enough to exert direct antimicrobial activity in particular microenvironments such as ASL or the paracellular space.
Effects of UTP on the interaction of HNECs with immune cells
CCL20 is secreted by airway epithelium in response to inflammatory stimuli and attracts immune cells at inflammation sites (Reibman et al., 2003). CCR6, the CCL20 receptor, is expressed on memory T cells, NK cells and some DC subtypes and plays an important role in DC localization and lymphocyte homeostasis in mucosal tissues, as demonstrated in CCR6 knockout mice (Lukacs et al., 2001; Lugering et al., 2003). Nevertheless, it has been shown that CCL20 had no effect on monocyte-derived immature DCs (Dieu et al., 1998) but rather attracted Langherans-type DCs that express CCR6 receptors or other leukocyte populations (memory T cells, γ/δ T cells) (Banchereau et al., 2000). In our experiments, monocyte-derived DCs and neutrophils failed to express CCR6 whereas about 60% of CD4+ T cells expressed CCR6. On the other hand, IL-8 is the most potent neutrophil chemoattractive chemokine. We therefore tested whether HNECs basolateral supernatants were capable of attracting neutrophils and CD4+ T cells. We found that CD4+ T cells and neutrophils chemotaxis was up-regulated ∼2-fold by basolateral supernatants of normal and CF TNF-α-treated HNECs. In presence of LPS or TNF-α, one of the factor produced during airway inflammatory diseases, UTP acted as an anti-inflammatory agent by preventing neutrophils and CD4+ T cells chemotaxis. This was correlated to the inhibition of CCL20 and IL-8 release. We could speculate that in CF therapy where CF airways are inflamed and infected, uridine nucleotides may act as anti-inflammatory agents by inhibiting CCL20 and IL-8 production induced by CF inflammatory environment and by preventing an excessive neutrophil or CCR6-expressing immune cell recruitment in CF airways.
Nucleotides induced CCL20 release from DCs and monocytes
A dense network of DCs has been described in the airways from nasal to alveolar mucosa. In airways, DCs sample and process pathogens in order to prime naïve T cells, either locally or within the draining lymph nodes (Lambrecht and Hammad, 2003). Here, we hypothesized that the stimulatory effect of nucleotides on CCL20 secretion by HAECs could be amplified by a similar effect on airway DCs. Epithelial cells and DCs can interact through cell–cell interactions and release of soluble mediators (Lambrecht and Hammad, 2003). One class of mediators which are abundantly released by epithelia during inflammation is extracellular nucleotides (Homolya et al., 2000).
Here, we showed that external nucleotides promoted CCL20 release not only by airway epithelia but also by DCs or monocytes but not by T CD4+ lymphocytes or neutrophils. To our knowledge, this is the first clear demonstration that DCs or monocytes could secrete CCL20. However, some caution is necessary since DCs differentiated from monocytes may not be perfectly representative of endogenous DCs residing in the airway mucosa in vivo. The release of CCL20 in response to ATPγS and UDP, but not in response to UTP/INS365, suggested the involvement of P2Y11 and P2Y6 receptors in DCs. Due to the lack of specific agonists or antagonists to efficiently discriminate P2X and adenine nucleotide P2Y receptors, we could not exclude a concomitant involvement of P2Y11 and P2X receptors in ATPγS-induced CCL20 release. On the other hand, since only UDP-induced CCL20 secretion in monocytes, the P2Y6 receptor seems to be preferentially involved in monocytes. Our data are in agreement with the up-regulation of the P2Y11 receptor during monocytes to DC differentiation (Wilkin et al., 2001). Monocytes and DCs have already been described to secrete IL-8 in response to P2Y6 receptor stimulation (Warny et al., 2001; Idzko et al., 2004) whereas ATP-induced P2Y11 receptor activation promoted thrombospondin-1 release in DCs (Marteau et al., 2005). The pharmacological differences that we observed between HAECs and DCs or monocytes on CCL20 release certainly depend on different expression of P2Y receptors in the distinct cell types. Our inhibitor experiments performed in DCs suggested that NF-κB, ERK1/2 as well as p38/MAPK pathways play a critical role in ATPγS-enhanced CCL20 release while UDP-induced CCL20 increase mainly involved NF-κB and p38/MAPK pathways.
Likewise, we found that LPS, a microbial agent that induces DC maturation (Banchereau et al., 2000), evoked CCL20 release by activating NF-κB and p38/MAPK pathways. Moreover, we observed that ATPγS but not UDP-potentiated CCL20 release from LPS-matured DCs. This could be explained by the observations that LPS induces nucleotide release and P2Y6 receptor activation in monocytes (Warny et al., 2001) and by the fact that the P2Y6 receptor is responsive to submicromolar concentrations of UDP whereas higher concentrations of ATP are required to activate the P2Y11 receptor (Communi et al., 1996, 1999). Finally, the P2Y11 receptor is coupled to both the cAMP and the phosphoinositide pathways while the P2Y6 receptor activation mainly triggers the Ca2+ signaling pathway (Communi et al., 1996, 1999). Thus, we could speculate that ATPγS-induced P2Y11 activation by stimulating the cAMP pathway may have synergistic effects with the LPS-induced signaling pathway.
In conclusion, we demonstrate for the first time that nucleotides, previously known as regulator of the mucociliary clearance, may also act as connecting molecules between the airway epithelium and the immune system by regulating inflammatory mediator release. UTP or analogs used in aerosol therapy in pulmonary and inflammatory diseases like CF might have anti-inflammatory properties that could be of interest for further clinical investigations.
Acknowledgements
We thank Frédéric Bulté and Dr. Anne Lefort for technical assistance. We thank Prof. Van Hummelen from the KUL microarray facility (KUL, Leuven, Belgium) and Nathalie Bles (ULB, IRIBHM, Belgium) for helpful advices and discussions. We are grateful to Dr. B. Yerxa (Inspire, Durham, NC, USA) for the gift of INS365 and INS48823 and Dr. B. Robaye (ULB, IRIBHM, Belgium) for the gift of TNF-α.




