ANG II-stimulated DNA synthesis is mediated by ANG II receptor-dependent Ca2+/PKC as well as EGF receptor-dependent PI3K/Akt/mTOR/p70S6K1 signal pathways in mouse embryonic stem cells
Abstract
Effect of angiotensin II (ANG II) on mouse embryonic stem (ES) cell proliferation was examined. ANG II increased [3H] thymidine incorporation in a time- (>4 h) and dose- (>10−9 M) dependent manner. The ANG II-induced increase in [3H] thymidine incorporation was blocked by inhibition of ANG II type 1 (AT1) receptor but not by ANG II type 2 (AT2) receptor, and AT1 receptor was expressed. ANG II increased inositol phosphates formation and [Ca2+]i, and translocated PKC α, δ, and ζ to the membrane fraction. Consequently, the inhibition of PLC/PKC suppressed ANG II-induced increase in [3H] thymidine incorporation. The inhibition of EGF receptor kinase or tyrosine kinase prevented ANG II-induced increase in [3H] thymidine incorporation. ANG II phosphorylated EGF receptor and increased Akt, mTOR, and p70S6K1 phosphorylation blocked by AG 1478 (EGF receptor kinase blocker). ANG II-induced increase in [3H] thymidine incorporation was blocked by the inhibition of p44/42 MAPKs but not by p38 MAPK inhibition. Indeed, ANG II phosphorylated p44/42 MAPKs, which was prevented by the inhibition of the PKC and AT1 receptor. ANG II increased c-fos, c-jun, and c-myc levels. ANG II also increased the protein levels of cyclin D1, cyclin E, cyclin-dependent kinase (CDK) 2, and CDK4 but decreased the p21cip1/waf1 and p27kip1, CDK inhibitory proteins. These proteins were blocked by the inhibition of AT1 receptor, PLC/PKC, p44/42 MAPKs, EGF receptor, or tyrosine kinase. In conclusion, ANG II-stimulated DNA synthesis is mediated by ANG II receptor-dependent Ca2+/PKC and EGF receptor-dependent PI3K/Akt/mTOR/p70S6K1 signal pathways in mouse ES cells. J. Cell. Physiol. 211: 618–629, 2007. © 2007 Wiley-Liss, Inc.
Angiotensin II (ANG II) is the most important effector peptide of the rennin-angiotensin system (RAS). In addition to the traditional actions of the humoral RAS, it has been suggested that all components of the RAS are present in many tissues, including embryos, and may play a major role in embryonic development and differentiation (Kon et al., 1989; Schutz et al., 1996; Tebbs et al., 1999; Leung, 2004). Indeed, the angiotensinogen mRNA from both embryos and yolk sac placenta was larger by approximately 200 bases than the mRNA obtained from an adult rat liver (Lee et al., 1987). Moreover, the angiotensinogen gene leads to an altered ovulatory capacity and early embryonic development in mice (Hefler and Gregg, 2001). During normal pregnancy, ANG II concentrations are elevated in maternal and fetal circulations, which are associated with placental angiogenesis, and growing fetus as well as these activations are mediated by ANG II receptors (Zheng et al., 2005).
It was also reported that one of the functions of ANG II was to maintain the anabolic pathways required for cell proliferation and survival (Touyz and Berry, 2002). A stem cell interacts with its environment through the cell surface receptors that activate the signaling pathways (Burdon et al., 2002). Indeed, the multiple actions of ANG II are mediated via specific, highly complex intracellular signaling pathways that are stimulated following the initial binding to its specific receptors (Matsusaka and Ichikawa, 1997; Vander Heiden et al., 2001; Touyz and Berry, 2002). In mammalian cells, ANG II binds to two distinct high-affinity plasma membrane receptors, AT1 and AT2 (Ardaillou, 1999). Most of the molecular and cellular actions of ANG II are mediated by the AT1 receptor. In various cell types, PKC has been found to be a key signaling pathway associated with the mitogenic response and growth induced by many growth factors including ANG II, EGF, and platelet-derived growth factor (PDGF) (Porreca et al., 1993; Itoh et al., 2001). In addition, previous results not only suggested that ANG II-induced DNA synthesis is partially dependent on extracellular signal-regulated kinase (ERK) and PKC, but also prompted us to hypothesize that another pathway, in addition to ERK and PKC, plays a substantial role in AT1-mediated mitogenesis of IEC-18 cells. In addition, several lines of evidence have shown that there is a complicated signaling crosstalk between ANG II and growth factors (Shah and Catt, 2002; Lautrette et al., 2005; Olivares-Reyes et al., 2005; Shah et al., 2006). This receptor cross talk can occur via extracellular release of EGF receptor ligands at the cell surface by matrix metalloproteases (MMP) that belong to the ADAM (a disintegrin and metalloprotease) family of zinc-dependent proteases (Prenzel et al., 1999). ANG II also increases the expression of the growth-associated nuclear proto-oncogenes and stimulates tyrosine phosphorylation of multiple substrates, including MAPKs (Murasawa et al., 1998; Ino et al., 2003). However, the signal transduction pathways involving the growth-regulatory effects of ANG II have not been reported yet in mouse ES cells, although the sequential proliferation is a tightly regulated process that is modulated by a broad spectrum of regulatory peptides.
The present study examined mouse ES cells, which were cultured in Dulbecco's modified Eagles medium (DMEM) supplemented with a leukemia inhibitory factor (LIF) in order to maintain an undifferentiated state and to support the derivation and expansion of the ES cells (Evans and Kaufman, 1981; Smith, 1992). ES cells are an attractive in vitro model system for examining their initial developmental decisions and molecular mechanisms during embryonic growth and development (Han et al., 2006; Heo et al., 2006). Therefore, the effects of ANG II on the proliferation of ES cells and its related signal pathways were examined in mouse ES cells.
MATERIALS AND METHODS
Materials
Mouse ES cells were obtained from the American Type Culture Collection (ES-E14TG2a). The fetal bovine serum (FBS) was purchased from Biowhittaker (Walkersville, MD). The ANG II, losartan, PD 123319, staurosporine, neomycin, U 73122, PD 98059, SB 203580, AG 1478, herbimycin A, β-actin, and fluorescence isothiocyanate-conjugated (FITC-conjugated) goat-anti mouse IgM were obtained from the Sigma Chemical Company (St. Louis, MO). The bisindolylmaleimide I and MMP inhibitor were purchased from Calbiochem (La Jolla, CA). [3H] thymidine and [3H] inositol phosphates were purchased from Dupont/NEN (Boston, MA). Fluo 3-AM was obtained from Molecular Probes, Inc. (Eugene, OR). The phospho-p44/42 and p44/42 MAPK antibodies were purchased from New England Biolabs (Herts, UK). The anti-Pan Protein Kinase C was obtained from Upstate Biotechnology (Charlottesville, VA). The AT1 receptor, PKC α, δ, ζ, CDK2, CDK4, cyclin D1, cyclin E, p21cip1/waf1, p27kip1, phospho-EGF receptor, total-EGF receptor, phospho-Akt, total-Akt, phospho-mTOR, total-mTOR, phospho-p70S6K1, and total-p70S6K1 antibodies were purchased from Santa Cruz Biotechnology (Delaware, CA). The goat anti-rabbit IgG was purchased from Jackson Immunoresearch (West Grove, PA). All other reagents were of the highest purity commercially available. The liquiscint was obtained from National Diagnostics (Parsippany, NY).
ES-cell culture
Mouse ES cells were cultured with or without a feeder layer in the DMEM (Gibco-BRL, Gaithersburg, MD) supplemented with 3.7 g/L sodium bicarbonate, 1% penicillin and streptomycin, 1.7 mM L-glutamine, 0.1 mM β-mercaptoethanol, 5 ng/ml mouse LIF, and 15% FBS. For each experiment, cells were grown on gelatinized 12-well plates or 60-mm culture dishes in an incubator maintained at 37°C with 5% CO2. The medium was removed and replaced with serum-free DMEM including all supplements containing LIF for 12 h prior to the experiments. After that, the cells were washed twice with PBS and then maintained in a serum-free DMEM including all supplements and indicated agents.
Alkaline phosphatase staining
Approximately 70% confluent mouse ES cells were washed twice with PBS and fixed with 4% formaldehyde (in PBS) for approximately 15 min at room temperature. The cells were washed with PBS and incubated with an alkaline phosphatase substrate solution [200 µg/ml Naphthol AS-MX phosphate, 2% N,N-dimethylformamide, 0.1 M Tris (pH 8.2), and 1 mg/ml Fast Red TR salt (4-chloro-2-methylbenzenediazonium salt; zinc chloride)] for 10 min at room temperature. After washing with PBS, the cells were photographed.
[3H] thymidine incorporation
The [3H] thymidine incorporation experiments were carried out as described by Brett et al. (1993). Zhang et al. (2005b) reported that most ES cells could be arrested in the G0/G1 phase using a serum deprivation culture. Furthermore, the synchronized ES cells could successfully re-enter a normal cell cycle after resupplying the serum. In this study, the cells were cultured in 1-well until they reached 50% confluence, washed twice with PBS, and maintained in serum-free DMEM including all supplements. After 24 h incubation, the cells were washed twice with PBS, and incubated with fresh serum free DMEM including all the supplements and indicated agents. After the indicated incubation period, 1 µCi of [methyl-3H] thymidine (specific activity: 74 GBq/mmol, 2.0 Ci/mmol; Amersham Biosciences, Buckinghamshire, UK) was added to the cultures. Incubation with [3H] thymidine continued for 1 h at 37°C. The cells were then washed twice with PBS, fixed in 10% trichloroacetic acid (TCA) at 23°C for 15 min, and then washed twice with 5% TCA. The acid-insoluble material was dissolved in 2 N NaOH for 12 h at 23°C. Aliquots were removed to determine radioactivity using a liquid scintillation counter (LS 6500, Beckman Instruments, Fullerton, CA). All values are means (±standard errors (S.E.)) of triplicate experiments. Values were converted from absolute counts to a percentage of the control to allow for comparison between experiments.
Cell counting
The cells were washed twice with PBS and trypsinized from the culture dishes. The cell suspension was mixed with a 0.4% (w/v) trypan blue solution and the number of living cells was determined using a hemocytometer. Cells failing to exclude the dye were considered to be non-viable.
Bromodeoxyuridine incorporation
The level of 5-bromo-2′-deoxyuridine (BrdU) (a thymidine analog) incorporation was measured in order to determine the level of DNA synthesis. The ES cells were serum-starved for 24 h prior to ANG II stimulation. The ES cells were then treated with ANG II for 24 h. Fifteen µM BrdU was added during last 16 h of incubation. After several washes with PBS, the cells were fixed with methanol [10% (vol/vol) for 10 min at 4°C], followed by incubation in 1 N HCl for 30 min at room temperature. The cells were then washed and incubated with 0.1 M sodium tetraborate for 15 min. Alexa Fluor 488-conjugate Mouse anti-BrdU mAb (diluted 1:200, Molecular Probes) in 2% BSA-PBS was incubated overnight at 4°C. After washing in PBS, coverslips were mounted onto glass slides with a Dako Fluorescent mounting medium using gelvatol and examined under an optical microscope (fluoview 300, Olympus, Tokyo, Japan).
For the double-labeling experiments, the cells were fixed in acid alcohol and processed for Oct-4 staining, followed by BrdU staining. The fixed cells were incubated with the rabbit anti-Oct-4 antibody (1:100, Santa Cruz Biotechnology) for 1 h at room temperature and Alexa Fluor 555 anti-rabbit IgG (1:100, Molecular Probes) for 1 h at room temperature. This was followed by incubation in 1 N HCl, neutralization with 0.1 M sodium tetraborate, and incubation with Alexa Fluor 488-conjugate Mouse anti-BrdU mAb for 1 h at room temperature. After washing with PBS, the BrdU/Oct-4 stained cells were examined under confocal microscopy (fluoview 300, Olympus).
Fluorescence activated cell sorter (FACS) analysis
The cells were incubated with ANG II (10−7 M) for 24 h and then cells were dissociated in trypsin/EDTA, pelleted by centrifugation, resuspended at approximately 106 cells/ml in PBS containing 0.1% BSA. When required (for Oct-4 staining), cells were fixed in 4% paraformaldehyde and permeabilized in 0.1% Triton X-100. The cells were labeled with the mouse anti-SSEA 1 antibody (1:50, Santa Cruz Biotechnology, Inc.) and then incubated with fluorescein isothiocyanate-conjugated (FITC-conjugated) second antibodies (1:50). The cells were washed and resuspended in PBS and then read by flow cytometry (Beckman Coulter). Samples were analyzed using CXP software (Beckman Coulter).
Inositol phosphates formation assay
The assay performed in this study was a modified version of the one described by Berridge et al. (1982). Cells were labeled myo-[3H]-inositol (2.5 µCi/ml, 2 ml final) for 24 h and followed by the addition of 10 mM LiCl for 15 min and were treated with the ANG II. The medium was removed, cells were scraped off the dish in 1.2 ml H2O and extracted in 1.8 ml chloroform/methanol (1:2, v/v), and the upper phase was applied to Bio-Rad AG 1-X8 columns (Bio-Rad Laboratories, Hercules, CA). After the cells were washed several times with 5 mM inositol and H2O, the fraction containing the [3H]-inositol phosphates (IP1, IP2, and IP3) was eluted with 1 M ammonium formate and 0.1 N formic acid.
Measurement of [Ca2+]i
Changes in [Ca2+]i were monitored by using Fluo-3/AM, which was initially dissolved in dimethylsulfoxide and stored at −20°C. Mouse ES cells in 35-mm culture dishes were rinsed twice with a Bath Solution [140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 0.5 mM MgCl2, 10 mM glucose, 5.5 mM HEPES (pH 7.4)], incubated in the Bath Solution containing 3 µM fluo-3/AM with 5% CO2–95% O2 at 37°C for 40 min, rinsed two more times with the Bath Solution, mounted on a perfusion chamber, and scanned every second using a confocal microscope (400×) (fluoview 300, Olympus). Fluorescence was excited at 488 nm and emitted light was observed at 515 nm. All analysis of [Ca2+]i were processed in a single cell, and expressed as relative fluorescence intensity (RFI).
PKC enzyme activity
The cells were incubated with ANG II (10−7 M) for 1 h and were lysed by ice-cold buffer [10 mM Tris(HCl (pH 7.5), 0.25 M sucrose, 0.2 mM CaCl2, 1 mM phenylmethylsufonyl fluoride (PMSF), 10 µg/ml leupeptin, and 10 mM benzamidine] and were separated into cytosolic and membrane fractions using ultracentrifugation. Aliquots of cytosolic and membrane fractions were assayed for PKC activity by using the PKC enzyme assay system kit (Amersham Biosciences) and expressed as picomoles per minute.
Preparation of cytosolic and total membrane fractions
The preparation of the cytosolic and total membrane fractions was performed using a modified version of the method reported by Mackman et al. (1991). The DMEM of the mouse ES cells was exchanged at 48 h before the experiments. The medium was then removed and the cells were washed twice with ice-cold PBS, scraped, harvested by microcentrifugation and resuspended in buffer A [137 mM NaCl, 8.1 mM Na2HPO4, 2.7 mM KCl, 1.5 mM KH2PO4, 2.5 mM EDTA, 1 mM dithiothreitol, 0.1 mM PMSF, 10 µg/ml leupeptin (pH 7.5)]. The resuspended cells were then mechanically lysed on ice by trituration with a 21.1-gauge needle. The lysates were first centrifuged at 1,000g for 10 min at 4°C. The supernatants were centrifuged at 100,000g for 1 h at 4°C to prepare the cytosolic and total particulate fractions. The supernatants (cytosolic fraction) were then precipitated with 5 vol. of acetone, incubated for 5 min on ice and centrifuged at 20,000g for 20 min at 4°C. The resulting pellet was resuspended in buffer A containing 1% (v/v) Triton X-100. The particulate fractions, which contained the membrane fraction, were washed twice and resuspended in buffer A containing 1% (v/v) Triton X-100. The protein in each fraction was quantified according to the Bradford (1976) procedure.
RNA isolation and RT-PCR
The total RNA was extracted from the cells using STAT-60, a monophasic solution of phenol and guanidine isothiocyanate from Tel-Test, Inc. (Friendwood, TX). Reverse transcription was carried out with 3 µg RNA using a reverse transcription system kit (AccuPower® RT PreMix, Daejeon, Korea) with oligo-dT18 primers. Five microliters of the RT products were then amplified using a PCR kit (AccuPower® PCR PreMix) under the following conditions: denaturation at 94°C for 5 min and 30 cycles at 94°C for 45 sec, 55°C for 1 min and 72°C for 1 min followed by a 5 min extension at 72°C. The primers were 5′-CGTTGCAGACTGAGATTGCC-3′(sense), 5′-ACCGGACAGGTCCACATCTG-3′ (antisense) for c-fos (356 bp), 5′-AACTCGGACCTTCTCACGTCG-3′(sense), 5′-TGCTGAGGTTGGCGTAGACC-3′ (antisense) for c-jun (355 bp), and 5′-TCCATTCCGAGGCCACAGCAAG-3′(sense), 5′-TCAGCTCGTTCCTCCTCTGACG-3′(antisense) for c-myc (266 bp). PCR of β-actin was also performed as a control for the quantity of RNA. The RT-PCR products were separated and visualized on 1.2% agarose gels. In order to analyze the PCR products, the quantitative values of the ANG II treatment group were compared with those calculated from the control group, and the data was then normalized to the data obtained for β-actin.
Real-time RT-PCR
The cells were treated with 10−7 M ANG II for 1 h prior to total RNA extraction. The real-time quantification of RNA targets was performed in the Rotor-Gene 2000 real-time thermal cycling system (Corbett Research, NSW, Australia) using the QuantiTect SYBR Green RT-PCR kit (QIAGEN, Valencia, CA). The reaction mixture (20 µl) contained 200 ng of total RNA, 0.5 µM of each primer, appropriate amounts of enzymes, and fluorescent dyes as recommended by the supplier. The Rotor-Gene 2000 cycler was programmed as follows: 30 min at 50°C for reverse transcription; 15 min at 95°C for DNA polymerase activation; 15 sec at 95°C for denature; 45 cycles of 15 sec at 94°C, 30 sec at 55°C, 30 sec at 72°C. The data collection was carried out during the extension step (30 sec at 72°C). The PCR reaction was followed by a melting cure analysis to verify specificity and identity of the RT-PCR products, which can distinguish the specific PCR products from the non-specific PCR product resulting from primer-dimer formation. The temperature of PCR products was elevated from 65 to 99°C at a rate of 1°C/5 sec, and the resulting data were analyzed by using the software provided by the manufacturer.
Western blot analysis
The cells were harvested, washed twice with PBS, and lysed in a buffer [20 mM Tris (pH 7.5), 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 1 mg/ml aprotinin, 1 mM phenylmethylsulfonylfluoride, 0.5 mM sodium orthovanadate] for 30 min on ice. The lysates were then cleared by centrifugation (10 min at 15,000 rpm, 4°C). The protein concentration was determined according to the Bradford procedure (1976). Equal amounts of the protein (20 µg) were resolved by electrophoresis on 10% SDS–PAGE and transferred to nitrocellulose. After the blots had been washed with TBST [10 mM Tris-HCl (pH 7.6), 150 mM NaCl, 0.05% Tween-20], the membranes were blocked with 5% skimmed milk for 1 h and incubated with the appropriate primary antibody at the dilutions recommended by the supplier. The membrane was then washed, and the primary antibodies were detected with goat anti-rabbit IgG or goat anti-mouse IgG conjugated to horseradish peroxidase, and the bands were then visualized by enhanced chemiluminescence (Amersham Pharmacia Biotech, England, UK).
Statistical analysis
The results are expressed as means ± S.E. All the experiments were analyzed by ANOVA followed in some experiments by a comparison of the treatment means with the control using the Bonnferroni–Dunn test. A P-value <0.05 was considered statistically significant.
RESULTS
Effect of ANG II on mouse ES cell proliferation
The undifferentiated state of the mouse ES cells used in this experiment was confirmed by examining the expression of the undifferentiated stem cells markers, including the alkaline phosphatase activity and Oct-4, FOXD 3, and SOX-2 expression levels. The cells in the presence of ANG II maintained the alkaline phosphatase enzyme activity (Fig. 1A). The mouse ES cells in both the presence and the absence of ANG II expressed Oct-4, FOXD 3, and SOX-2 mRNA (Fig. 1B). Moreover, flow cytometry analysis also showed that cells in the presence of ANG II expressed 89% SSEA 1 positive (control: 87.5%) (Fig. 1C). Therefore, the results demonstrate that mouse ES cells maintained an undifferentiated state under the experimental conditions used in this study.
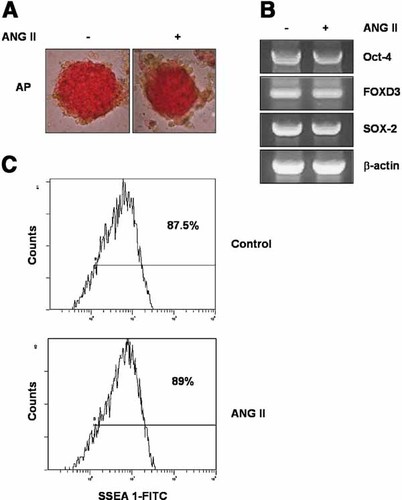
Effect of ANG II on the characterization of mouse ES cells. A: The alkaline phosphatase enzyme activity was measured cells in the presence or absence of ANG II (10−7 M), as described in “Materials and Methods.” B: Oct-4 (519 bp), FOXD3 (171 bp), SOX-2 (550 bp), and β-actin (350 bp) mRNA expression levels in the presence or absence of ANG II. C: Flow cytometry analysis to monitor the percentage of SSEA 1 positive in cells in the presence or absence of ANG II. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
In order to determine the time- and dose-response effect of ANG II on [3H] thymidine incorporation, the level of [3H] thymidine incorporation in mouse ES cells was observed for varying periods of time (0–24 h) and with various dosages of ANG II (0–10−6 M). As shown in Figure 2A, [3H] thymidine incorporation was stimulated in a time-dependent manner with the highest level of [3H] thymidine incorporation being observed at 8 h after incubation with 10−7 M ANG II (50 ± 7% increase vs. control; P < 0.05). When the mouse ES cells were treated with various ANG II doses for 8 h, a significant increase in [3H] thymidine incorporation was observed in the cells incubated with 10−7 M ANG II (52 ± 8% increase vs. control; P < 0.05) (Fig. 2B). Subsequently, there was a significant increase in the number of cells for 24 h incubation of ANG II (0–10−6 M) (Fig. 2C). Moreover, in order to validate ANG II exerts its growth-promoting effect on the undifferentiated ES cells, double labeling for Oct-4 and BrdU expression was performed. In these experiments, the ES cell population contained more than 90% of undifferentiated (Oct-4 positive) cells. The observed effects reflect the role of ANG II in the undifferentiated ES cells, not in the spontaneously differentiated progeny (Fig. 2D).
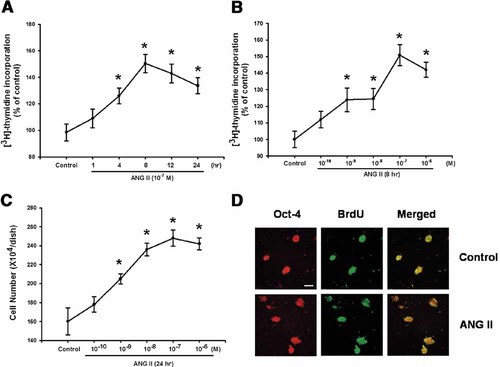
Effect of ANG II on cell proliferation. A: Mouse ES cells were incubated in the presence of ANG II (10−7 M) for varying periods of time (0–24 h) and were subsequently pulsed with 1 µCi of [3H] thymidine for 1 h prior to counting. B: Mouse ES cells were incubated for 8 h with various ANG II concentrations (0–10−6 M) and pulsed with 1 µCi of [3H] thymidine for 1 h. C: Mouse ES cells were treated with ANG II (0–10−6 M) for 24 h, and counted using a hemocytometer. The values represent the mean ± S.E. of four independent experiments with triplicate dishes. *P < 0.05 versus Control. D: Mouse ES cells were incubated with ANG II (10−7 M) for 24 h and double labeled with Oct-4 and BrdU antibody. The scale bars represent 20 µm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Involvement of AT1 receptor in ANG II-induced cell proliferation
In order to determine if the ANG II receptors are involved in ANG II-induced cell proliferation, the mouse ES cells were treated with losartan (10−6 M, an AT1 receptor antagonist) or PD 123319 (10−6 M, AT2 receptor antagonist) for 30 min prior to the ANG II treatment for 8 h. Losartan, not PD 123319, decreased the level of ANG II-induced increase in [3H] thymidine incorporation (P < 0.05) (Fig. 3A). Indeed, the mouse ES cells expressed the AT1 receptor, which was increased up to 6 h incubation of ANG II and then decreased gradually (Fig. 3B).
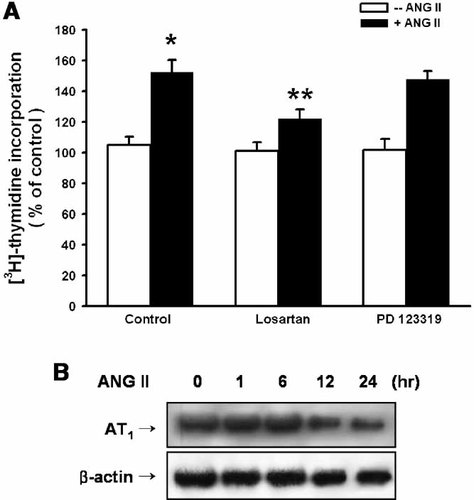
Effect of the ANG II receptor blockers on ANG II-induced stimulation of [3H] thymidine incorporation. A: Mouse ES cells were treated with losartan or PD 123319 (10−6 M) for 30 min prior to the ANG II (10−7 M) treatment for 8 h and subsequently pulsed with 1 µCi of [3H] thymidine for 1 h. The values represent the mean ± S.E. of four independent experiments with triplicate dishes. *P < 0.05 versus Control, **P < 0.05 versus ANG II alone. B: Mouse ES cells were treated with ANG II (10−7 M) for varying periods of time (0–24 h). The total protein was extracted and the protein blots were probed with the AT1 receptor or β-actin specific antibody. The bands represent ∼50 kDa of AT1, and 41 kDa of β-actin, respectively. Each of the examples shown is representative of four independent experiments.
Involvement of Ca2+/PKC in ANG II-induced cell proliferation
In experiment to examine the involvement of Ca2+/PKC pathways in ANG II-induced cell proliferation, ANG II increased [3H]-IPs formation up to 39 ± 8% of control (Fig. 4A) and induced a transient increase of [Ca2+]i followed by a rapid decline (Fig. 4B). Western blotting analysis was carried out to confirm the involvement of PKC in ANG II-induced cell proliferation. The redistribution of PKC from the cytosolic to the membrane compartment is believed to be the first step in PKC activation and was used to measure the level of PKC activation in response to ANG II in mouse ES cells. PKC was found to be present mainly in the cytosolic fraction of the untreated mouse ES cells. After a 1 h treatment with ANG II, ANG II increased PKC activity from cytosolic compartment to membrane compartment (Fig. 4C), which is consistent with Western blotting analysis (Fig. 4D). In addition, ANG II stimulated the translocation of the PKC α, δ, ζ isoforms to the membrane compartment (Fig. 4E). These results indicate that ANG II stimulates PKCs isotypes non-specifically. Consequently, as shown in Figure 4F,G, the pretreatment of the mouse ES cells with neomycin (10−4 M), U 73122 (10−6 M) (PLC inhibitors), or staurosporine, bisindolylmaleimide I (10−6 M, PKC inhibitors) decreased the level of ANG II-induced increase in [3H] thymidine incorporation (P < 0.05).
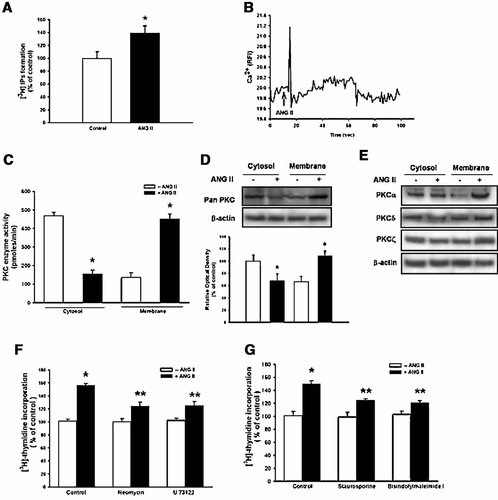
Effect of the PLC/PKC blockers on ANG II-induced stimulation of [3H] thymidine incorporation. A: Mouse ES cells were treated with ANG II (10−7 M) for 90 sec before inositol phosphates assay. B: Mouse ES cells were loaded with 2 µM fluo 3-AM in serum-free medium for 40 min and treated with ANG II (10−7 M) and then Ca2+ influx was measured. C: Mouse ES cells were treated with ANG II for 1 h and then PKC activity was measured as described in “Materials and Methods.” The values represent the mean ± S.E. of three independent experiments with triplicate dishes. *P < 0.05 versus Control. D: The Pan-PKC protein and (E) PKC α, δ, and ζ isoforms, which were present in either the cytosolic compartment or membrane compartment, was detected by Western blotting, as described in “Materials and Methods.” The bands represent the 80 kDa of Pan-PKC, 80–90 kDa of PKC α, δ, and ζ, and 41 kDa of β-actin. Each example shown is a representative of three experiments. The lower part of (D) depicting the bars denotes the mean ± S.E. of three experiments for each condition determined from densitometry relative to β-actin. *P < 0.05 versus Control. F, G: Mouse ES cells were treated with neomycin (10−4 M), U 73122 (10−6 M), staurosporine, or bisindolylmaleimide I (10−6 M) for 30 min prior to the ANG II (10−7 M) treatment for 8 h and subsequently pulsed with 1 µCi of [3H] thymidine for 1 h. The values represent the mean ± S.E. of three independent experiments with triplicate dishes. *P < 0.05 versus Control, **P < 0.05 versus ANG II alone.
Involvement of EGF receptor transactivation in ANG II-induced cell proliferation
In order to further determine if the mitogenic effect of ANG II is mediated by EGF receptor activation, the cells were pretreated with AG 1478 (10−5 M, receptor tyrosine kinase inhibitor) or herbimycin A (10−6 M, tyrosine kinase inhibitor) for 30 min prior to the 10−7 M ANG II treatment. Pretreatment with either AG 1478 or herbimycin A decreased the level of ANG II-induced increase in [3H] thymidine incorporation (31 ± 6% or 28 ± 7% decrease vs. control, respectively; P < 0.05). On the other hand, the combined treatment of AG 1478 and bisindolylmaleimide I completely blocked the ANG II-induced cell proliferation (50 ± 9% decrease vs. control; P < 0.01) (Fig. 5A). As shown in Figure 5B, ANG II and EGF stimulated the phosphorylation of the EGF receptor. However, AG 1478 inhibited the ANG II-induced phosphorylation of the EGF receptor. In experiments to examine the role of MMPs in ANG II-induced EGF receptor activation, MMP inhibitor also blocked the phosphorylation of ANG II-induced EGF receptor (Fig. 5B).
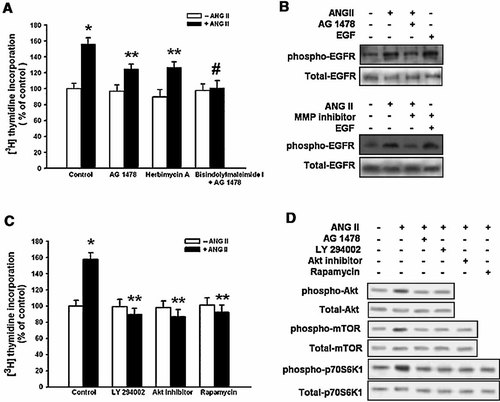
Effect of the ANG II on EGF receptor-induced PI3K/Akt activation. A: Mouse ES cells were treated with AG 1478 (10−5 M), herbimycin A (10−6 M), or combined AG 1478 and bisindolylmaleimide I (10−6 M) for 30 min prior to the ANG II (10−7 M) treatment for 8 h, and subsequently pulsed with 1 µCi of [3H] thymidine for 1 h. The values represent the mean ± S.E. of three independent experiments with triplicate dishes. *P < 0.05 versus Control, **P < 0.05 versus ANG II alone, #P < 0.01 versus ANG II alone. B: Mouse ES cells were pretreated with AG 1478 (10−5 M) or MMP inhibitor (10−7 M) for 30 min prior to the ANG II (10−7 M) treatment for 8 h. The total protein was extracted and blotted with the antibody against phospho-EGF receptor (175 kDa) or total EGF receptor, respectively. C: Mouse ES cells were preincubated with AG 1478, LY 294002 (10−6 M), Akt inhibitor (10−5 M), or rapamycin (10−9 M) for 30 min prior to the ANG II (10−7 M) treatment for 8 h, and subsequently pulsed with 1 µCi of [3H] thymidine for 1 h. The values represent the mean ± S.E. of three independent experiments with triplicate dishes. *P < 0.05 versus Control, **P < 0.05 versus ANG II alone. D: Mouse ES cells were preincubated with AG 1478, LY 294002, Akt inhibitor, or rapamycin for 30 min prior to the ANG II (10−7 M) treatment for 30 min. The total protein was extracted and blotted with the antibody against Akt (60 kDa), mTOR (289 kDa), p70S6K (70 kDa). Each of the examples shown is representative of three independent experiments.
To further examine the possible involvement of EGF receptor, we tested the involvement of Akt/mTOR/p70S6K1 pathways on ANG II-induced DNA synthesis. As shown in Figure 5C, the pretreatment with LY 294002 (PI3K inhibitor, 10−6 M), Akt inhibitor (10−5 M), or rapamycin (mTOR inhibitor, 10−9 M) inhibited ANG II-induced increase of DNA synthesis. In addition, ANG II increased the phosphorylation of Akt/mTOR/p70S6K1, which were inhibited by the pretreatment with AG 1478, LY 294002, Akt inhibitor, or rapamycin (Fig. 5D). These results suggest that ANG II-induced activation of the downstream pathways of PI3K is at least partly via transactivation of EGF receptor in mouse ES cells.
Involvement of p44/42 MAPKs in ANG II-induced cell proliferation
In experiments to elucidate if Ras/p44/42 MAPKs are activated by ANG II in mouse ES cells and these signaling pathways are involved in ANG II-induced cell proliferation, the cells were treated with PD 98059 (10−5 M, MEK inhibitor) or SB 203580 (10−6 M, p38 MAPK inhibitor) for 30 min prior to 10−7 M ANG II treatment. Figure 6A shows that PD 98059 decreased the ANG II-induced increase in [3H] thymidine incorporation (P < 0.05). However, SB 203580 had no effect. The maximum phosphorylation of p44/42 MAPKs appeared at 30 min after incubation with ANG II (48% increase vs. control; P < 0.05) (Fig. 6B). Western blotting analysis with the specific inhibitors was carried out to confirm involvement of the AT1 receptor, EGF receptor, and PKC in the ANG II-induced p44/42 MAPKs activation. As shown in Figure 6C, losartan, AG 1478, or bisindolylmaleiamide I inhibited the ANG II-induced p44/42 MAPKs phosphorylation. The changes in the mRNA levels of c-fos, c-jun, and c-myc were examined in order to further investigate the transcriptional regulation of ANG II. The result of real-time RT-PCR showed that ANG II increased the levels of these protooncogenes (Fig. 6D) and PD 98059 inhibited the mRNA level of c-fos, c-jun, and c-myc (Fig. 6E).
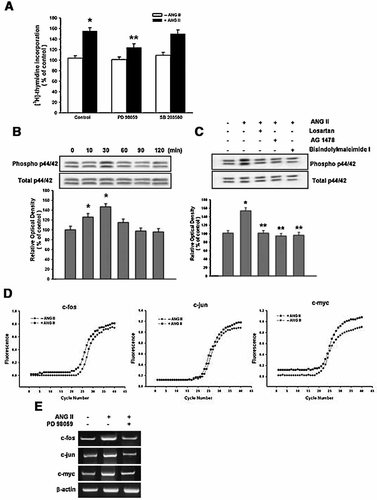
Effect of ANG II on p44/42 MAPKs activation. A: Mouse ES cells were pretreated with PD 98059 or SB 203580 (10−6 M) for 30 min prior to the ANG II (10−7 M) treatment for 8 h and then pulsed with 1 µCi of [3H] thymidine for 1 h. The values represent the mean ± S.E. of three independent experiments with triplicate dishes. *P < 0.05 versus Control, **P < 0.05 versus ANG II alone. B: Mouse ES cells were incubated with ANG II (10−7 M) for different periods (0–120 min), and total protein was then extracted and blotted with the antibody against phospho-p44/42 MAPKs or total-p44/42 MAPKs. *P < 0.05 versus Control. C: Mouse ES cells were pretreated with losartan (10−6 M), AG 1478 (10−5 M), or bisindolylmaleimide I (10−6 M) for 30 min prior to the ANG II (10−7 M) treatment, and the total protein was then extracted and blotted with the antibody against phospho-p44/42 MAPKs or total-p44/42 MAPKs. The lower parts of (B) and (C) depicting the bars denotes means ± S.E. of three experiments for each condition determined from densitometry relative to the total-p44/42 MAPKs. *P < 0.05 versus Control, **P < 0.05 versus ANG II alone. D: Mouse ES cells were treated with ANG II (10−7 M) for 1 h and then the c-fos, c-jun, and c-myc gene expression levels were then analyzed by real-time RT-PCR. E: Mouse ES cells were treated with ANG II (10−7 M) for 1 h after 30 min preincubation with PD 98059 (10−5 M). The c-fos, c-jun, and c-myc gene expression levels were then analyzed by RT-PCR. The genes were expressed in 356 bp for c-fos, 355 bp for c-jun, 266 bp for c-myc, and 350 bp for β-actin. Each example shown is representative of three independent experiments.
Effect of ANG II on expression of cell-cycle regulatory proteins
The effect of ANG II on the expressions of the cell-cycle regulatory proteins was examined in order to confirm the effect of ANG II on cell proliferation. ANG II (10−7 M) increased the protein levels of cyclin D1, cyclin E, CDK2, and CDK4 (Fig. 7A), which increased from 4 h treatment with ANG II and sustained up to 24 h after the ANG II treatment. In addition, the ANG II-induced stimulation of cyclin E, cyclin D1, CDK2, and CDK4 were blocked by losartan, neomycin, staurosporine, or PD 98059, and these inhibitors also blocked the ANG II-induced inhibition of p21cip1/waf1 and p27kip1 (Fig. 7B). In further experiments aimed at determining if the EGF receptor is involved in ANG II-induced stimulation of cell-cycle regulators, either AG 1478 or herbimycin A inhibited the ANG II-induced stimulation of the cell-cycle regulatory proteins (Fig. 7C).

Effect of ANG II on cyclin D1, cyclin E, CDK2, and CDK4 expression. A: Mouse ES cells were incubated with ANG II (10−7 M) for different periods (0–24 h) and the total protein was extracted and blotted with the antibody against cyclin D1, cyclin E, CDK2, and CDK4. B: Mouse ES cells were preincubated with losartan (10−6 M), neomycin (10−4 M), staurosporine (10−6 M), or PD 98059 (10−5 M) for 30 min prior to the ANG II (10−7 M) treatment for 8 h. The total protein was extracted and blotted with antibody against cyclin D1, cyclin E, CDK 2, and CDK 4, or p21cip1 and p27 kip1. C: Mouse ES cells were preincubated with AG 1478 (10−5 M) or herbimycin A (10−6 M) for 30 min prior to the ANG II (10−7 M) treatment for 8 h. The total protein was extracted and blotted with the antibody against cyclin D1, cyclin E, CDK 2, and CDK 4. The proteins were expressed in 35–55 kDa, respectively. Each of the examples shown is representative of three independent experiments. The lower parts depicting the bars denotes means ± S.E. of three experiments for each condition determined from densitometry relative to the total-p44/42 MAPKs. *P < 0.05 versus Control, **P < 0.05 versus ANG II alone.
DISCUSSION
This study demonstrated for the first time that ANG II-stimulated DNA synthesis is partially mediated through Ca2+/PKC-dependent p44/42 MAPKs and EGF receptor-dependent PI3K/Akt/mTOR/p70S6K1 signal pathways in mouse embryonic stem (ES) cells. Recently, we reported that ANG II increases the glucose transporter (GLUT1) activity through the PKC pathway via the AT1 receptor, which can be involved stimulating growth in mouse ES cells (Han et al., 2005). Therefore, this study examined the effect of ANG II on mouse ES cell proliferation by measuring the level of [3H] thymidine incorporation and counting the number of cells, which is the method used in previous reports of ANG II-induced human mesangial cell proliferation (Zhang et al., 2005a). In previous reports, the RAS was present in human ovary and ovarian RAS controlled follicle development, ovulation, and luteal function (Palumbo et al., 1989). The RAS, particularly ANG II, modulates primitive erythropoiesis in chick embryos (Savary et al., 2005). In human trophoblast cells, the AT1 receptor gene is expressed, the transcription factors interact within the AT1 receptor promoter (Duffy et al., 2004), and ANG II stimulates the proliferation of trophoblast cells via the AT1 receptor linked to the PKC/MAPK pathways (Ino et al., 2003). Therefore, based upon the previous reports and these results, we can hypothesize that ANG II acts as a growth factor in embryonic development and mouse ES cells.
The growth-promoting effects of ANG II and the associated signaling pathways have been studied extensively in various cell types. The activation of the AT1 receptor leads to a variety of signal pathways including Ca2+ influx, PKC activation, and MAPK in trophoblast and vascular smooth muscle cells (VSMC) (Force and Bonventre, 1998; Eguchi and Inagami, 2000; Ino et al., 2003). Consequently, these signal molecules stimulate DNA synthesis and cell proliferation. The results also showed that ANG II induced the activation of PKC in mouse ES cells. The inhibition of these pathways suppressed the ANG II-induced cell proliferation. Mouse ES cells were reported to express PKC α, β1, γ, δ, and ζ, and the activation of these isoforms in response to EGF were implicated in mouse ES cell proliferation (Heo et al., 2006). In various cell types, ANG II stimulated PKC isoforms to induce cell proliferation (Greco et al., 2003; Zhao et al., 2005) in consistency with our results that ANG II stimulated PKC α, δ, and ζ translocation.
It has been well established that ANG II exerts growth-promoting effects via activation of multiple signaling cascades including PI3K/Akt pathway in cardiovascular system (Berk and Corson, 1997). In the present study, we observed that ANG II activated PI3K/Akt pathway via AT1 receptor-mediated transactivation of EGF receptor in mouse ES cells. These results suggest that the AT1 receptor-operated PI3K/Akt pathways are highly conserved among different species and various cell types, and might play a crucial role in proliferation of mouse ES cells. In addition, mTOR can be regulated by the PI3K pathway. The recent results suggest that mTOR is essential for both ES cell proliferation and mouse development, and the treatment with rapamycin, which is a mTOR inhibitor, interferes with ES cell proliferation (Murakami et al., 2004; Takahashi et al., 2005). Thus, mTOR is a strong candidate for a downstream target of the PI3K signaling in ES cells. mTOR controls protein synthesis, in part by phosphorylating downstream substrates, including p70S6K kinase (Burnett et al., 1998) in consistency with our result that rapamycin decreased p70S6K1 phosphorylation. Furthermore, our results that Akt/mTOR/p70S6K1 phosphorylation was inhibited by the EGF receptor kinase blocker, AG 1478 can encourage ANG II-induced activation of Akt/mTOR/p70S6K1 through EGF receptor. This can be supported by the previous report that PI3K, Akt, and p70S6K1 are downstream effectors of EGF receptor tyrosine kinase contributing to cell-cycle progression into DNA synthesis in intestinal epithelial cells (Chiu et al., 2005). Further establishing the functional contribution of AT1 receptor-operated PI3K/Akt/mTOR/p70S6K1 cascade to ANG II-dependent proliferative effects during embryogenesis should be a crucial issue to be addressed.
In this study, ANG II stimulated the phosphorylation of p44/42 MAPKs in mouse ES cells via AT1 receptor-dependent PKC and EGF receptor transactivation. Previous studies have reported that PKC is a key regulator of ANG II-induced ERK activation in various cell types (Ino et al., 2003; Muscella et al., 2003; Zhao et al., 2005). It was reported that recombinant PKC ζ is associated with MAPK kinase (MEK) in oocytes (Diaz-Meco et al., 1994), which indicates that PKC ζ interacts directly with MEK to induce ANG II-mediated ERK1/2 activation. It was demonstrated that tyrosine kinases could mediate ANG II-induced ERK activation when PKC-dependent ERK activation is blocked (Li et al., 1998). The present result showed that ANG II increased DNA synthesis in activated mouse ES cells concomitant with activating the EGF receptor and its downstream ERK. Moreover, the EGF receptor kinase inhibitor AG1478 blocked ANG II-induced p44/42 MAPKs activation, and inhibited ANG II-stimulated DNA synthesis by approximately 31%. However, the inhibition of both PKC and EGF receptor tyrosine kinase completely blocked ANG II-induced DNA synthesis. Although these data suggest that ANG II stimulates mouse ES cells proliferation via an EGF receptor transactivation/ERK pathway, the 31% inhibition of ANG II-enhanced mouse ES cells proliferation by an EGF receptor kinase inhibitor suggests the existence of an alternate intracellular signaling pathway for the ANG II mitogenic effects on mouse ES cells. These observations provided the impetus to conduct the present study in an effort to elucidate this novel-signaling pathway for the ANG II mitogenic effect on mouse ES cells.
This study also showed that ANG II increased the expression of several early-response genes, including c-fos, c-jun, and c-myc, which were blocked by the inhibition of the p44/42 MAPKs pathway. It was previously reported that the ANG II-induced phosphorylation of c-jun is mediated by the Jun N-terminal kinase (JNK) pathway (Zhang et al., 2005a). In MCF-7 cells, PKC ζ-dependent MAPK in response to ANG II mediates cell proliferation and c-fos induction (Muscella et al., 2003). Therefore, it is suggested that ANG II-induced EGF receptor transactivation leads to the phosphorylation of specific tyrosine residues within the EGF receptor cytoplasmic domain that act as docking sites for effector molecules. This results in the activation of the downstream signal pathways such as p44/42 MAPKs, which contribute to DNA synthesis and mouse ES cell proliferation.
It has been known that the feature of the pluripotent cell cycle is unusually rapid and short G1 phase. Moreover, a contributing factor to this is likely to be the activity of cyclin E-associated kinases. This is also supported by previous reports demonstrating cyclin E to be a rate-limiting factor for the G1 to S transition (Wimmel et al., 1994; Resnitzky and Reed, 1995). Ectopic expression of cyclin E prematurely advances entry into S phase by shortening the length of G1, consistent with the meaning that the length of G1 is determined by the timing and/or rate at which cyclin E activity is accumulated. Therefore, cells that exhibit constitutively active cyclin E kinase activity may not be subjected to this rate-limiting step for G1–S progression. Several reports also suggest the role of cyclin D/CDK4 in ES cells. It was suggested the possibility that the functional significance of cyclin D/CDK4 complexes in ES cells might be to sequester p27kip1 and prevent this inhibitor acting on cyclin E/CDK2 kinase (Sherr and Roberts, 1995). Thus, the G1/S transition seems to be driven uniquely by cyclin E/CDK2 involving cyclin D/CDK4 activities during ES cell self-renewal. This study demonstrated that ANG II stimulated cyclin D1-CDK4 and cyclin E-CDK2, which phosphorylate the substrates, including the product of the retinoblastoma (RB) susceptibility gene pRB, thereby allowing the initiation of DNA synthesis (Lukas et al., 1996). Subsequently, these increases were mediated by Ca2+/PKC via the AT1 receptor as well as EGF receptor tyrosine kinase/cytosolic tyrosine kinases. Consistent with these results, ANG II increased the transcription of the D-type cyclins leading to the phosphorylation of pRb for cell-cycle progression (Nozato et al., 2000). In contrast, p21 and p27 are members of the Cip/Kip family of cell-cycle regulators that inhibit the activation of cyclin A, E, and D-dependent CDKs (Viallard et al., 2001). These results also show that ANG II downregulated both p21 and p27, which were blocked by PD 98059. A potent inhibitor of CDK2, p27, is also involved in the assembly of cyclin D/CDK4 complexes. Alternatively, ANG II may alter the properties of the inhibitors, reducing their ability to bind to the complex, or perhaps as a result of increased protooncogenes expression, inducing proteins that inhibit the interactions of p21 and p27 with cyclin E–CDK2 complexes. Therefore, the elucidation of ANG II-induced mechanisms regulating mouse ES cell proliferation can provide a novel paradigm for correlation between the AT1 receptor and EGF receptor signaling pathways (Fig. 8). In conclusion, our results indicate that ANG II-induced DNA synthesis may require two parallel signaling pathways in mouse ES cells: (1) AT1 receptor-mediated Ca2+/PKC and (2) AT1 receptor-induced transactivation of EGF receptor-dependent PI3K/Akt/mTOR/p70S6K1. This cascade could contribute to a better understanding of the molecular mechanism of embryonic growth and development as well as self-renewal of ES cells.

The hypothesized model for the signal pathways involved in ANG II-induced stimulation of ES cell proliferation. ANG II activates AT1 receptor, which stimulates PLC to generate IP3 and DAG. In turn, IP3 increases the concentration of intracelluar Ca2+. DAG activates PKC, which induces stimulation of protooncogenes (c-fos, c-jun, c-myc) and cell-cycle progression. In another pathway, AT1 receptor stimulates EGF receptor transactivation, which activates PI3K/Akt/mTOR/p70S6K1. In addition, ANG II can stimulate the phosphorylation of p44/42 MAPKs in mouse ES cells via AT1 receptor-dependent PKC and EGF receptor transactivation. AT1R, ANG II type 1 receptor; EGFR, EGF receptor; Gq, G-protein alpha (q) subunit; PLC, phospholipase C; IP3, 1,4,5-inositol-triphosphate; DAG, diacylglycerol; PKC, protein kinase C; MAPK, mitogen-activated protein kinase; PI3K, phosphatidyl inositol 3-kinase; mTOR, mammalian target of rapamycin; p70S6K1, p70 S6 ribosomal protein kinase 1; CDK, cyclin-dependent kinase. The solid line is the proposed pathway and the dashed line is suspected pathway.
Acknowledgements
This research was supported by Grant (SC 2210) from the Stem Cell Research Center of the 21st Century Frontier Research Program funded by the Ministry of Science and Technology, and the authors acknowledge a graduate fellowship provided by the Ministry of Education and Human Resources Development through the Brain Korea 21 project, Republic of Korea.




