Interaction of Integrin β4 With S1P Receptors in S1P- and HGF-Induced Endothelial Barrier Enhancement
ABSTRACT
We previously reported sphingosine 1-phosphate (S1P) and hepatocyte growth factor (HGF) augment endothelial cell (EC) barrier function and attenuate murine acute lung inury (ALI). While the mechanisms underlying these effects are not fully understood, S1P and HGF both transactivate the S1P receptor, S1PR1 and integrin β4 (ITGB4) at membrane caveolin-enriched microdomains (CEMs). In the current study, we investigated the roles of S1PR2 and S1PR3 in S1P/HGF-mediated EC signaling and their associations with ITGB4. Our studies confirmed ITGB4 and S1PR2/3 are recruited to CEMs in human lung EC in response to either S1P (1 µM, 5 min) or HGF (25 ng/ml, 5 min). Co-immunoprecipitation experiments identified an S1P/HGF-mediated interaction of ITGB4 with both S1PR2 and S1PR3. We then employed an in situ proximity ligation assay (PLA) to confirm a direct ITGB4–S1PR3 association induced by S1P/HGF although a direct association was not detectable between S1PR2 and ITGB4. S1PR1 knockdown (siRNA), however, abrogated S1P/HGF-induced ITGB4–S1PR2 associations while there was no effect on ITGB4–S1PR3 associations. Moreover, PLA confirmed a direct association between S1PR1 and S1PR2 induced by S1P and HGF. Finally, silencing of S1PR2 significantly attenuated S1P/HGF-induced EC barrier enhancement as measured by transendothelial resistance while silencing of S1PR3 significantly augmented S1P/HGF-induced barrier enhancement. These results confirm an important role for S1PR2 and S1PR3 in S1P/HGF-mediated EC barrier responses that are associated with their complex formation with ITGB4. Our findings elucidate novel mechanisms of EC barrier regulation that may ultimately lead to new therapeutic targets for disorders characterized by increased vascular permeability including ALI. J. Cell. Biochem. 115: 1187–1195, 2014. © 2013 Wiley Periodicals, Inc.
Lung endothelial cell (EC) barrier disruption is a key feature of syndromes associated with inflammation and increased vascular permeability including acute lung injury (ALI). We previously reported that sphingosine 1-phosphate (S1P) and hepatocyte growth factor (HGF) are both robust EC barrier-enhancing agonists that confer protection in murine models of ALI [Liu et al., 2002; Dudek et al., 2004; Peng et al., 2004; Singleton et al., 2007]. S1P is a phospholipid angiogenic factor released from activated platelets that, upon ligation of specific S1P receptors, enhances EC barrier integrity through activation of the small GTPase, Rac1, with subsequent actin and junctional protein rearrangement [English et al., 2000; Dudek et al., 2004; Belvitch et al., 2012]. HGF promotes EC barrier enhancement via ligation of c-Met, a cell surface receptor tyrosine kinase, also with downstream effects on Rac1 activation and actin cytoskeletal rearrangement [Liu et al., 2002; Singleton et al., 2007]. Most recently, we reported that HGF/c-Met-mediated EC barrier enhancement is dependent on the dynamic regulation of a signaling complex comprised of the S1P receptor, S1PR1, and integrin β4 (ITGB4) that are both recruited to membrane caveolin-enriched microdomains (CEMs) [Ephstein et al., 2013].
EC signaling by S1P is dependent on ligation of specific receptor subtypes. While five S1P receptors (S1PR1–5) have been identified EC primarily express the highly homologous S1PR1, S1PR2, and S1PR3 receptors [Hla et al., 2001]. Differential G-protein coupling of these receptors, however, affects distinct downstream signaling events as S1PR1 is coupled to Gi while S1PR2 and S1PR3 are coupled to Gi, Gq, and G12/13. Moreover, the functional roles of these receptors in vascular barrier regulation are somewhat unclear and appear to be variable depending on the experimental conditions. For example, in separate murine models, our lab has reported that decreased expression of either S1P2 or S1P3 is associated with decreased susceptibility to LPS-induced lung injury [Sammani et al., 2010] but increased susceptibility to radiation-induced lung injury [Mathew et al., 2011].
Integrins form transmembrane heterodimers consisting of α and β subunits that mediate both inside-out and outside-in signaling. In EC, integrins are known to regulate a variety of functions including cytoskeletal rearrangement [Giusti et al., 2013], barrier regulation [Eliceiri et al., 2002; Su et al., 2007], angiogenesis [Hood et al., 2003; Nikolopoulos et al., 2004], and inflammatory responses [Chen et al., 2012; Luu et al., 2013]. While eight integrin β subunits have been identified, ITGB4 is uniquely characterized by its long cytoplasmic tail comprised of 1,088 amino acids [Hogervorst et al., 1990]. Prior evidence suggests that integrins are involved in the regulation of CEM trafficking [Echarri and Del Pozo, 2006] and we have previously identified ITGB4 as an important mediator of EC protection by simvastatin [Jacobson et al., 2005; Chen et al., 2012]. We subsequently confirmed that ITGB4 is also a significant mediator of EC barrier enhancement induced by both S1P and HGF via effects on S1PR1 [Ephstein et al., 2013]. In this study, we investigated the role of S1PR2 and S1PR3 in EC barrier enhancement by S1P and HGF with specific focus on the role of ITGB4 in this context.
MATERIALS AND METHODS
CELL CULTURE AND REAGENTS
Human pulmonary artery EC obtained from Lonza (Walkersville, MD) were cultured as previously described in ECM-2 complete medium (Lonza) at 37 °C in 5% CO2 and 95% air, with cell passages 6–10 used for experimentation [Garcia et al., 2001]. Reagents for SDS–PAGE electrophoresis and immobilon-P transfer membrane were purchased from Bio-Rad (Richmond, CA), and gold microelectrodes from Applied Biophysics (Troy, NY). Sphingosine 1-phosphate (S1P) was purchased from Biomol (Farmingdale, NY). Recombinant human HGF was commercially obtained from PeproTech (Rocky Hill, NJ). Rabbit anti-caveolin-1, rabbit anti-S1PR1, mouse anti-S1PR2, goat anti-S1PR2, rabbit anti-S1PR3, goat anti-S1PR3 and rabbit anti-ITGB4 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit anti-S1PR2 antibody was purchased from GeneTex (Irvine, CA). Horseradish peroxidase-labeled secondary antibodies were obtained from Cell Signaling (Danvers, MA). Non-specific siRNA (nsRNA) and siRNA specific for S1PR1, S1PR2, and S1PR3 were purchased from Dharmacon (Layfayette, CO). All other reagents were purchased from Sigma (St. Louis, MO) unless otherwise specified.
CAVEOLIN-ENRICHED MICRODOMAIN (CEM) ISOLATION
CEMs were isolated from EC as previously described [Ephstein et al., 2013]. Briefly, EC were scraped in PBS, centrifuged at 2000 rpm at 4 °C and lysed with 0.2 ml of TN solution [25 mM Tris–HCl (pH 7.5), 150 mM NaCl, 1 mM DTT, protease inhibitors, 10% sucrose, 1% Triton X-100] for 30 min on ice. Triton X-100-insoluble materials were mixed with 0.6 ml of cold 60% Optiprep™ and overlaid with 0.6 ml of 40%, 30%, and 20% Optiprep™ in TN solution. The gradients were then centrifuged at 35,000 rpm for 12 h at 4 °C, different fractions were collected, and were analyzed by SDS–PAGE plus immunoblotting.
TRANSFECTION OF SMALL INTERFERING RNA (siRNA)
The siRNA sequences targeting human S1PR1, S1PR2, and S1PR3 were purchased from Dharmacon (Lafayette, CO). EC were transfected with siRNA using siPORT™ Amine (Ambion, Austin, TX) according to the manufacturer's instruction. Cells grown to 60–80% confluence, were treated with 100 nM siRNA in 2% FBS media. After incubating for 72 h, biochemical experiments and functional assays were conducted as described.
IMMUNOPRECIPITATION AND WESTERN BLOTTING
EC were incubated in immunoprecipitation buffer (50 mM HEPES (pH 7.5), 150 mM NaCl, 20 mM MgCl2, 1% Nonidet P-40, 0.4 mM Na3VO4, 40 mM NaF, 50 µM okadaic acid, 0.2 mM phenylmethylsulfonyl fluoride, and Calbiochem protease inhibitor mixture III at 1:250 dilution). The samples were then immunoprecipitated with anti-S1PR2, anti-S1PR3, or anti-ITGB4, followed by SDS–PAGE (4–15%), transferred onto immobilon-P membranes and subjected to immunoblotting using specific primary and HRP-labeled secondary antibodies. Visualization of immunoreactive bands was achieved by enhanced chemiluminescence (Thermo Scientific, Rockford, IL). Densitometric data were obtained with Image J software.
MEASUREMENTS OF TRANSENDOTHELIAL MONOLAYER ELECTRICAL RESISTANCE (TER)
EC were grown to confluence in polycarbonate wells containing evaporated gold microelectrodes and TER measurements were then performed using an electrical cell-substrate impedance sensing system (ECIS) as we have previously described [Garcia et al., 2001]. TER values from each microelectrode were pooled at discrete time points and plotted versus time as the mean ± SEM.
PROXIMITY LIGATION ASSAY (PLA)
EC were used for PLA in situ detection using the Duolink Detection Kit (Olin Bioscience, Uppsala, Sweden) according to the manufacturer's protocol. Briefly, Cells grown on coverslips were starved for 3 h and treated with HGF or S1P for 5 min, then washed with cold PBS and fixed with 4% paraformaldehyde for 20 min, permeabilized with 0.2% Triton and blocked with 1× blocking solution. Fixed cells were then incubated overnight with anti-ITGB4 and either anti-S1PR3 or anti-S1PR2, or incubated with anti-S1PR1 and anti-S1PR2 antibodies at 4 °C. Proximity ligation was performed with PLA PLUS and MINUS Probes for goat and rabbit. Dapi staining was included in the Duolink Detection Kit while anti-Phalloidin Alexa488 (Invitrogen, Carlsbad, CA) at 1:200 was added during the detection reaction. Lastly, slices were mounted and visualized with an epi-fluorescence microscope under a 60× oil objective. Texas-red signal was analyzed via BlobFinder Imaging Software, developed and optimized for the analysis of images generated by the in situ PLA (Uppsala Science Park, Sweden) [Ephstein et al., 2013]. Four fields were randomly chosen for analysis and averaged and three separate samples were examined per condition (approximately 60–80 cells total/condition).
STATISTICAL ANALYSIS
Data were analyzed using statistical software (SPSS version 15.0; SPSS, Inc., Chicago, IL) and are expressed as mean ± SEM. Differences between groups were analyzed, as appropriate, using t-tests and 1-way analysis of variance followed by the Fisher least significant difference test. P < 0.05 was considered significant.
RESULTS
S1P AND HGF-INDUCED TRANSLOCATION OF ITGB4, SP1PR2, AND S1PR3 TO EC CEMs
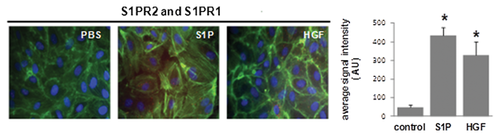
Western blotting for CEM fractions was conducted to assess ITGB4, S1PR2, and S1PR3 translocalization in response to S1P and HGF. Initially, CEM fractions were confirmed in 30% Optiprep fraction by Western blotting for caveolin-1 as we have previously described [Ephstein et al., 2013] (Fig. 1A). Subsequently, translocation of ITGB4, S1PR2, and S1PR3 to EC CEMs after either S1P (1 µM, 5 min) or HGF (25 ng/ml, 5 min) treatment was also confirmed (Fig. 1B).
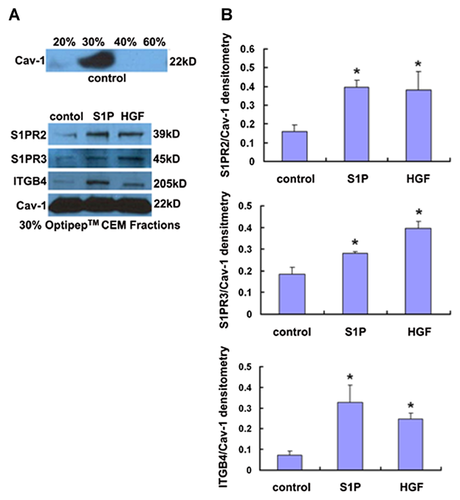
INTERACTION OF ITGB4 WITH S1PR2 OR S1PR3 IN RESPONSE TO S1P OR HGF
To investigate the potential associations of ITGB4 with S1PR2 and S1PR3 in both untreated EC and EC treated with either S1P or HGF, co-immunoprecipitation and Western blotting was performed. These experiments confirmed ITGB4–S1PR2 and ITGB4–S1PR3 associations in untreated EC that, in both cases, were signficantly increased after treatment with either S1P (1 µM, 5 min) or HGF (25 ng/ml, 5 min) (Fig. 2).
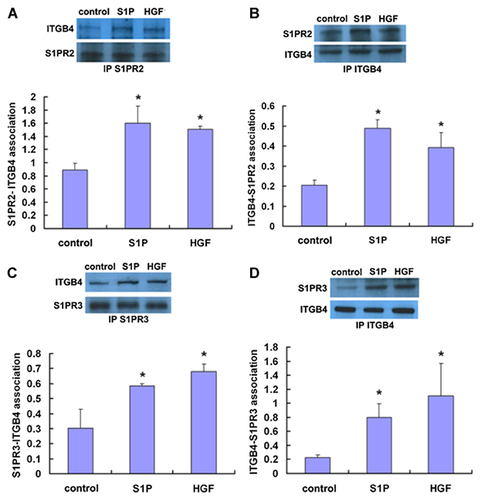
In complementary experiments, PLA studies were conducted to further explore the association of ITGB4 with S1PR2 and S1PR3. Complex formation was measured by in situ PLA using mouse anti-S1PR2 or anti-S1PR3 antibodies and rabbit anti-ITGB4 antibody, and corresponding secondary reagents. Secondary antibodies covalently linked to oligonucleotides were used as proximity probes, forming templates for circularization of two additional oligonucleotides by enzymatic ligation. This ligation requires coincident binding by two affinity reagents and thereby increases the selectivity compared with single recognition assays. Subsequently, one of the oligonucleotides serves as a primer for the RCA reaction, amplifying the circular DNA molecule ~1,000-fold in 1 h using φ29 DNA polymerase. The product represents a bundle of single-stranded DNA composed of tandem repeats of complements of the DNA circle. Individual bundles are easily visualized by hybridization of complementary fluorescence-labeled oligonucleotides. Thus, each red point represents the detection of a protein–protein interaction. Interestingly, these experiments did not identify a direct association between ITGB4 and S1PR2 (Fig. 3). However, there was a direct association between ITGB4 and S1PR3 that was further increased in response to either S1P (1 µM, 5 min) or HGF (25 ng/ml, 5 min), and was markedly attenuated in EC transfected with siRNA specific for S1PR3 (siS1PR3).
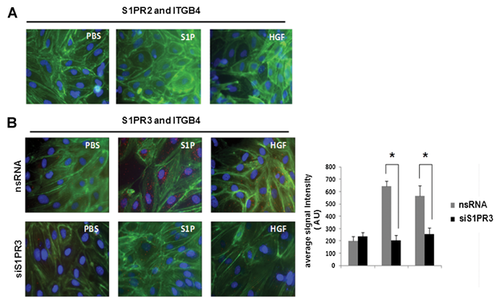
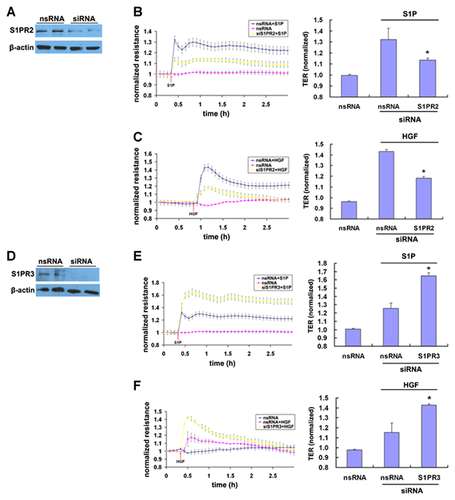
ROLE OF S1PR1 IN ITGB4–S1PR2 AND ITGB4–S1PR3 ASSOCIATIONS
To investigate the potential role of S1PR1 in ITGB4 associations with either S1PR2 or S1PR3, EC were transfected with siRNA specific for S1PR1 (siS1PR1) or non-specific siRNA (nsRNA) prior treatment with either S1P (1 µM, 5 min) or HGF (25 ng/ml, 5 min). Relative to control EC transfected with nsRNA, silencing of S1PR1 had no effect on the ITGB4–S1PR2 association at baseline but significantly attenuated S1P-induced increases in this association although the reduced association of ITGB4 and S1PR2 in response to HGF was not significant (Fig. 4). Conversely, there was no effect on the interaction of ITGB4 and S1PR3 after silencing of S1PR1 under any condition.
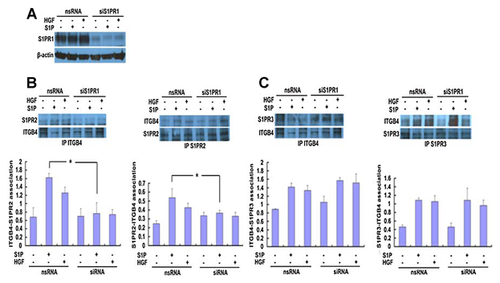
INTERACTION OF S1PR1 WITH S1PR2 INDUCED BY S1P AND HGF
As our results did not identify a direct association between ITGB4 and S1PR2 but did implicate S1PR1 as important determinant of an indirect ITGB4–S1PR2 association, we next explored a direct association between S1PR1 and S1PR2 in response to HGF and S1P by PLA. While there was little S1PR1–S1PR2 association in EC under basal conditions, both S1P and HGF treatment resulted in a significantly increased association (Fig. 5). Together, these results suggest S1PR2 translocation to CEMs in response to S1P- or HGF results in a complex formation characterized by an indirect association of S1PR2 with ITGB4 through S1PR1.
ROLE OF S1PR2 AND S1PR3 IN S1P- AND HGF-INDUCED EC BARRIER ENHANCEMENT
Finally, as we recently reported that ITGB4 and S1PR1 contribute to HGF- and S1P-mediated EC barrier enhancement, we next investigated the roles of S1PR2 and S1PR3 in this context as measured by TER. Reductions in the expression of S1PR2 by specific siRNA significantly attenuated S1P- and HGF-induced TER enhancement compared to control cells transfected with nsRNA (Fig. 6). Conversely, reductions in the expression of S1PR3 by siRNA knockdown resulted in augmented HGF- and S1P-induced barrier enhancement as measured by TER compared to control cells transfected with nsRNA.
DISCUSSION
The sphingolipid signaling pathway has emerged as an important mediator of EC barrier regulation particularly in the clinical context of ALI [Natarajan et al., 2013]. However, the relative roles of specific S1P receptors in mediating EC barrier responses and the mechanisms underlying these effects remain to be fully characterized. Having recently identified a critical role for ITGB4–S1PR1 complex formation localized to membrane CEMs in S1P- and HGF-induced EC barrier enhancement [Ephstein et al., 2013], we examined the potential association of ITGB4 with S1PR2 and S1PR3 in this same context. Our results indicate that both S1P and HGF induce translocation of ITGB4, S1PR2, and S1PR3 to EC CEMs and promote an indirect association of ITGB4 with S1PR2 through S1PR1 as well as a direct ITGB4–S1PR3 association. Moreover, knockdown of S1PR2 or S1PR3 (siRNA) significantly affected EC barrier enhancement by S1P and HGF. These studies further support growing evidence for ITGB4 as a prominent mediator of EC barrier function and inflammatory responses [Chen et al., 2010, 2012] and implicate for the first time a functional link between ITGB4 and S1PR2/S1PR3 in EC barrier regulation.
The complexities associated with the study of S1P receptor signaling are due in part to variable receptor expression profiles across cell types [Hla et al., 2001; Mazurais et al., 2002] including, potentially, different EC phenotypes, as well as evidence of variable functional roles for individual S1P receptors in different in vitro and in vivo models. For example, we have previously reported that both S1PR2−/− and S1PR3−/− mice demonstrate increased susceptibility to inflammatory lung injury induced by radiation [Mathew et al., 2011] while, in contrast, both S1PR2−/− mice and S1PR3-depleted mice (in vivo siRNA) were found to have decreased injury in an LPS-induced ALI model [Sammani et al., 2010]. Additionally, variable concentrations of the same agonist may induce highly discrepant S1P receptor signaling profiles in the same cell type. For example, activation of S1PR1 in response to physiologic concentrations of S1P (0.5 μM) results in EC barrier enhancement mediated by Rac1 while S1PR3 ligation in response to higher dose S1P (>5 μM) evokes EC barrier disruption predominantly through RhoA activation [Shikata et al., 2003]. Accordingly, to claim that S1PR2 mediates EC barrier enhancement and S1PR3 mediates barrier disruption on the basis of our findings now would likely be an inaccurate oversimplification, particularly given conflicting evidence in the literature in both cases. While our findings firmly support the idea that both receptors are important determinants of agonist-mediated EC barrier responses, an idea that is consistent with this growing literature, a full understanding of the relative effects of S1P receptors on EC barrier regulation in variable contexts is an important area of ongoing investigation.
Our identification of ITGB4 as a key component of a dynamically activated EC signaling complex involving S1PR1–3 as well as c-Met, a receptor tyrosine kinase, represents an entirely novel finding. As such, little is known about other potential complex components and it is likely that EC signaling induced by both S1P and HGF involves other proteins that have yet to be characterized. One particularly promising candidate in this regard is Gab-1, a docking protein that mediates a number of signaling pathways including MAPK signaling via binding to SHP-2, a protein tyrosine phosphatase [Cunnick et al., 2001; Cai et al., 2002]. This is notable as we previously reported that EC inflammatory responses mediated by ITGB4 are dependent on SHP-2 phosphorylation [Chen et al., 2010]. Moreover, separate reports have also characterized Gab-1 as an essential mediator of c-Met signaling [Sachs et al., 2000]. Finally, although there are no reports with respect to ITGB4 specifically, Gab-1 is also a known mediator of integrin signaling [Kuwano et al., 2007]. Indeed, our initial studies indicate that partial knockdown of Gab-1 via siRNA transfection significantly attenuates both S1P- and HGF-induced increases in EC barrier function [Ephstein et al., 2011]. Further study is ongoing as are efforts to identify additional complex components.
The potential clinical implications of our findings are significant as evidenced by the protective effects of both HGF and S1P in animal models of ALI [McVerry et al., 2004; Peng et al., 2004; Singleton et al., 2007]. Unfortunately, extending these findings to humans has been limited by potential adverse effects identified in pre-clinical studies including hypotension associated with HGF [Ido et al., 2011] and bradycardia induced by S1P [Forrest et al., 2004]. However, a number of pharmacologic agents that directly target sphingolipid signaling and that have potentially more favorable safety profiles are currently under investigation including a structural analog of S1P, FTY720 (2-amino-2-(2-[4-octylphenyl]ethyl)-1,3-propanediol) [Strader et al., 2011], as well as various analogs of phosporylated FTY720 [Hale et al., 2004; Foss et al., 2005; Clemens et al., 2005]. These agents have shown some early promise in relevant in vitro and in vivo models [Camp et al., 2009; Wang et al., 2013]. As these agonists are characterized by variable S1P receptor affinities, a full understanding of the functional roles of S1P receptors in vivo and the underlying mechanisms of downstream signaling events is imperative.
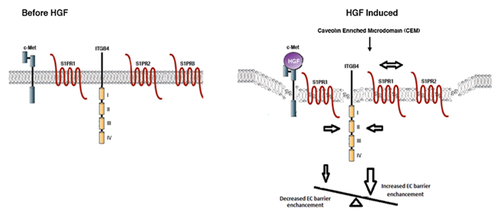
In summary, our results confirm for the first time a functional interaction of ITGB4 with both S1PR2 and S1PR3 that mediates EC barrier regulation induced by either S1P or HGF. Our study suggests that EC barrier function is regulated by the balance between signaling pathways mediated by S1PR1 and S1PR2/3 with induced recruitment of ITGB4, S1PR1, S1PR2, and S1PR3 to CEMs as key events in this setting (Fig.77). Finally, our results implicate individual complex components as potential therapeutic targets for diseases characterized by alterations in EC barrier integrity including ALI.
ACKNOWLEDGMENTS
This work acknowledges support from the NHLBI (HL 96687 and 98050, to J.R.J.) and the China Postdoctoral Science Foundation (2013M531067, to X.N.).




