Risks and Benefits of Bisphosphonate Therapies
ABSTRACT
Bisphosphonates are the mainstay of osteoporosis treatment but also play a fundamental role in treating other bone diseases such as Osteogenesis Imperfecta, Pagets' disease, and in the prevention of adverse skeletal effects in certain cancers such as prostate cancer or multiple myeloma. In the last decades, the refinement of bisphosphonates and an increase in the number of new bisphosphonates commercialized has altered the clinical management of these diseases. Despite differences between randomized controlled trials and observational studies, overall all bisphosphonates licensed have proven to reduce the risk of fracture through the inhibition of bone resorption. Other beneficial effects include pain reduction in bone metastasis and potentially a decrease in mortality. However, the chronic nature of most of these disorders implies long-term treatments, which can be associated with long-term adverse effects. Some of the adverse effects identified include an increased risk of atypical femur fractures, osteonecrosis of the jaw, gastrointestinal side effects, or atrial fibrillation. The harm/benefit thinking and the constant update regarding these medications are vital in the day-to-day decision-making in clinical practices. The aims of this review are to compile the basic characteristics of these drugs and outline the most important benefits and side effects and provide a clinical context as well as a research agenda to fill the gaps in our knowledge. J. Cell. Biochem. 117: 20–28, 2016. © 2015 Wiley Periodicals, Inc.
THERAPEUTIC USES OF BISPHOSPHONATES
Decreasing bone resorption has obvious advantages in patients with low bone mass and in patients with high turnover states of bone. Accordingly, in the past 3 decades, the introduction and refinement of the bisphosphonates has dramatically altered the clinical management of skeletal malignancies and of chronic diseases such as osteoporosis, Osteogenesis Imperfecta, and Fibrous dysplasia. The most striking and sustained effect of modern bisphosphonates occurs perhaps in Paget's Disease of Bone, where the course of disease has been transformed with extremely long duration of remission [Reid, 2012]. Today, bisphosphonates are the mainstay of osteoporosis treatment. In the European Union as a whole, about 5% of the population aged 50+ are treated with drugs for osteoporosis, chiefly bisphosphonates [Hernlund et al., 2013]. In 2012, 14.7 million retail prescriptions for bisphosphonates were filled in the United States [Wysowski and Greene, 2013]. Bisphosphonates are one of the most commonly prescribed drug classes in the elderly, with 12% of 75-year-old women and 17% of 85-year-old women in Denmark being current users (www.medstat.dk, statistics for 2013, accessed 27th of May 2015). Bisphosphonate use has also increased in children and adults with Osteogenesis Imperfecta though studies in these fairly rare disorders of bone have not been powered to detect fracture risk improvements or clinical outcomes other than BMD [Dwan et al., 2014]. Bisphosphonates are also effective in prevention of skeletal morbidity in metastatic bone disease, especially but not exclusively breast and prostate cancer, and in multiple myeloma [Coleman et al., 2014]. When used against metastatic bone disease, anti-resorptives are generally used with much shorter dosing intervals (equivalent to a many fold higher annual dose than that used in osteoporosis). A therapeutic area of particular growth in recent years is prevention of osteoporosis caused by anti-hormonal agents that are used in oncology. Concerns about long-term safety of bisphosphonates have ramifications not only within non-malignant bone disorders but also in cancer survivors.
PROPERTIES AND MODE OF ACTION
Bisphosphonates are stable analogs of pyrophosphate, which are deposited at bone surfaces in the first minutes or hours after uptake, with unbound bisphosphonate being rapidly cleared renally. The mode of action on the osteoclast is radically different between non-nitrogen-containing bisphosphonates (first generation) and nitrogen-containing bisphosphonates (second and third generation), with the former inducing apoptosis through forming a toxic analog of adenosine triphosphate and the latter targeting the enzyme farnesyl diphosphate synthase needed for post-translational modification of small GTP-binding proteins required for osteoclast function. Both potency and strength of binding to hydroxyappatite differ strongly between individual compounds [Russell et al., 2008]. This has led to introduction of novel and more convenient dosing regimens such as weekly alendronate and risedronate and yearly zoledronic acid for osteoporosis. It is recognized that there may also be clinically important differences in residence time in bone following cessation of treatment even among weekly dosed bisphosphonates such as risedronate versus alendronate [Peris et al., 2011].
AIMS OF REVIEW
The increase in the longevity of the population affects the incidence of certain diseases such as osteoporosis and certain cancers. Bisphosphonates are used regularly for the treatment of many disorders that affect not only the elderly, such as osteoporosis, but also children, such as osteogenesis imperfecta. Gaps remain in our understanding of the pathophysiology underlying both some of the potential adverse effects and also some of the potential extra-skeletal benefits and we shall endeavor to put these issues into a clinical context to develop a research agenda. The aims of this review also include compiling and synthesize the basic characteristics of these drugs and outline the most important benefits and side effects that can be produced by their prescription.
THE GLOBAL STAGE
When employing bisphosphonates in the health care system, clinicians and regulators will need answers to several questions: Do benefits outweigh the risks? Are we increasing the quantity or the quality of our patients' life? Important terms to consider are the number needed to treat (places the drug into perspective) and the number needed to harm (places the harm into perspective). These are difficult questions for medical doctors, which randomized clinical trials (otherwise the gold standard for efficacy) are unable to answer in most cases. Frequently scientific facts and results in trials are the main protagonists, leaving the harm/benefit thinking to medical doctors and health planners, who have to balance the long-term benefits against the short-term risks and vice versa. This is critical in old people (frailer and with a lower life expectancy) where bisphosphonates are frequently prescribed. No drug is exempt from risks, but talking about “harm” rather than “risks” implies that real-life bisphosphonate users may not be the optimal target population.
Osteoporosis is a chronic disease and patients are often diagnosed in their sixties or seventies, when their residual life expectancy despite chronic conditions can be estimated at 15–20 years [Abrahamsen et al., 2015]. Hence, osteoporosis management will typically involve drug changes, pauses in treatment, and re-starting treatment with the same or other drugs. Examples of this include following up a 24 month course of a PTH analog with long-term bisphosphonate treatment or using one or two courses of zoledronic acid as a sequel to osteoporosis drugs with rapid offset of treatment such as SERMs or denosumab. The need for a coherent evidence-based goal oriented therapy has been called for by many clinicians but there is a paucity of strong data from which such strategies can be developed. For Pagets' disease, the initial results with nitrogen-containing bisphosphonates have been so successful that it has made comparative studies challenging and the evidence for early institution of treatment in asymptomatic patients is subject to considerable debate [Langston et al., 2010; Reid, 2012].
BENEFITS OF BISPHOSPHONATES
The main drug benefits of bisphosphonates are summarized in Table I.
| Drug effect | Major indication | Strength of evidence | Effect size | |
|---|---|---|---|---|
| Prevention of fragility fractures | Inhibition of bone resorption | Primary osteoporosis [Kanis et al., 2013; Jansen et al., 2009; Freemantle et al., 2013] | RCT | 30–60% depending on the fracture site |
| Glucocorticoid-induced osteoporosis [Briot and Roux, 2015] | RCT observational studies | 6–48% | ||
| Prevention of SREa in cancers with bone metastasis | Interruption of the tumor-mediated osteolysis | Breast cancer [Coleman et al., 2014] | RCT | 50–70% |
| Prostate cancer [Coleman et al., 2014] | RCT | 11–36% | ||
| Multiple Myeloma [Coleman et al., 2014] | RCT | 26% | ||
| Mortality reduction | NCa | Multiple myeloma [Coleman et al., 2014] | RCT | 16% |
| Postmenopausal women with breast cancer [Coleman et al., 2014] | Cohort study meta-analysis | 7–18% | ||
| Subjects with recent surgical hip repair [Coleman et al., 2014] | RCT | 28% |
- a SRE, skeletal-related events; NC, mechanisms not clear.
SKELETAL EFFECTS
The main skeletal benefit of bisphosphonates is the prevention of fragility fractures, which are a great socio-economic burden to health care systems. Fracture risk reduction with bisphosphonates in primary or postmenopausal osteoporosis has been proven in clinical trials; alendronate was found to reduce 50% of vertebral fractures in subjects with a previous vertebral fracture or with low bone mineral density, risedronate proved to reduce the vertebral, non-vertebral fractures (40–50% and 30–36%, respectively), and hip fractures (only in women aged 70–79 years old) and ibandronate was found to reduce the risk of vertebral fractures by 50–60% and the non-vertebral fractures only in a high-risk population (bone mineral density <−3SD) [Kanis et al., 2013]. Nevertheless, the increasing number of approved medications raises doubts as to which ones to use. Prioritizing one bisphosphonate over another is essential for decision-making in clinical practice, and this can only be achieved through the head-to-head trials. Ideally, these would have to include all of the available bisphosphonates in the market but unfortunately these kinds of trials are not feasible for practical and economical reasons. The mixed treatment comparison (MTC) studies can partially solve this problem.
MTC studies are an extension of meta-analysis, where analysis of three or more interventions can be carried out simultaneously in one meta-analysis. MTC studies can be used to analyze studies with multiple intervention groups by synthesizing direct and indirect comparisons, in the absence of head-to-head trials.
Although based on indirect estimates (only including placebo-controlled trials), MTC studies allow us to report multiple comparisons between bisphosphonates regarding fracture prevention. These studies found that zoledronic acid provided a greater vertebral fracture reduction (40% lower number of fractures) compared with ibandronate, alendronate, and risedronate [Jansen et al., 2009]. Furthermore, in spite of the proven efficacy of non-vertebral fracture prevention with alendronate and zoledronic acid, risedronate was the only bisphosphonate that was found to reduce non-vertebral fractures using the indirect comparisons of the MTC studies [Freemantle et al., 2013].
Once initiating a treatment with bisphosphonates, a usual question that arises is how to monitor the effect. Is the absence of fracture a strong enough indicator to continue bisphosphonates or is the appearance of a new fracture a sign that the bisphosphonate is not working? Indirect surrogates of fracture such as the bone mineral density or the bone turnover markers have been proposed as indicators to help answer these questions but evidence is still missing and no general consensus has been achieved [Kanis et al., 2013].
The continued treatment with bisphosphonates has raised concerns of its long-term anti-fracture effects. This has been addressed in several extension studies; the FLEX (alendronate 5-year extension study after the pivotal trial FIT), the risedronate 7 years extension study, and the 6-year zoledronic extension study all reaffirmed their fracture risk reduction with continued bisphosophonate use. Intriguingly, the discontinuation of alendronate did not increase the risk of non-vertebral or morphometric vertebral fracture [Eriksen et al., 2014]. The ibandronic acid extension study, the only one where fractures were analyzed only as an adverse event and not as a main outcome, found that 8.5–11% of the subjects in the ibandronate arm reported fractures while on treatment [Eriksen et al., 2014]. These results lead us to think that the overall fracture reduction efficacy is sustained with long-term exposure for the majority of bisphosphonates.
Despite the fracture risk reduction demonstrated in the RCT and comparison studies, results in the real-life setting have shown to be somewhat less optimistic. The lower fracture risk reduction observed in observational studies with real-life patient's poses into question the external validity of the bisphosphonates clinical trials. One of the possible explanations is the poor adherence [Feldstein et al., 2009] of BPs in real-life settings. While clinical trials reported a very high adherence rates (needed in order to see any effect in fracture risk reduction [Feldstein et al., 2009]), a cohort study reported that only 44% of the subjects treated with BPs had MPR > 80%, which could explain the decreased anti-fracture effect [Feldstein et al., 2009]. Accordingly, Danish national observational study found a risk reduction in hip fractures of 40% (HR 0.60; 95% CI 0.53–0.68) in patients with a cumulative use of five dose years of alendronate, compared with patients with more limited use [Abrahamsen et al., 2010].
Bisphosphonates have also proven their efficacy in other types of osteoporosis including glucocorticoid-induced osteoporosis. Glucocorticoids are frequently used in primary and secondary healthcare services. Glucocorticoid users are 1.6 times more likely to have a vertebral fracture and 2.6 times more likely to have a hip fracture with the risk of fragility fracture increasing mostly in the first 3 months after the glucocorticoid initiation [Briot and Roux, 2015]. This type of induced-osteoporosis is not merely linked to high dosages of glucocorticoids; studies have reported that even 2.5 mg per day may increase bone fragility [Briot and Roux, 2015]. Despite the increased awareness of healthcare professionals detecting and treating this disorder, only 25% of the subjects at risk receive treatment for their glucocorticoid-induced osteoporosis [Majumdar et al., 1998]. Treatment for this disorder starts with an early detection and initiation of BPs. These drugs have already proven their anti-fracture efficacy in osteoporosis but there is a paucity of trials with a fracture as a main outcome in glucocorticoids users. Among the BPs that have proven to increase BMD and are recommended to prevent bone loss among glucocorticoid users are alendronate, risedronate, and zoledronic acid [Briot and Roux, 2015].
Other bone-related diseases also benefit from the bisphosphonates anti-resorptive action. Bisphosphonates have proven to be effective and cost-effective in patients with rheumatoid arthritis [Van Staa et al., 2007]. However, the major advances have been achieved in Pagets' disease where bisphosphonates have proven a remission of the illness for long time periods [Reid, 2012]. Zoledronic acid has proven efficacy in reducing the risk of adverse skeletal events, including bone loss, in men with prostate cancer or in women with breast cancer [Coleman et al., 2014].
Another benefit of bisphosphonates, besides the inhibition of bone resorption and fracture risk prevention, is the relief of bone pain. The relief of bone pain occurs not only in rare bone diseases such as the fibrous dysplasia but also in bone cancer and bone metastasis. Bisphosphonates act as co-adjuvants with radiotherapy in order to decrease bone pain especially in those subjects where this pain is poorly localized. Bisphosphonates have proven to reduce by 50% the frequency of skeleton-related events in breast cancer and to reduce bone pain in prostate cancer. As a result, the ESMO (European Society of Medical Oncology) stated recently in their recommendations that zoledronic acids should be used in patients with metastatic breast or prostate cancer, and in selected individuals with lung, renal, and other solid tumor with metastasis [Coleman et al., 2014].
The main mechanism through which bisphosphonates act is suppression of bone resorption. All BPs have an affinity for bone tissue, and more specifically to osteoclasts because during bone resorption, the acidic PH of the resorption lacuna of the osteoclasts causes an intracellular uptake of BPs, leading to the internalization of substantial amounts of these drugs. Independently of the pathway used to inhibit bone resorption, either inducing a cytotoxic effect in osteoclasts or inhibiting the production of farnesyl diphosphate, all of them lead to the apoptosis of osteoclasts [Russell et al., 2008].
Overall, the benefits of bisphosphonates have been proven not only in primary and postmenopausal osteoporosis, but also in other bone-related diseases. The limited evidence of head-to-head trials makes it difficult to recommend one bisphosphonate over another since all of them have their indications and contraindications, so the choice must remain in its cost-effectivity and the type of patient for which the BP is been prescribed. If we consider the main indication of bisphosphonates, which is the fracture prevention in osteoporosis, some guidelines recommend starting with alendronate [National Institute for Health and Care Excellence, 2008) and risedronate [Mendoza et al., 2013] because of their high cost-effectivity [Compston et al., 2009].
EXTRA-SKELETAL EFFECTS
The main extra-skeletal benefit of bisphosphonates identified in RCTs is the finding of a reduction in mortality in a hip fracture patients randomized to zoledronic acid. In the placebo group, 141 of 1,057 patients died during the study compared with 101 of 1,054 patients in the zoledronic acid treated arm, a risk reduction of 28%, chiefly explained by fewer deaths from cardiovascular causes [Lyles et al., 2007], though the mechanism is not clear. In rats, knock-down of farnesyl pyrophosphate synthase protects against hypertensive cardiac hypertrophy [Ye et al., 2010] and zoledronic acid could have a positive effect in some animal models of accelerated ageing. A number of observational studies have linked BP use to better survival but this could be due to elderly patients in poor health being potentially less likely to begin BP treatment. However, if BPs truly extend life expectancy—an outcome that clinical trials were not designed to or necessarily powered to pursue—it would not make sense for a high risk of mortality to be a barrier for treatment, rather the opposite.
Some observational studies, which should be regarded as hypothesis generating rather than proof of effect, have also suggested that oral bisphosphonates could reduce the risk of colon cancer and possibly gastric cancer [Abrahamsen et al., 2011; Pazianas et al., 2012; Singh et al., 2013]. Though a reduced risk of breast cancer was also suggested by the Women's Health Initiative [Chlebowski et al., 2010] (observational design), this was recently refuted by analysis of RCT data from the primary licensing trials of alendronate [Hue et al., 2014]. Finally, a twofold increased implant survival of the total hip or knee prosthesis was detected in a recent cohort study carried out in the United Kingdom. The protective effect was found to be greater in subjects with a knee rather than a hip prosthesis [Prieto-Alhambra et al., 2011].
POTENTIAL HARMS OF BISPHOSPHONATES
The main potential harms of bisphosphonates are summarized in Figure 1.
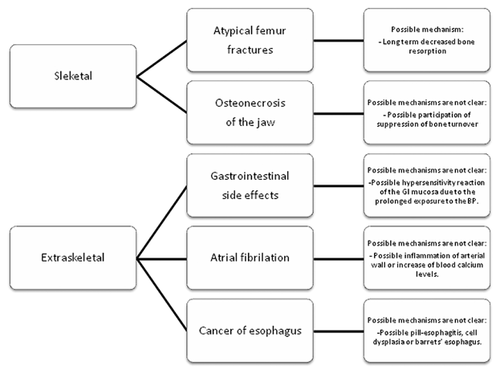
SKELETAL POTENTIAL HARM
Where early bisphosphonates had the capacity to inhibit bone mineralization and induce osteomalacia at high doses, the skeletal concerns with modern nitrogen-containing bisphosphonates (sometimes inappropriately referred to as aminobisphosphonates) relate to lowering of bone resorption itself. Lower bone resorption may indirectly reduce the rate at which bone microdamage is generated but it will also directly reduce the rate at which microdamage is removed and replaced by new bone. Atypical femur fractures (AFF) may be an indication that artificially induced low bone turnover over years may lead to unexpected novel fractures. The term atypical femur fracture refers to a rare group of femur fractures that have specific radiological features, which include a pronounced predilection for the lateral aspect of the subtrochanteric or diaphyseal femur, a mainly transverse fracture direction and a tendency for periosteal or endosteal thickening of the cortex. The diagnostic criteria are given in detail elsewhere [Shane et al., 2014]. The fractures may be bilateral and incomplete fractures often show delayed healing and may need surgical pinning. While such fractures are much more common in patients treated with bisphosphonates they do also occur, albeit rarely, in the background population.
Data from Kaiser California in the United States reported an age-adjusted incidence rate of 1.8 per 100,000 with up to 2 years of BP use, 16 per 100,000 with 4–6 years of use, and 107.5 per 100,000 with more than 10 years of use, suggesting an exponential increase in risk with increasing duration of use [Dell et al., 2012]. An exponential dose–response relationship has also been reported by Swedish researchers who first published findings based on reviewing all potential AFF X-rays in one calendar year for the Swedish nation [Schilcher et al., 2011]. More recently, the review period has been extended to 3 years. The updated analysis finds a relative risk of AFFs in Swedish BP users of more than 4 years of exposure of 126 (95%CI 55–288) or 110 excess fractures per 100,000 patient years. This rate is six times higher than expected from Kaiser California [Dell et al., 2012]. It is not however impossible as the rate falls within the 440 per 100,000 total rate of subtrochanteric and shaft fractures (of which they are a subgroup) reported in Danish women with an average of 4 years of BP use, a rate which declined to 320 per 100,000 with 9 years of use [Abrahamsen et al., 2010]. However, weaknesses of the Swedish dataset include the short prescription history provided by the national register, which could underestimate duration of BP use and inflate the dose–response association. The potential for harm should be balanced against the much higher rates of typical osteoporotic fractures; hence, Swedish women treated with BPs experience hip fractures at a rate of 1,500 per 100,000 [Schilcher et al., 2011] and are also at similarly high risk of other major osteoporotic fractures. It is unclear if all long-term users of BPs are at risk of AFF or if this risk is confined to a distinct subgroup of patients; at present, the risk of AFFs appears substantially higher in persons of East Asian heritage than in Europeans. Vitamin D deficiency, diabetes, and proton pump inhibitor use have been proposed as potential risk factors for AFF. It is unclear at present if the risk of AFF is lower with bisphosphonates with a shorter residence time in bone such as risedronate, the majority of observations having been made in alendronate users.
Among patients treated for osteoporosis, osteonecrosis of the jaw (ONJ) occurs at a much lower rate than AFF but ONJ is not rare when bisphosphonates are used in an oncology setting. The condition has been known for many decades in other contexts. It was first described in factory workers exposed to large amounts of phosphorous in the manufacture of matches and since identified as a consequence of local radiation therapy. Though the necrotic process may not initially be exposed to the naked eye, the working definition of ONJ remains a clinical diagnosis based on identification of an area of exposed bone in the oral cavity that fails to heal in 8 weeks [Khan et al., 2015]. An earlier stage, unfortunately termed non-exposed ONJ, has been proposed to cover cases of ONJ diagnosed by radiological imaging alone [Schiodt et al., 2014]. The epidemiological data for ONJ in patients treated with BPs for osteoporosis were recently reviewed by Solomon et al. [2013], finding broad variation—of a hundredfold or more—in reported incidence rates and relative risks between studies. The authors then analyzed two claims databases and found one confirmed case per 4,900 BP users in one cohort and one suspected case per 14,300 BP users in their other cohort, producing cross-sectional prevalence estimates that fell within the range reported in the prior literature. There is no clear indication that the risk of ONJ increases with exposure time with osteoporosis doses. In oncology, the risk of ONJ seems much higher than in osteoporosis, reflecting probably not only the shorter dosing interval (larger annual cumulative exposure) but also the effects of anti-neoplastic agents such as angiogenesis inhibitors, immune suppression, and prednisolone treatment. The highest cross-sectional prevalence of ONJ presently reported in the oncology field is eight cases out of a study population of only 43 patients with advanced prostate cancer who had extensive dental examinations. Incidence estimates [Khan et al., 2015] for ONJ with i.v. bisphosphonates in oncology have ranged from 0 to 12,200 per 100,000 patient years (so up to one case per eight patient years). There remains a substantial lack of solid data on the risk factors for ONJ, the absolute risk as a function of time and dose, and not least the underlying pathophysiology including the relative role of vascular, epithelial, and skeletal events and the sequence in which they develop and mature into clinically evident ONJ. Management of ONJ is beyond the scope of this review but readers are referred to the consensus paper by Khan et al. [2015].
EXTRA-SKELETAL SIDE EFFECTS
Gastrointestinal
While the effect of bisphosphonates basically takes place at the bone, the long-term extra-skeletal effect has generated much debate. Gastrointestinal (GI) effects are among the most important extra-skeletal adverse reactions linked to bisphosphonate use, together with cardiovascular events and some types of cancer.
Oral bisphosphonates have been associated with several gastro-intestinal side effects such as esophageal ulcers, esophagitis, and upper gastrointestinal hemorrhage. Drug-induced esophagitis is a common and often under-diagnosed side effect of many pharmacological treatments. Ulcers, erosions, bleeding, and even impacted pills fragments or coating with drug material are common endoscopic findings [Kim et al., 2014]. Not all of the pivotal trials of bisphosphonates reported GI side effects. The oral and endovenous ibandronic acid studies did find an increased risk of GI symptoms (1.7–7.4% and 14–20% for oral and endovenous ibandronate, respectively) compared to placebo [Eriksen et al., 2014]. Conversely, risedronate was not associated with any GI side effect [Eriksen et al., 2014] even when restricted to high-risk subjects, such as the ones >75 years old or those consuming NSAIDs [Pazianas et al., 2010]. Interestingly, the HORIZON trial (which studied the effects of intravenous zoledronate) also reported an unexpected increased frequency of GI symptoms such as nausea, vomiting, diarrhea, and dyspepsia among patients in the treatment arm [Pazianas et al., 2010]. Studies associating the upper gastrointestinal bleeding with bisphosphonate consumption have led to uneven results. Upper gastrointestinal bleeding is an infrequent but severe side effect of some drugs such as the NSAID and its association with BPs has also been studied; a population-based cohort study in Canada reported that subjects aged over 80 and those with a previous gastric ulcer had an increased risk of upper gastrointestinal bleeding in the first 120 days of BPs use [Knopp-Sihota et al., 2013] even after adjusting for NSAID use. The higher comorbidity of the population or the number of medications could have contributed to this increased risk of upper gastrointestinal bleeding. Conversely, another large case–control study found that BPs, taken alone, were not associated with an increased risk of upper gastrointestinal bleeding. The risk increased with BPs the intake of BPs occurred simultaneously with NSAIDs but then again this increased risk did not differ from the one found with NSAID alone [Etminan et al., 2009].
Mechanisms through which BPs could lead to any GI side effects are related with their poor GI absorption. Less than 1% of oral BPs are known to be absorbed leaving the gastric and intestinal mucosa exposed to a large amount of drug which could be responsible for these GI adverse events [Pazianas et al., 2010]. A hypersensitivity reaction of the GI mucosa due to the prolonged exposure to the BP could be the pathological pathway of the esophagic ulcers or even the GI bleeding [Naniwa et al., 2008]. Clear instructions on how to take oral bisphosphonates to minimize esophagic exposure to these drugs should be provided to all BP users.
Cardiovascular
Cardiovascular events, such as atrial fibrillation (AF), stroke or the cardio-vascular death, were analyzed in the major pivotal trials of BP with uneven results. Several studies reported in first instance a possible association that was not confirmed in latter analysis. This is the case of alendronate, where a numerical increase of cases was detected during its pivotal trial but was not confirmed by a latter meta-analysis [Eriksen et al., 2014]. In the HORIZON trial, cases of AF were more frequently reported among the zoledronic acid users compared to placebo (1.3% compared to 0.5%, respectively), although most of the AF were reported 30 days after the infusion of zoledronic acid, time by which the zoldronic acid is no longer detectable in blood samples [Grosso et al., 2009] which questions this association. Furthermore, the association between zoledronic acid and AF was not confirmed in the zoledronic extension trial where no evidence of this association was found [Pazianas et al., 2010]. Beyond the pivotal trials, no further light has been shed on this matter; a case–control study reported a possible association between the intake of alendronate and AF but the lack of dose–response challenged this finding [Heckbert et al., 2008]. The same happened with two Danish studies [Sørensen et al., 2008; Abrahamsen et al., 2009]; in the first one [Heckbert et al., 2008], no evidence of an association between the use of BP and AF was found and in the second one [Abrahamsen et al., 2009] despite finding a moderate increases risk among alendronate users, the risk decreased with increasing drug adherence. The increased risk in this study was also linked to the short-term use of bisphosphonates, which was also reported in a self-controlled case series study carried out in the United Kingdom [Sørensen et al., 2008]. The possibility of an increased risk of AF during the first weeks after bisphosphonate initiation found in these a forementioned studies, goes against what was previously found in the zoledronic acid study, where the increased risk of AF was mostly found 30 days after the infusion of the drug [Sørensen et al., 2008]. An already increased risk of cardiovascular events in the hip fracture population could contribute to the divergence between these results.
Considering the other cardiovascular events, no risk of stroke or myocardial infarction was found with the use of bisphosphonates [Abrahamsen et al., 2009] being the ibandronate trial the only to report cases of angina pectoris, myocardial ischemia, and hypertension in subjects that had received oral ibandronate [Eriksen et al., 2014].
There are still no plausible biological mechanisms linking bisphosphonate therapy to cardiac arrhythmia, still, some authors have postulated some hypothesis that aim to explain this possible increased risk. Among the mechanisms involved, there is the accumulation of BP in the arterial wall or the inflammation processes detected in animal studies [Abrahamsen et al., 2009]. The possible increase in calcium levels has also been pointed as a probable cause of arrhythmia, in spite of the little to null effect that zoledronic acid had on calcium serum levels after its infusion [Sørensen et al., 2008].
After reviewing the existing evidence the FDA concluded in 2008 that the association between the intake of BPs and the risk of AF had not been confirmed. Since then, despite than more recent reviews of clinical trial data and reports in Europe concluded that the risk of AF with the use of BP was low and that it should not influence the decision of initiating these drugs since the risk and benefits remained favorable, the information of the possible risk of AF with zoledronic acid was included in the characteristics of the drug [Pazianas et al., 2010].
Cancer
After the commercialization of alendronate, several case reports were published alerting of the possible risk of cancer of esophagus among alendronate users, which led to a change in the label of this drug [De Groen et al., 1996]. Since then, concerns have been raised regarding this association. Esophageal cancer is considered one of the deadliest cancers worldwide affecting over 450,000 people [Zhang, 2013]. Recently, two case–control studies supported the increased risk of esophageal cancer among BP users; the first was carried out in 2010 in the United Kingdom and found an increased risk in subjects with at least 10 prescriptions of BP independently of the type of BP used and independently of other risk factors [Green et al., 2010]. The second study was carried out with US veterans; here, a twofold increased risk of Barret's esophagus, considered to be a premalignant disease leading to esophagealcancer, was detected among BP users [Lin et al., 2013]. Conversely, there are observational and meta-analysis of observational studies were this association has not been confirmed [Oh et al., 2012; Ghirardi et al., 2014]. A difficulty with case–control studies is that they would include mainly male subjects because esophageal cancer is much rarer in women than in men, which poses a challenge when translating the findings to the population attending the osteoporosis clinic, which is strongly dominated by women. Finally, the histopathological distinction between adenocarcinomas of the gastric ventricle and the lower esophagus is highly challenging and borders on the impossible. BP users are more likely than non-users to undergo endoscopy and may be more likely to be diagnosed with adenocarcinomas at an earlier stage where distinction between gastric and esophageal origin remains possible, hence, leading to a seemingly reduced rate of gastric cancers and a corresponding increase in esophageal cancer as has been observed in some studies [Green et al., 2010].
One of the pathological pathways proposed to explain how BP would increase the risk of esophagic cancer include chronic esophagitis. Persistent injury of the mucosa due to the swallowing of the pill, called pill-esophagitis [De Groen et al., 1996] could lead to dysplasia of the esophageal cells and to an increased risk of Barretts' [Green et al., 2010].
The greater weight of evidence associating BPs with cancer of esophagus relies on case-reports. The observational studies published until today show inconsistent results, leading to the conclusion that the association between BPs and cancer of esophagus is still not clear [Pazianas et al., 2010]. However, it is important to bear in mind that previous oesophagitis remains a contraindication according to most oral bisphosphonate small product characteristic leaflets (SmPC).
DURATION OF TREATMENT
As our currently available treatments do not suffice to cure osteoporosis—we can increase bone mass and using bone building drugs we can improve microarchitecture but not restore it to the youthful state—it stands to reason that patients with osteoporosis will need long-term treatment; possibly with carefully chosen periods off drug (the so called “drug holidays”). It must, however, be said that the evidence for drug holidays with bisphosphonates remains relatively weak and the harms may exceed the benefits in many patients as far as we know [Ye et al., 2010]. The long carry over effect of bisphosphonates in bone is both the advantage and the main problem of these drugs, as the short duration of action is the strongest and weakest feature of alternative drugs like denosumab. How reversible do we want our anti-resorptive agents to be? In some patients, we want them long-lasting for dosing convenience, compliance, and the prospect of increasing the time interval for doses. But in other patients, we may appreciate the quick reversibility of short acting bone drugs even if the rebound bone resorption makes them difficult drugs to terminate.
EXPERT OPINION AND DIRECTIONS OF RESEARCH
The most important lesson from the past decade is perhaps that bisphosphonates are like all other medications: benefits come at a price and it is not clear how the harm/benefit may change over time as the effect of bisphosphonate treatment accumulates and how this may be different between patients. In this, the real challenge is not just getting better statistical power but more importantly gaining insights in the pathophysiological processes by which BPs may induce harm such as GI irritation, AFFs, or even ONJ.
There is also an urgent need for data on both the benefits (fracture reduction) and harms (the above listed adverse drug effects) among real-world BP users, who are fundamentally different from the participants recruited in most of the pivotal trials.
Basic research has helped us understand better the pharmacokinetics of bisphosphonates and how different bisphosphonates could have different anti-resorptive effects. In the same way, it is crucial to understand how these drugs increase the risk of certain events such as cancer, the GI side effects of the AF. The widening of bisphosphonate indications and the aging of the target population implies an increased risk of cardiovascular events and cancer at baseline. Despite the introduction of other medications for bone-related diseases, such as denosumab, bisphosphonates are still the main and most frequently prescribed treatment to decrease bone resorption. Knowing the pathological mechanisms through which bisphosphonates could worsen these conditions could help in their prevention.
DISCLOSURE
B.A. has received institutional research grants from or served as an investigator in studies for Novartis, Nycomed/Takeda, and Amgen. D.P.-A. has received unrestricted research grants from Amgen and Bioiberica. C.R. has no conflict of interests. M.H. has served as investigator for Amgen, MSD, and Novartis.