Therapies for bleomycin induced lung fibrosis through regulation of TGF-β1 induced collagen gene expression
Abstract
This review describes normal and abnormal wound healing, the latter characterized by excessive fibrosis and scarring, which for lung can result in morbidity and sometimes mortality. The cells, the extracellular matrix (ECM) proteins, and the growth factors regulating the synthesis, degradation, and deposition of the ECM proteins will be discussed. Therapeutics with particular emphasis given to gene therapies and their effects on specific signaling pathways are described. Bleomycin (BM), a potent antineoplastic antibiotic increases TGF-β1 transcription, TGF-β1 gene expression, and TGF-β protein. Like TGF-β1, BM acts through the same distal promoter cis-element of the COL1A1 gene causing increased COL1 synthesis and lung fibrosis. Lung fibroblasts exist as subpopulations with one subset predominately responding to fibrogenic stimuli which could be a specific cell therapeutic target for the onset and development of pulmonary fibrosis. J. Cell. Physiol. 211: 585–589, 2007. © 2007 Wiley-Liss, Inc.
TGF-β1 is an important modulator in the acute repair phase of a wound. It is a profibrotic factor that affects many cellular functions, including fibroblast proliferation and chemotaxis, stimulating the synthesis and deposition of connective tissue, and the inhibition of connective tissue breakdown. Type I collagen (COL 1) is the major fibrous collagen synthesized by wound fibroblasts in the repair process. When levels of TGF-β1 are chronically elevated, excessive fibrosis results which is characterized by increased collagen deposition. Fibrosis is the response to a traumatic event which terminates in the deposition of a connective tissue matrix. The composition and volume of that matrix differentiates between normal scar in acute wound repair and excessive fibrosis from chronic inflammation. In general, excess fibrosis contains increased concentrations of collagen, a rich blood supply, and myofibroblasts, identified by α-smooth muscle (SM) actin in cytoplasmic stress fibers. The development of fibrosis follows the sequence of overlapping phases; lag, proliferative, and remodeling. Either prolonging the proliferative phase and/or hindering the remodeling phase causes excess fibrosis.
Trauma initiates the repair process that can terminate in a normal flat scar or a raised hypertrophic scar. The acute wound repair process provides a rapid and efficient way to restore mechanical tissue integrity. On the other hand, excess fibrosis is a prolonged process that can terminate into functional morbidity. An early event in repair is the restoration of homeostasis by the deposition of a fibrin clot which forms the highway for the migration of cells into the defect. At the termination of fibrosis, the fibrin matrix is replaced with a collagen matrix. The initial cells to travel the fibrin highway are the inflammatory cells. First, neutrophils enter the wound site followed by macrophages. Inflammatory cells prevent microbial colonization, remove dead tissue, and release factors that promote the proliferative phase. Fibroblasts and endothelial cells migrate into the wound site, where they synthesize and deposit a new vascularized connective tissue matrix called granulation tissue. In granulation tissue endothelial cells initially form capillaries which develop into blood vessels, that is the process of angiogenesis. Fibroblasts in granulation tissue transform into myofibroblasts. Granulation tissue matures into scar during the remodeling phase in which the density of capillaries and blood vessels decline and myofibroblasts enter apoptosis. The scar unlike granulation tissue has few fibroblasts (no myofibroblasts), a low density of blood vessels, and collagen fibers arranged in random arrays. In excessive fibrosis, like hypertrophic scar, there is a high density of fibroblasts and myofibroblasts, a high density of blood vessels, and an exuberant immature collagen matrix.
Active TGF-β1 is a major cytokine that stimulates the transcription of the COL1A1 and COL1A2 genes which are located on different chromosomes (Fig. 1). COL 1 contains two polypeptide chains which are synthesized on ribosomes bound to the rough endoplasmic reticulum (Fig. 1). Certain prolyl and lysyl residues on each chain as they are being synthesized are hydroxylated by P-4-H and lysyl hydroxylase (LH) located in the cisternae of the RER (Fig. 1) and associated with ribosomes synthesizing the proCOL polypeptides (Cutroneo et al., 1977). P-4-H has also been shown to be associated with total lung tissue polysomes (Rokowski et al., 1981). ProCOL1A1 and proCOL1A2 of proCOL1 is in the ratio of 2 to 1 (Fig. 1). The triple helical portion of proCOL1 will constitute the COL1 molecule after the removal of the N and C termal globular regions.
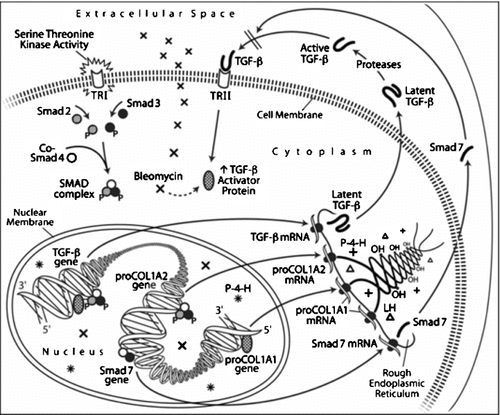
Diagrammatic representation of the mechanisms of extracellular and intracellular signaling of BM and TGF-β1 on the expression of the TGF-β1, proCOL1A1, proCOL1A2, and Smad 7 genes. This figure also demonstrates the synthesis and post translation hydroxylation modification of proCOL1A1, proCOL1A2 polypeptides, collagen triple helix formation, and propeptide globular extension formation.
Nuclear P-4-H is a distinct enzyme from the P-4-H located in the cisternae of the RER (Hofbauer et al., 2003; Marxsen et al., 2004). The nuclear enzyme's function is to hydroxylate specific prolyl residue(s) of hypoxia inducible factor-1, to ready this enhancer factor for ubiquinization, and subsequent 26 S proteosome degradation (Huang et al., 1998).
The synthesis of the different proCOL1 polypeptides is controlled by two separate pathways, the TGF-β-activating protein signaling pathway and the SMAD signaling pathway (Fig. 1). When TGF-β1 reacts with cell membrane receptors, the TGF-β activator protein signaling pathway is activated and the COL1A1 gene is transcribed. The transcription of the COL1A2 gene is via the SMAD pathway. Methods are available for reducing fibrosis advanced by excess TGF-β1 accumulation. Glucocorticoids diminish the synthesis of proCOL1 without affecting the degradation of pro COL1A1 and pro COL1A2 mRNAs (Cutroneo and Sterling, 2004). Silencing the signal pathways responsible for procollagen gene expression is another possible method for reducing COL1 synthesis associated with fibrosis. Cutroneo and Ehrlich [2006] reported the use of dsdecoys containing the TGF-β element in the phosphorothioate (PT) sense strands 5′-ATCCTGGCTGCCCACGGCCAGCCGGCC-3′ where the underlined base sequence represents the consensus TGF-β element, as a novel nonsteroidal antifibrotic to control fibrosis. Can dsdecoys alter the levels of proCOL1 and collagen synthesis? The hallmark of chronic fibrosis is increased extracellular matrix (ECM) protein synthesis by fibrotic fibroblasts continually stimulated by TGF-β1. The most fibrous collagen is COL1 which forms hypertrophic scar tissue and in the case of vital organs such as the heart, liver, or lung, leads to morbidity and in some patients death.
Bleomycin-induced Pulmonary Fibrosis
TGF-β1 the major profibrotic growth factor during fibrogenesis is produced by fibroblasts, myofibroblasts, recruited SM cells, and macrophages. This growth factor is stored in platelets. Bleomycin (BM), the antineoplastic antibiotic, treatment of lung fibroblasts results in an increased transcription of TGF-β1, an increase of TGF-β1 gene expression, and increased TGF-β protein (Breen et al., 1992; Fig. 1). BM was shown to act through the same distal cis-element of the COL1A1 gene as TGF-β1 by intracellular and extracellular signaling (King et al., 1994; Fig. 1). An autocrine mechanism maintains homeostasis for fibroblasts to limit the fibrogenic response to BM and TGF-β1 (Cutroneo, 2006); through intracellular signaling by decreasing the Smad 3 transcription factor binding to a distinct SMAD binding element in the proximal promoter of the Smad 7 inhibitory gene (Fig. 1). The early increase of total lung TGF-β1 (i.e., 7 days post-intratracheal BM) results from TGF-β1 production by alveolar macrophages, which generate latent TGF-β1 complexed with the glycoprotein thrombospondin (TSP-1) associated with CD36. This associated complex is necessary for plasmin release of active TGF-β1 (Yehualaeshet et al., 2000).
Alveolar epithelial cell (AEC) apoptosis is a prime factor in pulmonary fibrosis. The onset of AEC apoptosis by BM requires the synthesis and binding of angiotensin II to receptor AT1. The coadministration of the AT1 antagonist losartan with BM intratratracheally reduced DNA fragmentation, the active immunoreactive caspase 3, and total lung hydroxyproline (Li et al., 2003).
The Smads are also involved in the pathogenesis of BM-induced pulmonary fibrosis. Smad 3 deficient mice have decreased pro COL1 gene expression and lung hydroxyproline content as compared to wild-type mice treated with BM (Zhao et al., 2002). Therefore, inhibitors of Smad 3 may be potential therapy for lung fibrosis and also gene therapies directed at Smad 3. However, the SMAD signaling pathways is involved in the regulation of many diverse gene pathways. IL-7-inhibition of the TGF-β1 signaling pathway in lung fibroblasts results from increased Smad 7, the major inhibitory Smad of the SMAD signaling pathway (Huang et al., 2002). Recombinant IL-7 in vivo decreases BM-induced pulmonary fibrosis.
P38 mitogen-activator protein kinase (MAPK), a major signal transduction pathway of pro-inflammatory cytokines, and its substrate activating transcription factor (ATF)-2 were found to be phosphorylated in bronchoalveolavar fluid cells in intratracheal BM-treated mice (Matsuoka et al., 2002). In this study, the phosphorylation of (ATF)-2 was inhibited by the subcutaneous injection of a specific inhibitor of this MAP kinase. This inhibitor also decreased the expression of tumor necrosis factor-alpha, connective tissue growth factor (CTGF), apoptosis of lung cells, and pulmonary fibrosis in BM-induced lung fibrosis. Cytoplasmic phospholipase A(2) (cPLA-2), a key enzyme in the generation of pro-inflammatory eicosanoids, is important in the pathogenesis of murine BM-induced pulmonary fibrosis. Mutation of the cPLA2 gene abrogated fibrosis and inflammation in BM-induced lung fibrosis (Nagase et al., 2002). Lung collagen deposition during pulmonary fibrosis is a function of the ratio of tissue inhibitors of metalloproteinases (TIMPS) and matrix metalloproteinases (MMPs). TIMP-1 gene expression and protein are selectively increased during murine BM-induced pulmonary fibrosis (Madtes et al., 2001). In situ hybridization showed that the TIMP-1 mRNA was spatially restricted to the areas of lung injury.
TGF-β, Fibrosis, and Col1 Synthesis
TGF-β1 is the major profibrotic growth factor which stimulates fibroblast collagen production and deposition. The three known isoforms of TGF-β stimulate cultured fibroblast procollagen synthesis but are differentially expressed in BM-induced murine pulmonary fibrosis (Coker et al., 1997). However, these studies only determined gene expression and not protein profiles.
The SMAD signaling pathway transmits the signal from the TGF-β receptor I on the cellular membrane to the nucleus through the cytoplasm (for review see Heldin et al., 1997; Fig. 1). Increased transcription of the COL1A2 gene by TGF-β1 is determined by the binding of a Smad 3/Smad 4 to the TbRE cis-element in the proximal promoter region of this gene (Zhang et al., 2000; Fig. 1). Sp1 also binds to this complex. Recently, it has been shown that TGF-β cytostatic signals rely on a Smad 3 but not a Smad 2 dependent pathway (Kim et al., 2005). In addition, the TGF-β signaling level depends on the Smad 3/Smad 2 ratio. A Smad 3/Smad 4 complex is required for Smad 7 gene transcription (Fig. 1) by TGF-β1 which codes for this major inhibitory Smad 7 (Von Gersdorff et al., 2000). TGF-β1 induction of the COL1A2 gene depends on a 25 bp cis-element in the proximal promoter of this gene. This is an AP-1 site and this sequence is essential for the TGF-β response which may not require Sp-1 binding sites on this gene (Chung et al., 1996). Another study demonstrated that Sp-1 but not Ap-1 is required for the upregulation of COL1A2 gene transcription (Greenwell et al., 1997). Treatment of fibroblasts with TGF-β1 or BM, the latter which acts on fibroblasts through TGF-β1, increases collagen synthesis and the amount of TGF-β activator protein/TGF-β element complex in gel mobility shift assays. Glucocorticoids decrease collagen synthesis and the transacting protein/TGF-β element complex (Fig. 1; Shukla et al., 1999).
Gene Therapy for BM-Induced Lung Fibrosis
There exist many viral vectors coupled with certain genes which attenuate BM-induced pulmonary fibrosis. BM-induced murine lung receiving an intratracheal injection of a human adenovirus vector carrying mouse Smad 7 cDNA resulted in significant decreases of pro COL1 mRNA and lung hydroxyproline content with no morphological evidence of fibrosis as compared to mice given Smad 6 cDNA (Nakao et al., 1999). A single adenovirus-decorin transgene treatment of BM-induced murine lung, decreased the fibrotic response as determined by lung hydroxyproline as compared to control virus (Kolb et al., 2001). Murine BM-induced pulmonary fibrosis was reduced after intra-venous injection of prostaglandin D2 synthetase cDNA-expressing fibroblasts (Ando et al., 2003). This treatment decreased basic fibroblast growth factor, CTGF, and the procollagen mRNAs. After the transfer of the flt-1 gene in vivo into mouse skeletal muscle, this tissue served as a biofactory for anti-vascular endothelial growth factor (VEGF) since flt-1 is a specific receptor for VEGF and soluble flt-1 binds to VEGF and competitively inhibits it from binding to its receptors. This therapeutic strategy was used to attenuate BM-induced pulmonary fibrosis (Hamada et al., 2005). When rats were given intratracheal BM plus adenoviral vector expressing the tissue factor pathway inhibitor (TFPI), there was a decreased BM-induced procoagulant and thrombin generation, inhibition of pulmonary fibrosis, and decreased CTGF gene expression (Kijiyama et al., 2006).
The elucidation of molecular and signaling pathways in eukaryotic cells is often achieved by targeting regulatory element(s) found in the promoter or the enhancer region of eukaryotic gene(s) using a double-stranded (ds) oligodeoxynucleotide (ODN) containing a specific cis-element. Our laboratory is focusing on dsODN decoys containing the TGF-β element found in the distal promoter of the 5′-flanking region of the proCOL1A1 gene as a novel nonsteroidal antifibrotic for achieving normal wound healing.
Dsdecoys have been useful in searching and unraveling molecular mechanisms of the initiation of disease development. The failure of a specific transcription factor to bind to the gene's cis-element may totally knock out specific gene expression. Our dsdecoys have the ability to either silence or totally knockout gene transcription depending upon decoy dose (Cutroneo and Ehrlich, 2006). Knockout of specific mice genes is an in vivo genomic method to observe the physiological, biochemical, and the molecular effect(s) for eliminating the expression of a specific gene(s) and how it alters physiology. However, often it renders a lethal progeny. With SiRNA therapies a transfection agent is required. However, no transfection agent is needed in vivo when locally using the naked linearized PT dsdecoy containing the TGF-β element to inhibit COL1. Therefore, there are major advantages of using linearized PT dsoligo decoy gene therapy for either silencing or knocking out specific gene expression. First, the degree of silencing may be controlled by dsdecoy dose. Secondly, our present in vivo studies demonstrate that transfection methods were not required to obtain biochemical effects of these PT dsdecoys (Cutroneo and Chiu, 2000; Boros et al., 2005).
The central issue is that PT dsdecoys or other modified decoys presently in the pipeline which are more potent, have better delivery, and a longer duration of action could contain the consensus TGF-β element, decrease proCOL1 gene expression, proCOL1 synthesis, and COL1 deposition during fibrosis. The rationale is that the decoys containing the TGF-β element or homologous cis-elements bind the transacting protein preventing the latter from binding to the cis-element in the 5′ flanking region of the natural gene or other genes resulting in transcription inhibition. We are focusing on aspects involved in TGF-β1 induced fibrosis and the use of the TGF-β element containing PT dsdecoys to control excessive COL1 synthesis and deposition resulting from persistent TGF-β1. In our model of regulation of COL1 synthesis, these PT dsdecoys act as promoter competitors, binding to the activator protein (i.e., the transacting factor) both in the cytoplasm and nucleus. The significance of our studies is that these novel natural antifibrotics mimic the effect of glucocorticoids on COL1 synthesis during fibrosis without the unwanted side effects of these steroids. Based on our previous studies on the molecular mechanisms by which glucocorticoids selectively decrease collagen synthesis, designed PT dsdecoys resistant to nuclease action mimic the effects of gluocorticoids at the molecular, cellular and in vivo levels of collagen synthesis (Cutroneo and Sterling, 2004). However, the glucocorticoids significantly inhibit noncollagen protein synthesis. Both the PT single-stranded (ss) and the PT dsdecoys specifically decrease COL1 synthesis without inhibiting total noncollagen protein synthesis (Cutroneo and Chiu, 2000; Cutroneo and Boros, 2002). In future studies, we will determine if you specifically inhibit TGF-β1-induced synthesis by using these PT dsdecoys, will this inhibit pulmonary fibrogenesis?
Protein Kinase C Epsilon (PKC-ε) and Lung Fibrosis
PKC-ε signaling protein is involved in lung fibrogenesis. Following microvascular injury in vivo chronic exposure to thrombin results in the conversion of fibroblasts to myofibroblasts with the activation of PKC-ε being required (Bogatkevich et al., 2001). Associated with these myofibroblasts which resemble the phenotype of scleroderma lung fibroblast, protein-activated receptor expression is increased (Bogatkevich et al., 2005). Scleroderma lung tissue is association with inflammatory and fibroproliferative foci. Thrombin increases tenacin in lung fibroblasts which is mediated by PKC-ε. In fibrotic systemic sclerotic (SS) lung fibroblasts, thrombin decreased PKC-ε mediated tenacin secretion (Tourkina et al., 2001). SS lung fibroblasts in culture overexpressed collagen, contained more activated MEK/ERK as compared to normal fibroblasts (Tourkina et al., 2005). This same study demonstrated that antisense to PKC-ε resulted in concomitant decreases of collagen and PKC-ε. The cis-PKC-ε element in the distal promoter of the PKC-ε gene has a sequence very homologous differing to TGF-β cis-element found in the distal promoter of the COL1A1 gene by only two bases (Cutroneo and Ehrlich, 2006). Will PKC-element dsdecoys be as effective or more so in silencing or knocking out COL1 synthesis and attenuating BM-induced pulmonary fibrosis? Or will TGF-β element containing dsdecoys silence or knockout PKC-epsilon synthesis?
Lung Fibroblast Heterogeneity
There are many cell types involved in BM-induced pulmonary fibrosis including alveolar macrophages, type II AECs, neutrophils, esinophils, myofibroblasts, both resident and collagen synthesizing fibroblasts derived from bone marrow progenitor cells (Hashimoto et al., 2004). Normal lung fibroblasts exist as a heterogenous population of cells (Phipps, 1992) which can be separated and isolated by flow cytometry and cell sorting based upon the presence of the glycophosphatidylinositol-linked protein on the cell surface THY-1(+) and THY-1(−) lung fibroblast subpopulations (Phipps, 1992). The subpopulations when treated with BM were assessed for active TGF-β1, Smad 3 phosphorylation, alpha-SM actin, and fibronectin expression (Zhou et al., 2004). THY-1(−) fibroblasts responded to BM treatment by increases in all four parameters, while the THY-1 (+) cells did not show any stimulating effects. THY-1-1-C57 BL mice treated with BM showed a greater fibrotic response both histopathologically, greater TGF-β1 activation and collagen content (Hagood et al., 2005). Breen et al. [1990] based on fibroblast cell surface proCOL1 or proCOL3 previously separated by flow cytometry and cell sorting the heterogeneous population of lung fibroblasts into subpopulations, one mainly expressing the proCOL1A1 and the COL1A2 genes and the other population mainly expressing the COL3A1 gene. Breen et al. [1992] later demonstrated that the total lung fibroblast population treated with BM before flow cytometry and cell sorting could be separated into one population which responded to BM with increased proCOL1A1 and proCOL1A2 mRNAs which lasted for at least three passages (Breen et al., 1992). Would this population of lung fibroblasts make an excellent specific cell target for dsdecoy attenuation of BM-induced fibroblast proCOL1 synthesis during BM-induced pulmonary fibrosis?
Acknowledgements
This publication was made possible by NIH Grant Number P20 RR16435 from the COBRE Program of the National Center for Research Resources and NIH grants (HL52285 (SHP) and GM056581 (HPE). We appreciate Gary J. Nelson's efforts for putting into one figure many dynamic cellular, biochemical, and molecular concepts of references of others and ours cited in the text of this review. We also would like to thank Mrs. Tammy L. Provencher for her superb typing skills and Mrs. Nancy A. Bianchi for her excellent literature searches.




