Reduced chondrogenic potential of adipose tissue derived stromal cells correlates with an altered TGFβ receptor and BMP profile and is overcome by BMP-6†
Thea Hennig and Helga Lorenz are contributed equally to this work.
Abstract
Recent interest has focused on mesenchymal stem cells (MSC) for tissue engineering and regenerative therapy of cartilage defects. MSC originating from adipose tissue (ATSC) are attractive as they are easily available and abundant. They have similar properties like bone marrow derived MSC (BMSC), except for a reduced chondrogenic potential under standard culture conditions driven by TGFβ. Aim of this study was to search for possible differences explaining the reduced differentiation capacity of ATSC and to eliminate it by adaptation of induction protocols. Expanded MSC were analyzed for their growth factor and related receptor repertoire and ATSC spheroid cultures were supplemented with BMP-2,-4,-6,-7, TGFβ, FGFa, FGFb, IGF-1, and PTHrP alone or in combination with TGFβ. In contrast to BMSC, ATSC showed reduced expression of BMP-2, -4, and -6 mRNA and did not express TGFβ-receptor-I protein. Consistent with this, increased concentrations of TGFβ did not improve chondrogenesis of ATSC. BMP6 treatment induced TGFβ-receptor-I expression and combined application of TGFβ and BMP-6 eliminated the reduced chondrogenic potential of ATSC inducing a gene expression profile similar to differentiated BMSC. Like in BMSC, chondrogenesis of ATSC was associated with hypertrophy according to premature collagen Type X expression, upregulation of alkaline-phosphatase activity and in vivo calcification of spheroids after ectopic transplantation in SCID mice. In conclusion, a distinct BMP and TGFβ-receptor repertoire may explain the reduced chondrogenic capacity of ATSC in vitro, which could be compensated by exogenous application of lacking factors. Further studies should now be directed to induce chondrogenesis in the absence of hypertrophy. J. Cell. Physiol. 211: 682–691, 2007. © 2007 Wiley-Liss, Inc.
Mesenchymal stem cells (MSC) are considered to be an attractive cell source for application in regenerative medicine due to their excellent capacities in proliferation and differentiation. MSC are present in various tissues like bone marrow, adipose tissue (ATSC), muscle, synovial tissue, dermis, and others (Young et al., 2001; Zuk et al., 2002; Tintut et al., 2003; Sakaguchi et al., 2005). The most thoroughly characterized population are bone marrow derived mesenchymal stem cells (BMSC), but lately stem cells derived from ATSC gain in importance as they can easily be accessed in large quantities and show comparable characteristics to BMSC (De Ugarte et al., 2003). Though MSC surface markers have been extensively characterized (Haynesworth et al., 1992b; Bruder et al., 1997; Yoo et al., 1998; Barry et al., 1999, 2001; Lodie et al., 2002), no MSC specific marker was identified. MSC are, therefore, mainly defined by their multilineage capacity. It remains unclear, if MSC derived from different tissues are identical cell populations or if each tissue has its own stem cell population with slightly distinct capacities.
Extensive characterization of BMSC and ATSC presented no differences concerning cluster of differentiation (CD) marker profile, proliferation characteristics, and differentiation capacity for the osteogenic and the adipogenic lineage (Jaiswal et al., 1997, 2000; Dennis et al., 1999). The two cell populations varied, however, strongly in their potency to undergo chondrogenesis under standard conditions (Winter et al., 2003), an observation that was confirmed in a comparison of various stem cell sources by Sakaguchi et al. (2005). Standard culture conditions for chondrogenic induction of MSC include high-density spheroid culture in serum-free medium supplemented with ascorbate, dexamethasone and transforming growth factor beta 3 (TGFβ3) (10 ng/ml). While under these conditions about 90% of BMSC cultures accumulated proteoglycan and collagen Type II, less than 20% of the ATSC donor cultures underwent successful chondrogenic differentiation and collagen Type II protein expression remained restricted to parts of the spheroids (Winter et al., 2003).
It is well known that growth factors, especially of the TGFβ superfamily can influence the success of chondrogenic induction of MSC in vitro and in vivo. TGFβ was demonstrated to be crucial for in vitro induction of chondrogenic differentiation in BMSC (Yoo et al., 1998; Bai et al., 2004; Huang et al., 2004; Lee et al., 2004a). Further, bone morphogenetic proteins (BMPs), which form a subgroup of the TGFβ superfamily, secrete signaling factors regulating cartilage and bone formation (Massague and Chen, 2000; Yoon et al., 2004). BMP-4 can upregulate the levels of collagen Type II protein and aggrecan in cultured articular chondrocytes (Luyten et al., 1994) and BMP-2 and -6 are expressed in prehypertrophic chondrocytes (Vortkamp et al., 1996). In vitro, BMP-2 (Majumdar et al., 2001; Palmer et al., 2005; Sekiya et al., 2005), BMP-4 (Sekiya et al., 2005) and BMP-6 (Indrawattana et al., 2004; Sekiya et al., 2005) were reported to have supportive effects on the chondrogenic differentiation of BMSC.
In cartilage development and endochondral ossification endogenous BMPs promote the differentiation of chondrocytes and can activate MSC towards producing the same factors by autocrine stimulation. Specific heteromeric Type I/Type II serine/threonine kinase receptor complexes mediate BMP action. Type I receptors, also termed activin-receptor-like kinase (ALKs) act downstream of Type II receptors and determine the specificity within the receptor complex. The Type I receptors activate their intracellular downstream targets, known as Smads (Heldin et al., 1997). So, differences in the level of endogenously secreted growth factors and/or in the expression of their specific receptors might modulate the resulting phenotype of the target cell and be responsible for the success of chondrogenic differentiation in MSC.
The aim of this study was to search for differences in the repertoire of expressed growth factors and growth factor receptors between ATSC and BMSC, which might lead to the reduced chondrogenic differentiation potential of ATSC. Based on this background we wanted to improve chondrogenic induction conditions for ATSC to obtain a chondrogenic phenotype similar to BMSC.
MATERIALS AND METHODS
Materials
For all experiments human recombinant growth factors were used. Human platelet-derived growth factor BB (PDGF-BB) and epidermal growth factor (EGF) were purchased from Strathmann Biotech (Hamburg, Germany). TGFβ3, BMP-2, -4, -6, -7 and insulin like growth factor-1 (IGF-1) were obtained from R + D Systems (Wiesbaden, Germany) and Parathyroid hormone-related protein (PTHrP) from Bachem (Bubendorf, Switzerland).
Human samples
ATSC stromal cells were isolated from 21 lipoaspirates generated in elective liposuction procedures (mean age 30.7 years male and female). Bone marrow samples for isolation of mesenchymal stem cells (BMSC) were obtained after informed consent from three patients (mean age 47.8 years male and female) undergoing total hip replacement or iliac bone graft harvest. Studies were approved by the local ethics committee. Informed consent was obtained from all individuals included in the study.
Cell isolation and cultivation
BMSC were isolated from fresh bone marrow samples as described previously (Haynesworth et al., 1992a). Briefly, cells were fractionated on a ficoll density gradient (Ficoll-Paque™-PLUS, Amersham Pharmacia, Uppsala, Sweden) and the low-density MSC-enriched fraction was washed, seeded in culture flasks and maintained at 37°C in a humidified atmosphere and 6% CO2. MSC expansion culture medium consisted of Dulbecco's modified Eagle's medium (DMEM) high glucose with 40% MCDB201 medium (Sigma-Aldrich, Deisenhofen, Germany), 2% fetal calf serum (FCS), 2 × 10−8 M dexamethasone, 10−7 M ascorbic acid-2-phosphate, 5 µg/ml insulin, 5 µg/ml tranferrin, 5 µg/ml selenous acid, 100 units/ml penicillin, 100 µg/ml streptomycin, 10 ng/ml recombinant human EGF (Strathmann Biotech) and 10 ng/ml recombinant PDGF-BB (Strathmann Biotech). After 24–48 h, MSC cultures were washed with phosphate-buffered saline (PBS) to remove non-adherent material. During expansion, medium was replaced twice a week. Cells were used after 2–6 passages.
ATSC were isolated according to the method described by Hauner et al. (1989). Lipoaspirates were digested with Krebs–Ringer solution buffered with 25 mM HEPES, 20 mg/ml bovine serum albumin (BSA) and 1.5 mg/ml collagenase (CLS Type I, Worthington Biochemical Corp., Freehold, NJ) and filtered with a 250 µm nylon mesh. Erythrocytes were removed by erythrocyte lysis buffer (0.154 M NH4Cl, 10 mM KHCO3, 0.1 mM EDTA). The remaining cells were seeded in culture flasks at 5,000–6,000 cells/cm2 and maintained under conditions identical to those of BMSC.
Induction of differentiation
Spheroids of 4–5 × 105 MSC were formed by centrifugation at 300g in 1.5 ml microcentrifuge tubes (Eppendorf, Hamburg, Germany). After incubation at 37°C, 6% CO2 for 4 days spheroids were transferred to 96-well U-bottomed plates. Cells were kept in induction medium for 2, 4, 7, 14, 21, 28, or 42 days. Basal medium consisted of DMEM high glucose supplemented with 5 µg/ml insulin, 5 µg/ml tranferrin, 5 ng/ml selenous acid, 0.1 µM dexamethasone, 0.17 mM ascorbic acid-2-phosphate, 1 mM sodium pyruvate, 0.35 mM proline, 1.25 mg/ml BSA, and was supplemented with various human recombinant growth factors (TGFβ3, BMP-2, -4, -6, -7, IGF-1, fibroblast growth factor 1 acidic (FGFa) or basic (FGFb) or PTHrP) in a concentration of 10 ng/ml as indicated.
RNA isolation
Stem cell spheroids were minced in a polytron (Kinematica, Littau-Luzern, Switzerland). Total RNA was then isolated using a standard guanidinium thiocyanate/phenol extraction (peqGOLD Trifast; Peqlab, Erlangen, Germany). Polyadenylated mRNA was isolated from total RNA using oligo-d(T) coupled to magnetic beads (Dynabeads; Dynal, Oslo, Norway) according to the manufacturer's instruction.
RT-PCR
First strand cDNA was generated of 20 ng of each mRNA by using reverse transcriptase (Omniscript®, Quiagen, Hilden, Germany) and oligo-d(T) primers. First strand cDNA was diluted 1:5 and 1 µl was subjected to PCR using gene-specific upstream and downstream primers (Table 1). Following PCR, 5 µl of the samples were loaded onto a 1.5% agarose gel containing ethidium bromide, electrophoresed and visualized under UV light. β-Actin-specific bands were used as controls for equal amounts of cDNA in each sample.
| Gene | Sequence | Size (bp) |
|---|---|---|
| β-actin | 5′-CTCTTCCAGCCTTCCTTCCT-3′ | 244 |
| 5′-CGATCCACACGGAGTACTTG-3′ | ||
| Activin receptor like kinase Type 1 (ALK-1) | 5′-ATCTGAGCGGGCGACAC-3′ | 124 |
| 5′-GGTTGCCTTGGTGGAGTGT-3′ | ||
| Activin receptor Type I (ALK-2) | 5′-AAGGCAGGTATGGTGAGGT-3′ | 252 |
| 5′-AGAGTAGTAAGCTGAAGATAG-3′ | ||
| Activin receptor IIA (Act RII/ACVR2A) | 5′-TCAGGTGCTATACTTGGTAGA-3′ | 350 |
| 5′-TGGCTTAGGTGTAACTGGATT-3′ | ||
| Bone morphogenetic protein 2 (BMP-2) | 5′-ACGAGGTCCTGAGCGAGTTC-3′ | 312 |
| 5′-GAAGCTCTGCTGAGGTGATAA-3′ | ||
| Bone morphogenetic protein 4 (BMP-4) | 5′-AAGAGCAGATCCACAGCAC-3′ | 323 |
| 5′-TCTCGTGTCCAGTAGTCGT-3′ | ||
| Bone morphogenetic protein 6 (BMP-6) | 5′-ATTACAACAGCAGTGAATTGA-3′ | 156 |
| 5′-TTCATGTGTGCGTTGAGTG-3′ | ||
| Bone morphogenetic protein 7 (BMP-7) | 5′-ATGGTGGCTTTCTTCAAGG-3′ | 248 |
| 5′-CAGTAGTAGGCGGCGTAGC-3′ | ||
| Bone morphogenetic protein receptor Type IA (BMPRIA) | 5′-TATGGAGAAGTATGGATGGG-3′ | 559 |
| 5′-AGATGTCAGCCATGATGTAG-3′ | ||
| Bone morphogenetic protein receptor Type IB (BMPRIB) | 5′-GACACTCCCATTCCTCATC-3′ | 356 |
| 5′-GCTATAGTCCTTTGGACCAG-3′ | ||
| Bone morphogenetic protein receptor Type II (BMPRII) | 5′-TCAAGAACGGCTATGTGCGT-3′ | 588 |
| 5′-CGCTCATCCAAGGAGCCTTT-3′ | ||
| Collagen alpha 1 type 2 (COL2A1) | 5′-TGGCCTGAGACAGCATGAC-3′ | 373 |
| 5′-AGTGTTGGGAGCCAGATTGT-3′ | ||
| Collagen alpha 1 type X (COL10A1) | 5′-CCCTTTTTGCTGCTAGTATCC-3′ | 468 |
| 5′-CTGTTGTCCAGGTTTTCCTGGCAC-3′ | ||
| Fibroblast growth factor 1 basic (FGFb) | 5′-CGTTACCTGGCTATGAAGGA-3′ | 142 |
| 5′-TCAGTGCCACATACCAACTG-3′ | ||
| Osteocalcin (OC) | 5′-TCACACTCCTCGCCCTATTG-3′ | 362 |
| 5′-GAAGAGGAAAGAAGGGTGCC-3′ | ||
| Osterix | 5′-CCAGGCAACACTCCTAC CC-3′ | 329 |
| 5′-TATCCACCACTACCCCCAGT-3′ | ||
| Transforming growth factor ß receptor I (TGFβ RI) | 5′-ATTACCAACTGCCTTATTATGA-3′ | 133 |
| 5′-CATTACTCTCAAGGCTTCAC-3′ | ||
| Transforming growth factor ß receptor II (TGFβ RII) | 5′-GGAAATGGAGGCCCAGAAAGATG-3′ | 108 |
| 5′-CACTGACAACAACGGTGC-3′ |
Quantitative real time PCR
cDNA was synthesized as described for RT-PCR. Expression levels of individual genes were analyzed by quantitative PCR using LightCycler™ technology (Roche diagnostics, Mannheim, Germany) and the LightCycler™ FastStart DNA Master SYBR Green I Kit was used according to the manufacturer's instructions.
PCR amplification conditions were: 10 min denaturation at 95°C followed by 40 cycles of denaturation at 95° C, annealing at 58° C for 7 sec and extension at 72° C for 30 sec. Specificity of the PCR products was confirmed by melting curve analysis and agarose gel electrophoresis of PCR products. The expression of the gene of interest in each sample is given as the percentage of β-actin expression. The number of cDNA copies is correlated with the apparent threshold cycle (CT). Building the difference between CT of gene of interest and CT of β-actin from one sample gives ΔCT values which can be expressed as percentage of β-actin. A ΔCT value of 0 corresponds to an expression level similar to β-actin signal, a ΔCT value of 4 corresponds to an expression level of 10-fold β-actin signal. Standard curves for COL10A1 and COL2A1 confirmed identical efficiencies for both primer pairs in real-time PCR demonstrating that the earlier detection of Col10A1 was not an artifact of the used primer sets (data not shown).
Histology
Staining procedures were performed using standard protocols. In brief, spheroids were fixed with 4% paraformaldehyde/PBS (2 h, 20°C), dehydrated in alcohol, rinsed in acetone, and infiltrated with paraffin. Sections of 4–5 µm were dried, deparaffinized using XEM-200 (Vogel, Giessen, Germany) and rehydrated in alcohols. To detect proteoglycan synthesis as an indication of cartilage production, sections were stained with 1% Alcian Blue (Chroma, Köngen, Germany) for 10–30 min. Counterstaining was performed for 3–5 min with fast red before permanently mounting with Eukit (HICO-MIC, Hirtz&Co, Cologne, Germany).
For immunohistochemical staining of collagen Type II, sections were treated with 2 mg/ml hyaluronidase (Merck, Darmstadt, Germany; 15 min, 37°C) followed by digestion with 1 mg/ml pronase (Roche Diagnostics; 30 min, 37°C). Non-specific background was blocked using 5% BSA/PBS (30 min). Sections were incubated overnight at 4°C with a monoclonal mouse anti-human collagen Type II antibody (clones I-8H5 and II-4C11, ICN Biomedicals, Aurora, OH) in PBS/1% BSA. The detection of collagen Type X was performed as described in detail previously (Aigner et al., 2000). In brief, de-paraffinized sections were pre-treated with 0.02 mg/ml protease XXIV (Sigma Aldrich, Deisenhofen, Germany; 60 min, 20°C) to optimize staining intensity before detection with a monoclonal mouse anti-human collagen Type X antibody (X-53, undiluted; Girkontaite et al., 1996). Following, the sections were washed with tris-buffered saline and incubated with a biotinylated goat anti-mouse secondary antibody (1:500; Dianova, Hamburg, Germany). Furthermore, sections were pre-treated with streptavidin-alkaline phosphatase (Dako, Glostrup, Denmark; 30 min, 20°C), and following incubated with Fast Red (Roche Diagnostics; 15 min, 20°C). At last, sections were permanently mounted with Aquatex (Merck) and examined by light microscopy.
Western blot analysis
Expanded BMSC or ATSC were washed in PBS, homogenized, lysed in 100 µl of 10 mM Tris-HCl (Merck) and 0.5% NP-40 (Calbiochem, Bad Soden, Germany) for 30 min at 4°C and centrifuged at 12,000g for 15 min to remove cellular debris. Proteins were separated on 8% polyacrylamide gel and transferred to nitrocellulose membranes (Amersham, Braunschweig, Germany). After blocking in TBS supplemented with 5% BSA (Sigma, Deisenhofen, Germany) and 0.1% Tween 20 (Sigma) (TBST), membranes were incubated for 2 h at room temperature with a monoclonal mouse anti-human collagen Type II antibody (1:250; clones I-8H5 and II-4C11, ICN Biomedicals), a monoclonal mouse anti-human collagen Type X antibody (X-53) (Girkontaite et al., 1996) or a polyclonal rabbit anti-human TGFβ RI antibody (1:200; Santa Cruz Biotechnology, Heidelberg) in TBST/1% BSA and washed three times with TBST for 10 min. For detection of collagen Type II and X protein, membranes were incubated with a biotinylated goat anti-mouse secondary antibody (1:500; Dianova), pre-treated with streptavidin-alkaline phosphatase (Dako; 30 min, 20°C), and incubated with Fast Red (Roche Diagnostics; 15 min, 20°C). To detect TGFβ RI protein, membranes were incubated with an AP-conjugated goat anti-rabbit IgG (1:500; Santa Cruz Biotechnology), washed and protein was visualized with NBT/BCIP according to the manufactures instruction.
cDNA array production
Analyzed genes included cartilage-, bone-, and ATSC-specific genes as well as housekeeping genes (GAPDH and β-actin, RPS9, RPL13) and negative controls (Arabidopsis thaliana genes). Selected cDNAs (size range 400–850 base pairs) were cloned into pBluescript SK+ vector (Stratagene, Amsterdam, Netherlands). cDNAs were PCR amplified using vector specific primers and 50 ng of plasmid as template. PCR products were purified (PCR purification kit, Qiagen, Hilden, Germany), concentrated, and standardized amounts arrayed onto positively charged nylon filters (Hybond N+, Amersham Pharmacia Biotech, Freiburg, Germany). Gene fragments (10 ng/dot) of 48 selected genes (Steck et al., 2005), including four housekeeping genes and negative controls (Arabidopsis thaliana), were spotted twice on each filter.
cDNA array hybridization
32[P]-labeled cDNA probes were prepared from isolated mRNA according to the manufacturer's protocol (SuperScript II, Life Technologies, Karlsruhe, Germany). The labeled cDNAs were denatured and hybridized to cDNA arrays overnight at 68°C. Arrays were washed 3 × 30 min in 0.04 M phosphate buffer pH 7.2/1% SDS at 68°C before exposure to a Fuji imaging plate for 18 h. Images were captured on a Bio-Imaging Analyzer BAS-1800 II using BAS Reader 2.26 beta software (Fuji/Raytest, Straubenhardt, Germany) and analyzed using the AIDA software (Fuji/Raytest). Expression levels in mRNA samples of different sources were normalized to signal strength of the housekeeping genes.
Alkaline phosphatase enzyme activity assay
Culture supernatants were collected at day 14, 21, 28, and 42 of the same pellet after induction and stored at −80°C until further use. For measurement of alkaline phosphatase (ALP) activity 100 µl of each supernatant was incubated with 100 µl of substrate solution (10 mg/ml p-nitrophenylphosphate (Sigma-Aldrich) in 0.1 M glycine, 1 mM MgCl2, 1 mM ZnCl2 (pH 9.6)). After incubation for 150 min, measurement was carried out at 405 nm in an ELISA reader and enzyme activity was referred to a standard curve made from p-nitrophenol (Sigma-Aldrich) and normalized to the total DNA content of the pellet.
DNA quantification
Spheroids were homogenized in 500 µl of 0.01% Triton X-100 after 14, 21, 28, and 42 days of induction. Twenty five microliters of the cell extract was mixed with 150 µl of cold EDTA, pH 12.3 incubated at 37°C for 20 min and neutralized with 10 µl of KH2PO4. After addition of 50 µl of Hoechst 33258 solution (Sigma-Aldrich; 200 µg/ml in water) fluorescence was measured at 355 nm. Data were referred to DNA standard curve prepared from sheared salmon sperm DNA.
In vivo implantation of spheroids into SCID mice
After 5 weeks of culture under chondrogenic conditions in vitro, micromasses were transplanted into subcutaneous pockets, which were prepared in the upper dorsal area of anesthetized SCID mice. Before transplantation, pellets were attached to a non-resorbable surgical suture with a small amount of fibrin glue to facilitate transplantation and harvest. The mice were killed 4 weeks after transplantation, and the transplants were excised and subjected to histological and immunohistological procedures. Animal experiments were approved by the Local Animal Experimentation Committee Karlsruhe.
RESULTS
Reduced expression of BMP-2, -4, and -6 and of TGFβ RI in ATSC
Analysis of expanded and undifferentiated ATSC revealed a reduced mRNA expression of BMPs compared to BMSC. In ATSC faint BMP-2 and BMP-6 expression was found in 1 of 3 donor samples, while BMP-4 expression was not detected (Fig. 1A). However, when a larger collection of ATSC samples was analyzed for BMP-4 expression, 3 of 21 donors were positive. Interestingly, the ATSC positive for BMP-2 and -6 also revealed some collagen Type II immunostaining after chondrogenic induction in contrast to the others, which remained negative (data not shown). No differences in mRNA expression were evident for FGFb (Fig. 1A), FGFa, IGF-1, TGFβ1, TGFβ3, BMP-7, and connective tissue growth factor (CTGF; not shown).
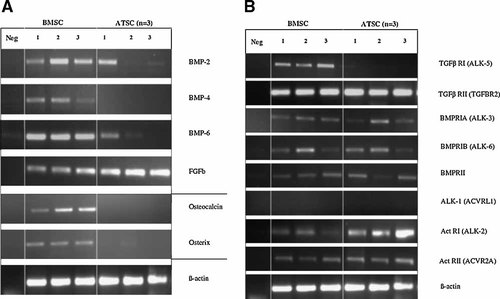
Endogenous growth factor expression in undifferentiated mesenchymal stem cells from bone marrow (BMSC) and adipose tissue (ATSC). Cells obtained from three donors each were cultured for two passages before mRNA was isolated and gene-specific RT-PCR was performed. A: bone morphogenetic protein (BMP)-2, -4, and -6, basic fibroblast growth factor 1 (FGFb) and bone specific molecules osteocalcin and osterix. B: mRNA expression of growth factor receptor molecules. Expression of β-actin was used as control.
BMPs like BMP-2, -4, -6, and -7 act via heterodimeric receptors encoded by the genes BMP receptor Type IA (BMPRIA), Type IB (BMPRIB) or activin receptor-like kinase ALK-2 (Act RI) and BMP receptor Type II (BMPRII) or activin receptor IIA (Act RII). No differences for mRNA expression of these receptors were evident between BMSC and ATSC except for Act RI (ALK-2), which was detected at higher levels in ATSC by RT-PCR (Fig. 1B). TGFβ in contrast, signals via TGFβ Type I receptor ALK-5 in a heterodimer with TGFβ RII (Derynck and Zhang, 2003). Remarkably, TGFβ RI (ALK-5) provided signals only in BMSC but not ATSC samples indicating a reduced responsiveness to TGFβ in ATSC.
Further, low levels of mRNA expression of the osteogenic markers osteocalcin and osterix were detected in BMSC, but not in ATSC (Fig. 1A). This might suggest the presence of osteoprogenitor cells or a predetermination of BMSC towards the osteogenic pathway, which may also account the higher BMP expression.
Effect of TGFβ on chondrogenic induction of ATSC
10 ng/ml TGFβ were insufficient for effective induction of a chondrocyte like phenotype in ATSC according to immunohistochemistry (Fig. 2A) and alcian blue staining. When enhanced TGFβ concentrations (25 and 50 ng/ml) were applied over 4 weeks, no collagen Type II deposition was detected (Fig. 2B,C). Even after application of 50 ng/ml TGFβ for 6 weeks pellets remained negative for collagen Type II (not shown). Thus, consistent with a lack of TGFβ RI expression, enhanced TGFβ concentrations could not improve chondrogenesis of ATSC.
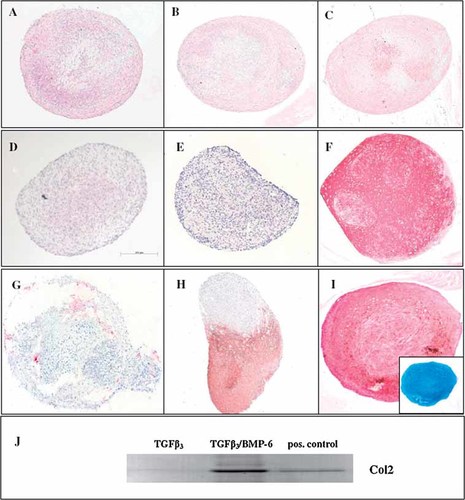
Detection of collagen Type II in spheroids after induction of ATSC by distinct growth factors (10 ng/ml each). Spheroids were either stained by immunohistochemistry (A–I) or subjected to Western Blot analysis (J). A: TGFβ3, (B) 25 ng/ml TGFβ3, (C) 50 ng/ml TGFβ3, (D) BMP-4, (E) BMP-6, (F) BMSC induced with TGFβ3 (positive control), (G) ATSC induced with TGFβ3 plus BMP2, (H) TGFβ3 plus BMP-4, and (I) TGFβ3 plus BMP-6, Inset: Alcian blue staining. J: Spheroids were pooled, homogenized in lysis buffer and subjected to PAGE and Western blotting together with purified collagen Type II used as a positive control (55 kDa).
Single and combined application of growth factors
To improve chondrogenic induction of ATSC we tested whether the following single growth factors could replace TGFβ when applied for up to 6 weeks at 10 ng/ml: TGFβ, BMP-2, BMP-4, BMP-6, BMP-7, FGFa, FGFb, IGF-1, and PTHrP. In some of the donors BMP-2, BMP-4, BMP-6, BMP-7, and FGFa induced mRNA expression of COL2A1, while addition of IGF-1, FGFb, and PTHrP to the induction medium was never followed by upregulation of COL2A1 mRNA (Table 2). None of the single growth factors, however, reproducibly resulted in positive immunohistological staining for collagen Type II (Table 2, Fig. 2D,E).
| Growth factor (10 ng/ml each) | RT-PCR | Immunohistochemistry |
|---|---|---|
| COL2A1 | Collagen Type II | |
| Number of positive/total donorsa | Number of positive/total donorsb | |
| TGFβ3 | 3/6 | 1/9 |
| BMP-2 | 1/4 | 0/5 |
| BMP-4 | 1/3 | 0/4 |
| BMP-6 | 4/6 | 0/7 |
| BMP-7 | 1/3 | 0/4 |
| FGFa | 1/3 | 0/4 |
| IGF-1 | 0/2 | 0/3 |
| FGFb | 0/3 | 0/4 |
| PTHrP | 0/2 | 0/3 |
| TGFβ3 + BMP-2 | 6/6 | 2/7 |
| TGFβ3 + BMP-4 | 3/3 | 3/7 |
| TGFβ3 + BMP-6 | 7/7 | 7/9 |
| TGFβ3 + BMP-7 | 3/3 | 0/6 |
| TGFβ3 + IGF-1 | 2/3 | 0/3 |
| TGFβ3 + FGFa | 4/5 | 0/6 |
| TGFβ3 + FGFb | 1/3 | 0/4 |
| TGFβ3 + PTHrP | 1/3 | 0/3 |
- Shown are the expression of COL2A1 mRNA and the deposition of collagen Type II.
- a 4–7 spheroids per donor were pooled for mRNA analysis.
- b 2–3 spheroids per donor were evaluated.
When TGFβ was combined with one of the other growth factors, induction of COL2A1 mRNA was enhanced (Table 2). However, in contrast to RT-PCR data, no immunohistochemical staining occurred in any of the spheroids, if TGFβ was administered together with BMP-7, IGF-1, FGFa, FGFb, or PTHrP. Deposition of collagen Type II was observed only for combinations of TGFβ with BMP-2, BMP-4, and BMP-6, respectively, (Table 2, Fig. 2G–I) and thus for those growth factors endogenously produced by BMSC (Fig. 1A). The most potent induction occurred by TGFβ and BMP-6 (Table 2, Fig. 2I) in all tested donors while spheroids induced with TGFβ/BMP-2 (Fig. 2G) or TGFβ/BMP-4 (Fig. 2H) were only partially positive for collagen Type II. Alcian blue staining, indicating proteoglycan deposition, always correlated with immunohistological staining for Type II collagen (Fig. 2I, inset).
Detection of collagen Type II protein by Western Blot analysis correlated with immunohistochemistry but not with faint COL2A1 mRNA signals obtained by RT-PCR (Fig. 2J and Table 2).
TGFβ RI protein expression is induced in ATSC after treatment with BMP-6
Consistent with RT-PCR results, Western Blot analysis confirmed discrepant expression of TGFβ RI protein between BMSC and ATSC in cells derived from three donors (Fig. 3A). However, when expanded ATSC were exposed to 10 ng/ml BMP-6 for 2 weeks in monolayer culture TGFβ RI expression was induced (Fig. 3B), providing a possible mechanism of rendering the cells responsive to TGFβ.
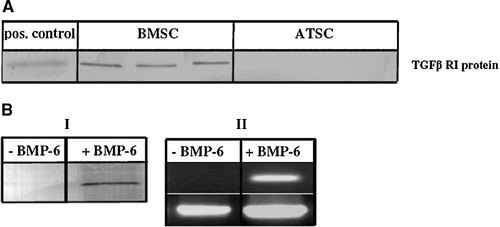
Analysis of endogenous TGFβ RI expression of BMSC and ATSC before and after BMP-6 treatment. A: Expanded ATSC and BMSC (n = 3, passage 2) were analyzed by Western blotting and chondrocytes were used as positive control (52 kDa). B: BMP6 treated ATSC were subjected to Western blotting (I) and gene-specific RT-PCR (II).
Successful chondrogenic induction leads to a similar expression profile in ATSC and BMSC
Cartilage and stem cell relevant gene expression was quantified by cDNA array analysis before and after successful induction of chondrogenesis (Fig. 4C). The gene expression pattern of uninduced ATSC and BMSC was almost identical (Fig. 4A,D). In addition, successfully induced BMSC (use of TGFβ) and ATSC (use of TGFβ and BMP-6) showed similar gene expression profiles with upregulation of cartilage-related genes like AGC, BGN, COL2A1, COL11A1, COMP, and DCN (Fig. 4B,E). In contrast, in ATSC incubated with TGFβ alone, only upregulation of COMP and downregulation of YKL-40 was evident and no major shift in gene expression occurred under these conditions (Fig. 4F). This is consistent with the lack of a collagen Type II and proteoglycan positive extracellular matrix in these spheroids.
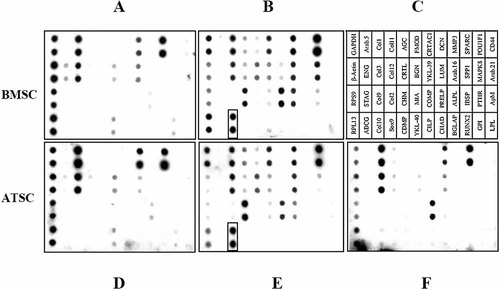
cDNA array analysis of BMSC and ATSC before and after chondrogenic induction. A: Non-induced BMSC, (B) TGFβ3 induced BMSC, (C) annotation of cartilage and bone specific genes (Steck et al., 2005), (D) non-induced ATSC, (E) TGFβ3 plus BMP-6 induced ATSC, and (F) TGFβ3 induced ATSC. COL10A1 expression is marked in boxes.
Induction of ATSC with TGFβ and BMP-6 leads to a hypertrophic phenotype and calcification in vivo
cDNA-array analysis revealed an upregulation of the hypertrophic marker COL10A1 in both BMSC and ATSC after successful chondrogenic induction (Fig. 4B,E). Remarkably, upregulation of COL10A1 mRNA occurred before that of COL2A1 during successful chondrogenesis of ATSC according to RT-PCR (Fig. 5A) and real-time PCR confirmed this on a quantitative level (Fig. 5B). This phenomenon was described before for BMSC (Mwale et al., 2006; Pelttari et al., 2006). Standard curves for COL10A1 and COL2A1 confirmed identical efficiencies for both primer pairs in real-time PCR (data not shown), demonstrating that the earlier detection of COL10A1 was not an artifact of the primer sets used. Deposition of collagen Type X protein in spheroids (Fig. 6B) and upregulation of ALP enzyme activity in culture supernatants (n = 3, Fig. 5C) further confirmed the induction of a hypertrophic phenotype in ATSC. No upregulation of ALP activity occurred when ATSC were induced with TGFβ alone for 6 weeks (data not shown). When spheroids were chondrogenically induced for 5 weeks and implanted subcutaneously in SCID mice for further 4 weeks, they showed distinct areas of calcification and microossicle formation demonstrating that no stable phenotype like in articular cartilage was obtained (Fig. 6C, D).
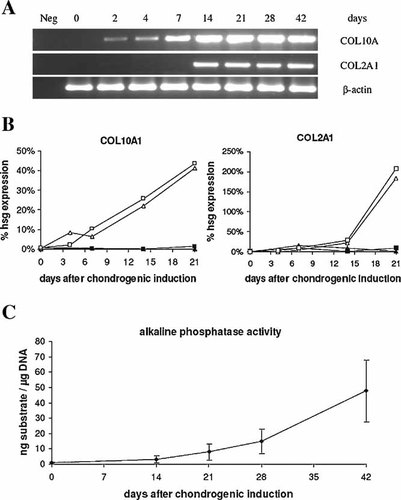
Evaluation of a hypertrophic phenotype of ATSC during chondrogenic induction for 6 weeks. A: Time course of COL10A1 and COL2A1 mRNA expression detected by RT-PCR after chondrogenic induction with TGFβ3/BMP-6. The signal for COL10A1 mRNA is evident before that of COL2A1 mRNA.Identical signal intensities for β-actin indicate equal amounts of cDNA template in each sample. B: Time course of COL10A1 and COL2A1 mRNA expression detected by quantitative real time PCR. The expression was normalised to the expression of the house keeping gene β-actin. Two donor cell populations of ATSC were induced with TGFβ3/BMP6 (10 ng/ml each) (open symbols) or with TGFβ3 (closed symbols). C: Time course of alkaline phosphatase (ALP) enzyme activity. ALP-activity in culture supernatants was measured by turn-over of p-nitrophenylphosphate substrate at day 0, 14, 21, 28 and 42 after induction and normalized to the total DNA content.
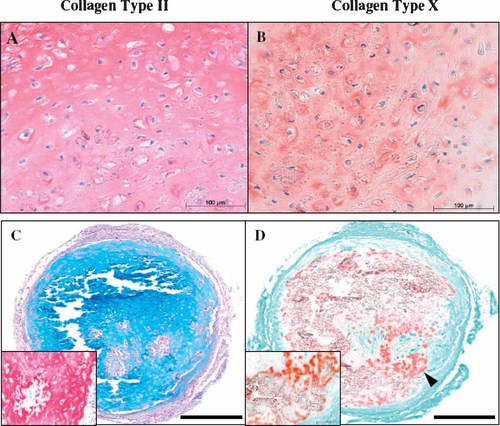
Histology of ATSC spheroids. Immunohistochemical staining of ATSC spheroids for collagen Type II (A) and collagen Type X (B) after chondrogenic induction for 6 weeks with TGFβ3 and BMP-6 in vitro. C, D: Ectopic cartilage formation capacity of ATSC was analysed by subjecting 5x105 cells/spheroid to a 5 week induction period under chondrogenic conditions followed by transplantation of two spheroids (n = 5 donors) into a subcutaneous pocket of a SCID mouse. Four 4 weeks later, explants were harvested and sections were stained with Alcian Blue (C) and Alizarin Red (D). All spheroids were rich in proteoglycans (C), and collagen Type II (C inset, 20-fold higher magnification of a selected area) but also showed calcification and microossicle formation (arrow head in D, inset). Representative data of one experiment are shown. Bar = 200 µm.
DISCUSSION
Due to their ability to develop into various types of tissue, MSCs have an outstanding role in regenerative medicine. As the recovery of bone marrow, the major source of adult stem cells, requires a painful procedure, the focus has been on alternative stem cell sources recently. Especially adipose tissue derived MSCs are estimated advantageous because of their abundance and easy accessibility (Huang et al., 2004). For successful application of these cells in tissue engineering it is important to thoroughly characterize their differentiation potential and improve cultivation protocols in order to enhance the quality of regenerative therapies. Though ATSC have been reported to display similar characteristics to BMSC (De Ugarte et al., 2003; Winter et al., 2003; Lee et al., 2004b), their potential for chondrogenic differentiation in 3D culture, and thus, their quality in tissue engineering of cartilage is controversial (Zuk et al., 2002; Winter et al., 2003; Huang et al., 2004; Sakaguchi et al., 2005). While some authors report the beginning of chondrogenic differentiation of ATSC as early as 14 days after induction for single donors, we found a 90% reduced chondrogenic potential of ATSC in micromass cultures, when more than 100 donor cultures were compared to BMSC (Winter et al., 2003 and unpublished results) and our observation was confirmed by others (Sakaguchi et al., 2005).
Our data indicate that BMPs are necessary for successful chondrogenic induction of MSC. While all tested BMSC populations apparently produced BMP-2, -4, and -6 endogenously, ATSC showed expression of these BMPs only in exception and if so they contained cells that were able to undergo chondrogenesis and deposited some collagen Type II protein in the matrix. Remarkably, BMP-2, -4, and -6 mRNA expression in undifferentiated ATSC was seen at a similar frequency like successful differentiation of pellets under stimulation with TGFβ alone. When one of the lacking BMPs (BMP-2, -4, or -6), however, was added to the TGFβ containing chondrogenic medium, deposition of collagen Type II protein was evident in the pellets, indicating a close correlation between the presence of BMP and successful chondrogenesis. Whether discrepancies in BMP expression of ATSC cultures is donor-dependent or related to a contamination with other or partially differentiated cell populations, remains to be determined. We speculate that in BMSC cultures, osteoprogenitor cells may account for a consistent secretion of BMPs facilitating chondrogenesis. Alternatively, a small fraction of BMSC may undergo direct osteogenesis already during expansion conditions and provide BMPs for the culture. Beside BMPs, undifferentiated ATSC also lacked expression of TGFβ receptor Type I and had higher ALK-2 mRNA levels, the main Type I receptor for BMP-6 (Ebisawa et al., 1999; Nifuji et al., 2004). Interestingly, treatment of ATSC with BMP6 induced expression of the TGFβ receptor I protein (Fig. 3) and eliminated the reduced chondrogenic capacity of ATSC most likely by enhancing the sensitivity of ATSC for TGFβ3. This is consistent with the fact that BMP-6 alone was ineffective and combined addition of TGFβ and BMP-6 was necessary to induce chondrogenesis. In sum, the expression profile and response to growth factors demonstrated that expanded BMSC and ATSC are not identical cell populations which require distinct growth factor supplementation for successful chondrogenesis.
According to the present study TGFβ and BMP-6 was the best combination among a multitude of growth factors to induce chondrogenic differentiation of ATSC. Although other combinations were capable to provide COL2A1 and COL10A1 mRNA signals, these observations rarely correlated with a phenotypic shift to chondrocytes and also to deposition of a cartilage like matrix, which is positive for collagen Type II and proteoglycan. Since up to seven spheroids had to be pooled for mRNA isolation, the high sensitivity of the RT-PCR detection method may have depicted even a very small number of differentiating cells, which might have escaped detection in immunohistological staining or Western Blotting. Alternatively, induction of COL2A1 mRNA may be only a first step towards chondrogenesis of MSC, which is, however, not sufficient for a shift of the cell phenotype towards chondrocyte-like cells and the successful deposition of collagen Type II and proteoglycan in the surrounding matrix. Thus, mRNA detection of single marker genes like COL2A1 by RT-PCR has to be interpreted with care, when evaluating chondrogenic differentiation of MSC. In contrast, a chondrogenic phenotype always correlated with a broad shift of gene expression profiles in cDNA-array analysis. Evaluation of spheroids by histology or cDNA array analysis is, therefore, crucial to measure the success of chondrogenesis in culture.
BMSC could, due to their origin from bone, be predetermined towards osteogenesis and preferential induction of a differentiation program related to fracture healing or endochondral ossification is conceivable. We previously demonstrated that successful in vitro chondrogenesis of BMSC was always associated with COL10A1 upregulation and induction of ALP activity (Pelttari et al., 2006). Hypertrophic chondrocytes in the growth plate express COL10A1 and show ALP activity during endochondral bone formation before they mineralize and eventually enter apoptosis (Enomoto-Iwamoto et al., 1998; Shum and Nuckolls, 2002). In contrast, chondrocytes in normal articular cartilage do not alter their phenotype, remain ALP negative and COL2A1 is the major collagen expressed. Except for low levels in cells bordering the tidemark, COL10A1 is not expressed in healthy articular chondrocytes, whose development seems arrested before hypertrophy. We here demonstrate that MSC derived from ATSC undergo a similar program like BMSC leading to a phenotype, which rather resembles hypertrophic chondrocytes of the growth plate than resting chondrocytes of normal articular cartilage. The cartilage formation of chondrocytes can be measured in vivo by ectopic transplantation in immune deficient mice (Lipman et al., 1983; Dell'Accio et al., 2001; 2003), a very good model to further prove the concept of tissue engineering from stem cells. However, most of the current papers always used cell culture, a concept which cannot reflect the real situation. After subcutaneous transplantation of spheroids into SCID mice, BMSC and ATSC pellets both underwent vascular invasion, calcification, and microossicle formation and did not form stable ectopic cartilage-like transplants like spheroids made of human articular chondrocytes (Pelttari et al., 2006). This indicates that not a predisposition of BMSC to undergo osteogenesis or fracture healing seems to be responsible for this result, since ATSC from liposuction material should not be predetermined to form bone. Rather, standard in vitro conditions for induction of MSC chondrogenesis seem to be suboptimal and should be improved to allow chondrogenesis in the absence of hypertrophy. When MSC are considered as a source for chondrocytes in tissue-engineered repair or regeneration of cartilage, their functional suitability and phenotypic stability are imperative for clinical application and any risk for graft instability should be avoided. It is, however, important to emphasize that the subcutaneous environment in mice is not representative for a cartilage repair situation and further studies should address the performance of MSC with and without prior chondrogenic induction after transplantation in cartilage defects in a joint environment.
Acknowledgements
The authors thank Regina Foehr for excellent technical assistance and Christoph Lee, MD (Department of Orthopaedic Surgery, University of Heidelberg) and Proaesthetic (Private Clinic, Heidelberg) for providing bone marrow and adipose tissue samples. The authors thank Eric Steck for his helpful contribution in revising the manuscript, and also Prof. von der Mark for kindly providing the X 53 antibody for detection of collagen Type X.




