A pair of chiral Cu(II)-Tb(III)-Fe(III) heterotrimetallic enantiomers: Structural, luminescent, and magnetic studies
Funding information: National Natural Science Foundation of China, Grant/Award Numbers: 21101096, 21371104, 21771111; Natural Science Foundation of Hebei Province, Grant/Award Number: B2016202280; Hebei Province 2020 Overseas Student Introduction Program, Grant/Award Number: C20200317
Abstract
One chiral heterotrimetallic compound, [Cu(H2O){(R,R)-valcy}Tb(CH3OH)2(H2O)(μ-CN)Fe(CN)5]·[Cu(H2O){(R,R)-valcy}Tb(CH3OH)1.5(H2O)1.5(μ-CN)Fe(CN)5]·7.85H2O·2CH3OH (1) (H2(R,R)-valcy = N,N′-bis(3-methoxysalicylidene)-1R,2R-1,2-diaminocyclohexane), has been prepared and structurally characterized by X-ray single-crystal diffraction. In 1, the asymmetric trinuclear {CuIITbIIIFeIII} units are assembled into 1-D supramolecular single chain by hydrogen-bonds and the supramolecular chains are further connected into 2D sheets. Magnetic studies show overall weak ferromagnetic interaction and magnetic anisotropy. The enantiomer of 1, denoted as complex 2, derived from N,N′-bis(3-methoxysalicylidene)-1S,2S-1,2-diaminocyclohexane was also synthesized and characterized by PXRD, Infrared spectra, CD spectra, and fluorescence spectra. The results indicated that complexes 1 and 2 are isomorphous exhibiting similar fluorescence properties.
1 INTRODUCTION
In recent years, interest has grown tremendously in developing molecularly designed materials with predictable and controllable architectures and therefore tailor-made applications. At the heart of a transition from a single molecule to a material, a molecular building block plays an important role.[1] Rational design of ligands to coordinate multiple metal ions in a controlled way has been proven to be a successful strategy for the preparation of heterometallic complexes, which can provide unique sets of properties for a variety of applications. For an example of the metalloligand approach, the compartmental Schiff-base ligands derived from o-vanillin (or o-vanillin analogs) (Scheme 1) were originally designed by Costes,[2] in order to obtain 3d-4f complexes, since the open O2O2′ compartment can easily accommodate the large and oxophilic lanthanide cations, while the inner N2O2 compartment hosting the 3d metal ions, such as Cu(II), Ni(II), and Zn(II).[3]
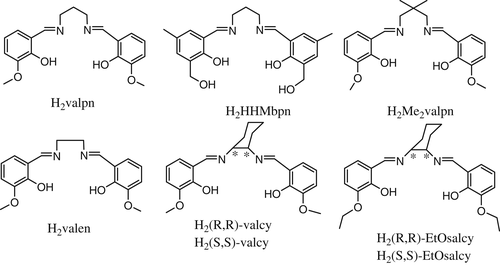
After the discovery of the first heterodinuclear single-molecule-magnet (SMM) behavior in the complex [(Me2valpn)Cu(O2COMe)Tb(thd)2] (thd = tetramethylheptanedione),[4] by the node-and-spacer approach, a lot of coordination polymers based on [CuLn] heterodinuclear complex stabilized by compartmental Schiff base have been reported. Some of them are SMM or SCM, such as [(μ3-C9H3O6){CuLn(HHMbpn)(NO3)2}3]·3THF (Ln = Gd, Tb, Dy; C9H3O6 = benzene-1,3,5-tricarboxylic acid trivalent anion)[5]; [Co(CN)5{(μ-CN)CuTb(valpn)(H2O)x}]·7H2O[6]; [{(Cu(valpn))2Dy}{Mo(CN)8}]·CH3CN·H2O[7]; [Cu(Me2valpn)]2Ln(H2O)Fe(CN)6·xH2O·yCH3CN}n,[8] [Ni(valen)Dy(H2O)4][M(CN)6]·3H2O(MCr, Fe, Co) and [Ni(valen)Tb(H2O)4][Co(CN)6]·3H2O[9] etc.
However, self-assembly based on the [CuLn] or [NiLn] units stabilized by chiral compartmental Schiff base ligands has been rarely reported.[10, 11] In order to obtain chiral molecular-based magnetic materials, we synthesized a chiral CuII-TbIII-FeIII heterotrimetallic compound [Cu(H2O){(R,R)-valcy}Tb(CH3OH)2(H2O)(μ-CN)Fe(CN)5]·[Cu(H2O){(R,R)-valcy}Tb(CH3OH)1.5(H2O)1.5(μ-CN)Fe(CN)5]·7.85H2O·2CH3OH (1) by self-assembly of the [CuTb] building block derived from chiral compartmental Schiff base ligands and [Fe(CN)6]3−. The crystal structure, FTIR spectra, CD spectra, as well as fluorescent and magnetic properties were characterized. The structure–function relationships were also discussed. The enantiomer of 1, denoted as complex 2, was prepared and characterized by PXRD, Infrared spectra, CD spectra, and fluorescence spectra.
2 RESULTS AND DISCUSSION
2.1 Description of the crystal structures
Complex 1 crystallizes in the monoclinic chiral space group P21. As shown in Figure 1, the crystal structure reveals that 1 is composed of two similar asymmetric {CuIITbIIIFeIII} trinuclear units, ([Cu(H2O){(R,R)-valcy}Tb(CH3OH)2(H2O)(μ-CN)Fe(CN)5] abbreviated as Cu(1)Tb(1)Fe(1) and·[Cu(H2O){(R,R)-valcy}Tb(CH3OH)1.5(H2O)1.5(μ-CN)Fe(CN)5] abbreviated as Cu(2)Tb(2)Fe(2), and crystallized solvent molecules (H2O and CH3OH). One {CuIITbIII} binuclear unit and one [Fe(CN)6]3− are connected into a trinuclear {CuIITbIIIFeIII} unit by the cyano-bridge.
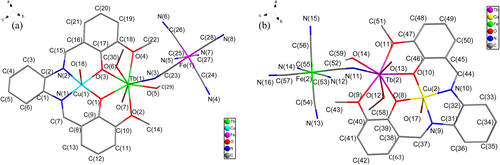
This paper takes Cu(1)Tb(1)Fe(1) as an example to describe the structure of the trinuclear units. As far as the {CuIITbIII} binuclear moiety is concerned, the copper(II) ion occupies the inner coordination site of the H2(R,R)-valcy Schiff base (N2O2 pocket), whereas the oxophilic Tb(III) cation is housed in the outer site (O2O2′ pocket). The copper(II) displayed a slightly distorted tetragonal-pyramidal geometry (Addison parameter τ = 0.053).[12] The basal plane is formed by the four atoms of the N2O2 chromophore (1.913–1.925 Å), and the apical position is occupied by one oxygen atom arising from the water (Cu(1)O(18) = 2.891 Å) indicating the presence of Jahn–Teller effect.[13] The dihedral angle between the Cu(1)N(1)N(2) and Cu(1)O(1)O(3) planes (5.1°) indicates that the N2O2 moiety affords an approximately square plane centered on Cu(1). In the inner cavity, the Cu(II) and Tb(III) centers are doubly bridged by two phenolato oxygen atoms, with the intramolecular Cu(II)···Tb(III) separation of 3.368 Å. Although the cyclohexyl moiety is flexible, the CuO2Tb bridging moiety in 1 is nearly planar, which is consistent with the literature.[14, 15] The dihedral angle between the CuO2 and TbO2 planes is 0.1°, which is smaller than that between the CuO2 and GdO2 planes (8.1°) in the related dinuclear complexes [Cu((S,S)-EtOsalcy)Gd(NO3)3∙CH3OH]n and [Cu((R,R)-EtOsalcy)Gd(NO3)3∙CH3OH]n.[10]
In the trinuclear unit, the angles of C(23)≡N(3)Tb(1) and Fe(1)Tb(1)Cu(1) are 158.1° and 147.5°, respectively, which obviously deviate from 180°, with the intramolecular Fe(III)···Tb(III) distance of 5.375 Å. The Tb(III) is eight-coordinate with four oxygen atoms from the outer site of the Schiff-base ligand, two oxygen atoms from coordinated methanol molecules, one oxygen atom from the coordinated water molecule, and one cyanide-nitrogen atom from the [Fe(CN)6]3−. The TbO bond lengths fall in the range 2.296–2.501 Å, and the TbN bond length is 2.410 Å. Intra-molecular hydrogen-bonds are formed between the coordinated H2O from Cu(II) and the coordinated CH3OH from Tb(III) (O(18A)···O(6A) = 2.691 Å, O(17A)···O(12A) = 2.672 Å) (Figure 2).
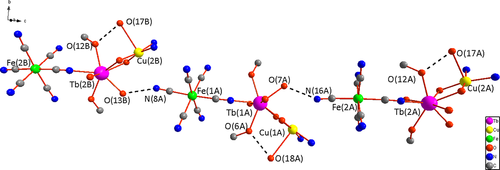
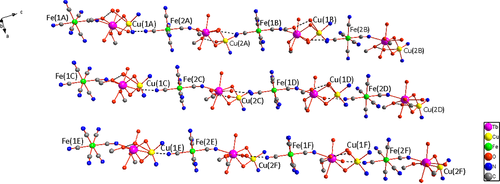
The trinuclear units are organized into 1D supramolecular single chain by means of hydrogen bonds established between the water molecules coordinated to Tb(III) and the terminal cyano groups from [Fe(CN)6]3− (O(13B)···N(8A) = 2.764 Å, O(7A)···N(16A) = 2.808 Å), with the Cu((R,R)-valcy) moiety displayed above and below the 1D single chain alternately (Figure 2). In the supramolecular chain, the nearest intermolecular Fe(III)···Cu(II) and Fe(III)···Tb(III) distances are 5.561–5.676 and 7.549–7.587 Å, respectively. With the parallel arrangement of the 1D supramolecular chains, a unique 2D sheet come into being (Figure 3). Furthermore, in the crystal lattice there are numerous intermolecular hydrogen-bonds between the crystallized solvent molecules (H2O and CH3OH), coordinated solvent molecules (H2O and CH3OH), and the terminal cyano groups from [Fe(CN)6]3−. Consequently, the neighboring layers are connected into 3D supramolecular frameworks via the hydrogen bonds and van derWaals force, where the nearest intermolecular distances of Tb(III)···Tb(III), Cu(II)···Cu(II), and Fe(III)···Fe(III) are 8.949, 9.373, 10.960 Å, respectively.
2.2 Spectroscopic properties
2.2.1 Infrared spectra
The infrared spectra of 1 (Figure S1) exhibit a strong broad band centered at about 3,376 cm−1, which can be assigned to the ν(OH) of the methanol or water molecules.[16, 17] The strong imine group C═N stretching vibrations of the Schiff base ligand were observed at 1636 cm−1.[10, 18] The single peak of medium intensity at 2119 cm−1 should be ascribed to the stretching vibrations of νasym(C≡N), which is at higher wavenumbers than that of free [Fe(CN)6]3− (νasym(C≡N) = 2096 cm−1) and consistent with the formation of FeC≡NTb bridges.[8, 19] The IR spectra of complex 2 were similar with complex 1 (Figure S2).
2.2.2 Circular dichroism spectra
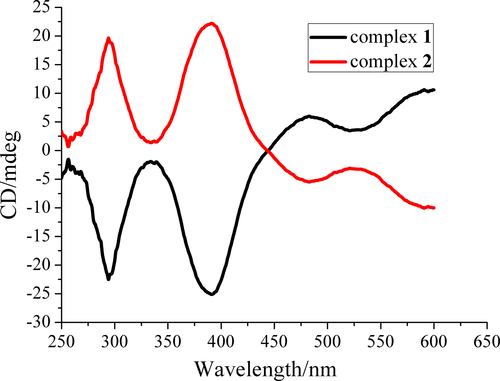
The circular dichroism (CD) spectrum measurements in KBr pellets confirm the optical activity of 1 and 2 (Figure 4). The CD spectrum of 1 exhibits negative Cotton effects at λmax = 294 and 390 nm, positive Cotton effects at λmax = 482 and about 600 nm, due to the inherent chiralities of the chiral Schiff base ligand H2(R,R)-valcy.[14, 20] Complex 2 presents similar intensity and completely opposite Cotton effect compared to complex 1 at the same wavelength. The CD peaks can be assigned to the charge-transfer of the UV–Vis absorption spectra of 1 and 2 and the d-d transition of Cu(II).[11] CD spectra confirmed the chirality has been successfully transferred from the chiral ligands to the coordination environment of Cu(II) ions.
2.2.3 Fluorescence spectra
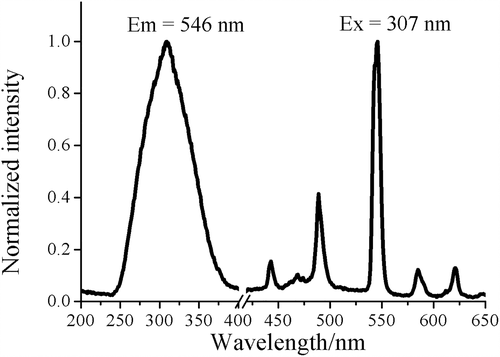
The fluorescence property of the polycrystal of 1 was investigated at room temperature (Figure 5). There are conjugate rigid-planes in the ligand H2(R,R)-valcy, so upon excitation the ligand can emission through π* → π transition[21] and effectively sensitize the fluorescence of Tb(III) ion.[22, 23] The absence of the emission from (R,R)-valcy2− indicates the ability of the ligand to act as an antenna group to transfer energy to Tb(III) ion.[24] The emission spectrum excited at 307 nm shows four weak characteristic luminescence of Tb(III) at 445 nm (5D3 → 7F4), 489 nm (5D4 → 7F6), 546 nm (5D4 → 7F5), 585 nm (5D4 → 7F4) and 612 nm (5D4 → 7F3).[25-27] As mentioned above, in 1 the Schiff base ligand coordinated to the Tb(III) ion closely and can sensitize the emission of Tb(III) efficiently (antenna effect).[28] However, the H2O molecules and cyano groups from paramagnetic [Fe(CN)6]3− unit coordinated to Tb(III) center are quenching path for the fluorescence.[29] As a result, the fluorescent cyanide-bridged 3d-4f complexes based on paramagnetic [Fe(CN)6]3− or [Cr(CN)6]3− anions have been rarely reported.[24] Therefore, the antenna effect and quenching effect must be considered simultaneously to explain the experimental phenomenon. In one trinuclear unit of 1, each Tb(III) ion has only one FeC≡NTb (Tb-N = 2.410 Å) bridging moiety and a small quantity (1–1.5) of coordinated H2O molecules in the inner coordination sphere, which can explain the relatively weak quenching effect and further illuminate the resulting intensity of the partly quenched characteristic fluorescence of Tb(III). Complex 2 exhibited similar fluorescence properties with complex 1 (Figure S3).
2.3 Magnetic properties
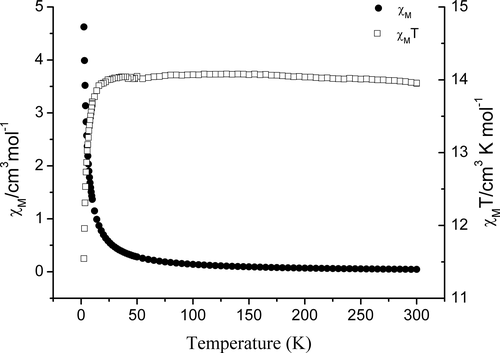
Magnetic measurements on crystalline samples of 1 were performed under an applied direct current (dc) field of 1,000 Oe. The temperature dependence of χM and χMT for 1 in the temperature range 2–300 K is shown in Figure 6. Complex 1 shows a magnetic property similar to that of complex [Cu(valpn)(H2O)Tb(H2O)3Fe(CN)6]·3H2O.[8] The χMT value for 1 is 13.95 cm3 K mol−1 at 300 K, which is a little higher than the spin-only value of 12.57 cm3 K mol−1 expected for uncoupled one Cu(II) (S = 1/2, g = 2.0), one Tb(III) (J = 6, S = 3, g = 1.5), and one low-spin Fe(III) ion (S = 1/2, g = 2.0).[30-32] Upon cooling, the χMT value remains practically constant (13.95–14.07 cm3.K·mol−1) until 14 K, which might be due to the thermal depopulation of the excited Stark sub-levels for Tb(III) that is nearly offset by the Cu(II)-Tb(III) ferromagnetic interaction and the Fe(III)-Tb(III) negligible or weak ferromagnetic coupling.[8, 33, 34] With further drop of temperature, the value decreases sharply until reach a minimum value of 11.55 cm3 K mol−1 at 2 K, which may be due to the magnetic anisotropy of Tb(III) and/or intermolecular antiferromagnetic interactions mainly through the intermolecular hydrogen-bonds. The magnetic susceptibility obeys the Curie–Weiss law with a Curie constant C = 14.015 cm3 mol−1 and a small positive Weiss constant θ = 9.01 × 10−4 K. The small positive Weiss value indicates the presence of predominant weak ferromagnetic interactions in 1.
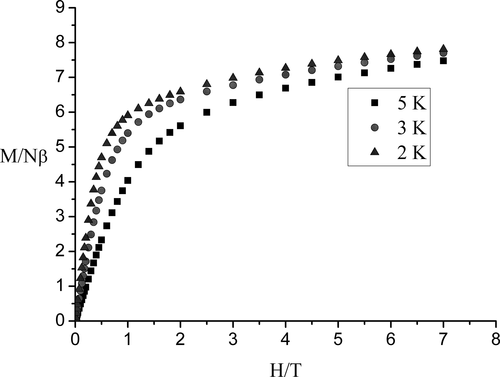
To verify the magnetic behaviors, the field dependences of the magnetization up to 7 T for 1 were measured at a low temperature region (Figure 7). At 2, 3, and 5 K, upon increasing the applied external magnetic field to 7 T, the magnetization of 1 increased monotonically to 7.81, 7.72, and 7.50 Nβ, respectively, but all did not reach the expected saturation value of 11 Nβ (calculated from 9 Nβ for Tb(III) ion, 1 Nβ for Cu(II) ion, and 1 Nβ for Fe(III)). This is mainly due to the crystal-field effects leading to the significant magnetic anisotropy of the Tb(III) ions and/or the population of low-lying excited states.[31-33]
In order to probe the magnetic dynamic behavior of 1, the ac susceptibilities of 1 were measured in the temperature range 2–10 K with zero dc field and a 3 Oe ac field oscillating at the frequency of 100 Hz (Figure S4). The in-phase ac magnetic susceptibility (χ′M) is temperature dependent and the out-of-phase ac magnetic susceptibility (χ′′M) is negligibly small. It reveals that 1 shows no slow magnetization relaxation.
3 EXPERIMENTAL SECTION
3.1 General procedure
All of the reagents and solvents were commercially available and used without further purification. The C, H, and N elemental analyses were performed with a Perkin–Elmer 240C elemental analyzer. The IR spectra of KBr pellets were recorded with a Vector-22 Fourier FTIR spectrophotometer in the wavenumber range 4,000–400 cm−1. The CD spectra were recorded on a JASCO J-715 spectropolarimeter with KBr pellets. The fluorescence spectra were performed with a near-infrared spectrometer (FS920P, Edinburgh Instruments) with a 450 W xenon lamp as the steady-state excitation source, a double-excitation monochromator (1800 lines·mm−1), an emission monochromator (600 lines·mm−1), a semiconductor-cooled Hamamatsu RMP928 photomultiplier tube. The magnetic susceptibilities were measured with a Quantum Design MPMS-7 superconducting quantum interference device (SQUID) magnetometer. Diamagnetic corrections were made with Pascal's constants for all the constituent atoms. Powder X-ray diffraction (PXRD) data were recorded on a Rigaku D/max-2,400 X-ray diffractometer with Cu-Kα as the radiation source (λ = 0.15406 nm) at room temperature in the range of 2θ = 5°–50°.
3.2 Preparation of compounds
3.2.1 Preparation of H2(R,R)-valcy and H2(S,S)-valcy
The Schiff base ligand H2(R,R)-valcy was synthesized according to the literature method.[34] A methanol solution (50 ml) of 1R,2R-1,2-diaminocyclohexane (1.141 g, 10.000 mmol) was mixed with a methanol solution (100 ml) of 2-hydroxy-3-methoxybenzaldehyde (o-vanillin)(3.043 g, 20.000 mmol), and the mixture was refluxed for 4 hr. After cooling to room temperature, the brown precipitate of the product was collected by filtration, and dried under vacuo. Yield: 3.12 g (82.02%). H2(S,S)-valcy was prepared by the same procedure using 1S,2S-1,2-diaminocyclohexane instead of 1R,2R-1,2-diaminocyclohexane. Yield: 3.14 g (82.28%).
3.2.2 Preparation of [Cu{(R,R)-valcy}] and [Cu{(S,S)-valcy}]
An aqueous solution (15 ml) of copper(II) acetate monohydrate (1.002 g, 5.000 mmol) was added dropwise to a methanol solution (50 ml) of H2(R,R)-valcy (1.914 g, 5.000 mmol) with stirring. An ash-colored precipitate was given immediately. After stirring for 6 hr and resting for one night, the compound [Cu{(R,R)-valcy}] was collected by filtration and air-dried.[14] Yield: 1.98 g (89.39%). [Cu{(S,S)-valcy}] was prepared by the same procedure using H2(S,S)-valcy instead of H2(R,R)-valcy. Yield: 1.88 g (84.88%).
3.2.3 Preparation of 1
Under stirring, an acetonitrile solution (7 ml) of Tb(NO3)3·6H2O (0.0223 g, 0.050 mmol) was added dropwise to a methanol solution (20 ml) of [Cu{(R,R)-valcy}] (0.0234 g, 0.050 mmol). After 2 hr, an aqueous solution (8 ml) of K3[Fe(CN)6] (0.0173 g, 0.050 mmol) was added, and the resulting mixture was stirred for another 1 hr. The solution was filtered, and the solvent from the filtrate was allowed to evaporate slowly at room temperature. The dark red, block-like crystals of 1, suitable for X-ray diffraction, were obtained in 3 weeks. Anal. Calcd. for C30.75H46CuFeTbN8O13 (%) (1,014.06): C, 36.42; H, 4.57; N, 11.05; Found: C, 36.61; H, 4.48; N, 11.24. Complex 2 was synthesized by the same procedure using [Cu{(S,S)-valcy}] instead of [Cu{(R,R)-valcy}]. Despite many attempts, the ideal single crystal of complex 2 suitable for X-ray diffraction could not be obtained.
3.3 X-ray crystallographic study
Diffraction intensities for 1 were collected on a computer-controlled Bruker SMART 1000 CCD diffractometer equipped with graphite-monochromated Mo-Kα radiation with radiation wavelength 0.71073 Å by using the ω-scan technique. Data processing, Lorentz-polarization, and absorption corrections were performed using SMART, SAINT, and SADABS computer programs. The structure was solved by the direct method using the SHELXS program of the SHELXTL package and refined by full-matrix least-squares methods with SHELXL.[35, 36] Anisotropic thermal parameters were assigned to all nonhydrogen atoms. The hydrogen atoms were set in calculated positions and refined as riding atoms with a common fixed isotropic thermal parameter. Analytical expressions of neutral atom scattering factors were employed, and anomalous dispersion corrections were incorporated. Crystallographic data and details of structural determination and refinement were summarized in Table 1. The selected bond distances and angles for 1 are given in Table 2. The experimental PXRD patterns of complex 2 are in good agreement with the simulated patterns of complex 1 (Figure S5), confirming the phase purity and isomorphism of complexes 1 and 2.[37]
| Complex | 1 |
|---|---|
| Empirical formula | C30.75H46CuFeN8O13Tb |
| Formula weight | 1,014.06 |
| Crystal system | Monoclinic |
| Space group | P21 |
| a (Å) | 8.8485(5) |
| b (Å) | 18.0604(11) |
| c (Å) | 24.8330(15) |
| α (°) | 90 |
| β (°) | 94.2960(10) |
| γ (°) | 90 |
| V (Å3) | 3,957.3(4) |
| Z | 4 |
| ρ (Mg/m3) | 1.702 |
| Absorption coefficient (mm−1) | 2.732 |
| F(000) | 2042 |
| Crystal size (mm3) | 0.22 × 0.18 × 0.16 |
| Theta range for data collection (°) | 3.14 to 27.54 |
| Reflections collected/unique | 40,543/16782 |
| Completeness to θ = 27.54 | 0.989 |
| Max. and min. transmission | 0.7353 and 0.6113 |
| Data/restraints/parameters | 16,782/71/1041 |
| Goodness-of-fit on F2 | 1.097 |
| R1 (I > 2σ(I)) | 0.0336 |
| wR2 (I > 2σ(I)) | 0.0827 |
| R1 (all data) | 0.0364 |
| wR2 (all data) | 0.1012 |
| Flack parameter | 0.021(8) |
| Bond lengths | Bond lengths | ||
|---|---|---|---|
| Tb(1)O(3) | 2.331(6) | Tb(2)O(10) | 2.300(7) |
| Tb(1)O(2) | 2.498(5) | Tb(2)O(8) | 2.317(6) |
| Tb(1)O(6) | 2.362(7) | Tb(2)O(13) | 2.329(6) |
| Tb(1)O(5) | 2.395(7) | Tb(2)O(14A) | 2.335(14) |
| Tb(1)O(7) | 2.332(7) | Tb(2)O(14) | 2.402(15) |
| Tb(1)O(4) | 2.501(5) | Tb(2)O(12) | 2.402(6) |
| Tb(1)O(1) | 2.296(7) | Tb(2)N(11) | 2.418(7) |
| Tb(1)N(3) | 2.410(7) | Tb(2)O(11) | 2.503(6) |
| Tb(2)O(9) | 2.506(6) | ||
| Cu(1)O(1) | 1.917(6) | Cu(2)O(10) | 1.893(6) |
| Cu(1)N(1) | 1.916(7) | Cu(2)O(8) | 1.900(6) |
| Cu(1)O(3) | 1.913(6) | Cu(2)N(10) | 1.903(7) |
| Cu(1)N(2) | 1.925(6) | Cu(2)N(9) | 1.931(7) |
| Cu(1)O(18) | 2.891(1) | Cu(2)O(17) | 2.693(1) |
| Bond angles | Bond angles | ||
|---|---|---|---|
| O(1)Cu(1)O(3) | 83.8(3) | O(8)Cu(2)O(10) | 82.3(3) |
| N(1)Cu(1)O(1) | 95.6(3) | O(8)Cu(2)N(9) | 95.1(3) |
| N(1)Cu(1)O(3) | 177.9(3) | O(8)Cu(2)N(10) | 167.6(3) |
| N(2)Cu(1)O(1) | 174.7(3) | O(10)Cu(2)N(9) | 170.5(3) |
| N(2)Cu(1)O(3) | 93.6(3) | O(10)Cu(2)N(10) | 96.4(3) |
| N(2)Cu(1)N(1) | 87.2(3) | N(9)Cu(2)N(10) | 88.0(3) |
| O(18)Cu(1)O(1) | 79.8(1) | O(17)Cu(2)O(8) | 94.1(1) |
| O(18)Cu(1)O(3) | 96.5(3) | O(17)Cu(2)O(10) | 83.7(1) |
| O(18)Cu(1)N(1) | 85.3(1) | O(17)Cu(2)N(9) | 87.3(3) |
| O(18)Cu(1)N(2) | 96.0(7) | O(17)Cu(2)N(10) | 98.0(3) |
| C(23)N(3)Tb(1) | 158.1(5) | C(52)N(11)Tb(2) | 161.5(6) |
| Cu(1)O(1)Tb(1) | 104.0(2) | Cu(2)O(8)Tb(2) | 105.6(2) |
| Cu(1)O(3)Tb(1) | 104.0(2) | Cu(2)O(10)Tb(2) | 106.6(3) |
- a Symmetry transformations used to generate equivalent atoms:#1 −1 + X, 1 + Y, +Z.
4 CONCLUSIONS
By using chiral compartmental Schiff base ligand H2(R,R)-valcy, the heterotrimetallic complex [Cu(H2O){(R,R)-valcy}Tb(CH3OH)2(H2O)(μ-CN)Fe(CN)5]2·[Cu(H2O){(R,R)-valcy}Tb(CH3OH)1.5(H2O)1.5(μ-CN)Fe(CN)5]·7.85H2O·2CH3OH (1) has been synthesized and structurally characterized by X-ray single-crystal diffraction. CD spectra of 1 confirmed the fact that the optical activity of the ligand has been transferred to the complex. Luminescent studies illustrate that the partly quenched characteristic Tb(III) fluorescence of 1 was observed. Static magnetic properties reveal that 1 showed overall weak ferromagnetic interaction and significant magnetic anisotropy. Dynamic magnetic properties indicate that 1 showed no slow magnetization relaxation. The enantiomer of 1, complex 2, exhibited similar fluorescence properties with 1.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China under Grant (Nos. 21771111, 21101096, and 21371104), the Natural Science Foundation of Hebei Province (No. B2016202280) and the Hebei Province 2020 Overseas Student Introduction Program (No. C20200317).
CONFLICT OF INTEREST
No potential conflict of interest was reported by the authors.




