Abstract
Vacuolization of the cytoplasm is one of the dramatic and frequently observed phenomena in various cell types. Cellular vacuoles occur spontaneously or via a wide range of inductive stimuli, but the molecular mechanism involved in this process remains largely unknown. In this study, we investigated the role of the p38 and JNK pathways in the formation of cytoplasmic vacuoles. We found that p38 and JNK agonist anisomycin abolishes spontaneous cytoplasmic vacuolization of HepG2 cells through p38 activation, but not through JNK activation. Importantly, blocking the activity of p38 or suppression the expression of p38 elicits cytoplasmic vacuoles formation in various cancer cells. Furthermore, cytoplasmic vacuoles induced by p38 blocking are derived from the perinuclear region. These observations provide direct evidence for a role of p38 signaling in regulating the formation of cytoplasmic vacuoles. J. Cell. Biochem. 114: 1789–1799, 2013. © 2013 Wiley Periodicals, Inc.
In respond to environmental changes, cells experience a number of morphological changes, including shrinkage, swelling, detachment, and vacuolization. Vacuolization of the cytoplasm is one of the more dramatic and frequently observed phenomena when cells are exposed to chemicals and bioactive substances [Belkin et al., 1962; Yang et al., 1965; Finnin et al., 1969; Ohkuma and Poole, 1981; Henics and Wheatley, 1999; Morissette et al., 2004; Wang and Chen, 2012]. In addition to be induced by a wide variety of agents, vacuolization also occurs spontaneously [Henics and Wheatley, 1999; Kar et al., 2009]. The degree of cytoplasmic vacuolization depends to a considerable degree on the cell type. Some cells becoming vacuolated very easily, whereas others might be quite resistant [Ohkuma and Poole, 1981; Henics and Wheatley, 1999]. Although the appearances of the vacuoles look similar under light microscopy, cytoplasmic vacuoles can be derived from distinct membrane sources, such as endoplasmic reticulum (ER), lysosome, mitochondria, and nuclear [Henics and Wheatley, 1999; Hirokawa et al., 2000; Kobayashi et al., 2002; Nakajima et al., 2008; Wasik et al., 2011; Funakoshi et al., 2012; Komiya et al., 2012]. Cytoplasmic vacuoles have been identified as the cellular digestive acidic compartment [Valdeira and Geraldes, 1985; Alcami et al., 1989; Kobayashi et al., 2002; Siebert et al., 2004; Hiruma and Kawakami, 2011; Funakoshi et al., 2012]. In addition, vacuolization of cytoplasmic components other than acidic compartment has also been reported [Aki et al., 2012]. It has been accepted that the origin, mechanism, and consequences of cytoplasmic vacuolization depending on the nature of the vacuolization inducer as well as the cell type.
The implicated function of cytoplasmic vacuolization has been reported. It has been accepted that autophagy can induce cytoplasmic vacuolization, and vacuolization may represent certain typical feature of autophagy [Kosta et al., 2004; Martinet and De Meyer, 2008; Alonso et al., 2012]. However, some cytoplasmic vacuolization are not resulted from autophagy, and not all autophagy are accompanied by vacuolization within the cytoplasm [Tasdemir et al., 2007; Zhang et al., 2010]. Furthermore, the vacuolization of the cytoplasm is closely related with cell apoptosis. Some apoptosis inducers initiate the formation of cytoplasmic vacuoles, and which plays a role in apoptosis regulation [Monack et al., 1996; Chatellard-Causse et al., 2002; Dutta and Das, 2011; Wang et al., 2012]. Importantly, some cytoplasmic vacuole inducers do not induce apoptosis, although the vacuolization is sustained in these inducers-treated cells [Morissette et al., 2004; Menon et al., 2011]. p38, a mitogen-activated protein kinase (MAPK), plays an important role in converting extracellular stimuli into a wide range of cellular responses, including inflammatory response, differentiation, proliferation, apoptosis, and survival [Enslen et al., 1998; Maher, 1999; Matsumoto et al., 1999; Ono and Han, 2000; Wagner and Nebreda, 2009; Dai et al., 2012a]. A cellular stress response regulating role of p38 has been proposed [Nebreda and Porras, 2000; Hsieh et al., 2003; Smith et al., 2003; Takebe et al., 2011]. Cytoplasmic vacuolization is correlated with cellular stress response. However, whether the vacuolization of the cytoplasm is regulated by stress response signaling pathways, such as the p38 and JNK pathways remains unknown.
In this study, we investigated the role of p38 and JNK in the vacuolization of the cytoplasm. We found that spontaneous vacuolation occurs in HepG2 cells. Interestingly, spontaneous vacuolation of HepG2 cells was abolished by anisomycin-mediated p38 activation, but not by anisomycin-mediated JNK activation. Moreover, p38 blocking elicited vacuolization of the cytoplasm in various cancer cell lines. However, JNK blocking has no effect on the vacuolization of the cytoplasm. These data indicate that p38 plays a potential role in regulating the vacuolization of the cytoplasm.
MATERIALS AND METHODS
Materials
JNK inhibitor SP600125, p38 MAPK inhibitor SB203580, p38 and JNK agonist anisomycin and protein synthesis inhibitor cycloheximide (CHX) were purchased from Merck. Antibody against β-actin was purchased from Santa Cruz Biotechnology. p38 MAPK siRNA and antibodies against GRP78, phospho-MAPKAPK2, MAPKAPK2, p38 MAPK, and phospho-c-Jun were purchased from Cell Signaling Technology. Cy3-conjugated secondary antibody was purchased from Invitrogen. ER-tracker red and Lyso-tracker red were purchased from Invitrogen Molecular Probes.
Cell Culture and Treatments
HepG2, LM3, QBC, A549, and HeLa cells were cultured in Dulbecco's Modified Eagle Medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin in a humidified incubator containing 5% CO2 and 95% ambient air at 37°C. The protocol used for p38 MAPK knockdown has been previously described [Dai et al., 2012a].
Western Blot
Cells were lysed in Triton lysis buffer (20 mM Tris, pH 7.4, 137 mM NaCl, 10% glycerol, 1% Triton X-100, 2 mM EDTA, 1 mM PMSF, 10 mM NaF, 5 mg/ml aprotinin, 20 mM leupeptin, and 1 mM sodium orthovanadate), and centrifuged at 12,000g for 15 min. Protein concentrations were measured using the BCA assay. Protein samples were denatured with 4 × SDS-loading buffer (200 mM Tris, pH 6.8, 8% SDS, 400 mM DTT, 0.4% bromophenol blue, 40% glycerol) at 100°C for 5 min and subjected to standard SDS–PAGE and Western blot analysis as previously described [Dai et al., 2012b].
Immunofluorescence Staining and Confocal Microscopy Analysis
Indirect immunofluorescence of paraformaldehyde-fixed cells was described previously. Cells were re-plated on chamber slides. When cultured to 60% confluence, cells were immunostained with anti-GRP78 primary antibody and Cy3-conjugated secondary antibody. Cells were labeled with the organelle-specific probes: ER-tracker red for ER and Lyso-tracker red for lysosomes and other acidic organelles. Confocal microscope (Leica, Heidelberg, Germany) was used to observe the subcellular fluorescence distribution in cells and to capture time-lapse images of cells.
Electron Microscopy Imaging
Cells were centrifuged at 1,000g for 3 min. After fixed in 2% paraformaldehyde and 0.1% gluteraldehyde in 0.1 M phosphate buffer (pH 7.4) at 4°C for 6 h, the cell pellets were then postfixed with 1% osmium tetroxide for 2 h on ice and then rinsed three times with distilled water. The fixed cell pellets were dehydrated through an ethanol (ETOH) dilution series up to 100% ETOH and then immersed in propylene oxide (PO) for 2 min, three times. Pellets were then infiltrated in PO/eponate resin mixture overnight and were subsequently embedded in 100% eponate resin and allowed to harden in a 65°C oven overnight. After hardening, tissue blocks were ultrathinsectioned at a 70 nm thickness and placed on 300 mesh copper grids. Grids were next counterstained with saturated uranyl acetate and lead citrate and then viewed on electron microscope.
RESULTS
Anisomycin Abolishes the Vacuolization of the Cytoplasm
To investigate the role of p38 and JNK signaling pathways in the vacuolization of the cytoplasm, we first examined the effect of p38 and JNK agonist anisomycin on the vacuolization of the cytoplasm of HepG2 cells. As shown in Figure 1A, anisomycin abolished the cytoplasmic vacuolization of HepG2 cells in a dose-dependent manner. Moreover, anisomycin inhibited the vacuolization of the cytoplasm of HepG2 cells in a time-dependent manner (Fig. 1B). These data indicate that the p38 and JNK pathways might be involved in the vacuolization of the cytoplasm.
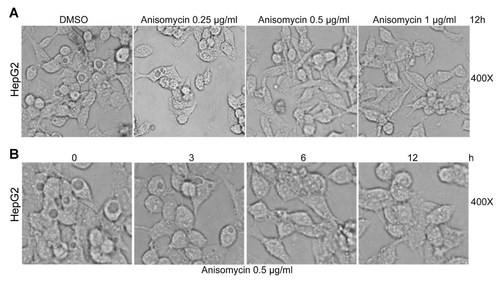
Anisomycin abolishes cytoplasmic vacuolization. A: After treated with various doses of anisomycin for 12 h, images of HepG2 cells were recorded by light microscopy. Images were taken at a magnification of 400×. B: After treated with 0.5 µg/ml anisomycin for the indicated time periods, images of HepG2 cells were recorded by light microscopy. Images were taken at a magnification of 400×.
p38 Activation Is Responsible for Anisomycin-Induced Cytoplasmic Vacuolization Abolishment
Given that both p38 and JNK are activated by anisomycin, we tested whether vacuolization of the cytoplasm can be abolished by anisomycin-mediated p38 or JNK activation. As shown in Figure 2A, p38 inhibitor SB203580 pre-treatment blocked the inhibitor effect of anisomycin on cytoplasmic vacuolization of HepG2 cells. However, JNK inhibitor SP600125 had no effect on anisomycin-induced the abolishment of cytoplasmic vacuolization (Fig. 2A). To confirm the effect of anisomycin on p38 and JNK activation, we assessed the levels of phosphorylated MAPKAPK2 and c-jun, a downstream effector of p38 and JNK, respectively. The data showed that anisomycin increased the phosphorylation levels of MAPKAPK2 and c-jun (Fig. 2A), indicating that anisomycin increased the activity of p38 and JNK in HepG2 cells. Furthermore, the effect of SB203580 and SP600125 on p38 and JNK activity inhibition were confirmed by the levels of phosphorylated MAPKAPK2 and c-jun in SB203580- and SP600125-treated HepG2 cells (Fig. 2A).
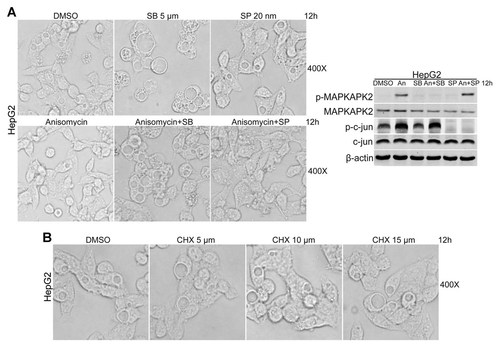
p38 activation is responsible for anisomycin-induced cytoplasmic vacuolization abolishment. A: p38 inhibitor, but not JNK inhibitor blocked the inhibitor effect of anisomycin on cytoplasmic vacuolization of HepG2 cells. After treated with 0.5 µg/ml anisomycin for 12 h with or without SB203580 (SB, 5 µM) or SP600125 (SP, 25 nM) pre-incubation for 1 h, images of HepG2 cells were recorded by light microscopy. Images were taken at a magnification of 400×. The effects of anisomycin, SB203580, and SP600125 on the activity of p38 and JNK were measured by Western blot. B: Protein synthesis inhibition has no effect on cytoplasmic vacuolization. After treated with CHX (10 µM) for the indicated time periods, images of HepG2 cells were recorded by light microscopy. Images were taken at a magnification of 400×.
Since anisomycin is known as a potent protein synthesis inhibitor, we investigated whether protein synthesis inhibition is involved in anisomycin-mediated the abolishment of cytoplasmic vacuolization. The data showed that protein synthesis inhibitor CHX had no effect on vacuolization of the cytoplasm of HepG2 cells (Fig. 2B), indicating that anisomycin-induced the abolishment of cytoplasmic vacuolization is independent of protein synthesis inhibition. Thus, these data suggest that p38 activation is required for the abolishment of cytoplasmic vacuolization events caused by anisomycin.
p38 Blocking Initiates Cytoplasmic Vacuoles Formation
To confirm the role of p38 in regulating cytoplasmic vacuoles formation, the most widely used p38 inhibitor SB203580 was used in our studies. Treatment with various doses of SB203580 for 12 h induced vacuoles formation in LM3 cells (Fig. 3A). Moreover, treatment LM3 cells with 5 µm SB203580 induced vacuoles formation in a time-dependent manner (Fig. 3B). These results indicate that SB203580 induced vacuoles formation in a dose- and time-dependent manner. To further confirm the role of p38 inhibition in cytoplasmic vacuoles induction, specific small-interfering RNA (siRNA) was used to block the expression of p38 in LM3 cells. The vacuolization of the cytoplasm was induced by p38 knockdown (Fig. 3C), confirming that p38 blocking plays an important role in cytoplasmic vacuoles induction. Moreover, SB203580 treatment initiated cytoplasmic vacuoles formation in QBC939, A549, and HeLa cells (Fig. 3D). However, JNK inhibitor SP600125 treatment had no effect on cytoplasmic vacuoles formation (data not shown). It is notable that, another p38 inhibitor VX-745, but not the negative analog of SB203580, also initiated cytoplasmic vacuoles formation in LM3 cells (Supplementary Fig. 1). Taken together, these results suggest that p38 blocking, but not JNK blocking is involved in cytoplasmic vacuoles induction.
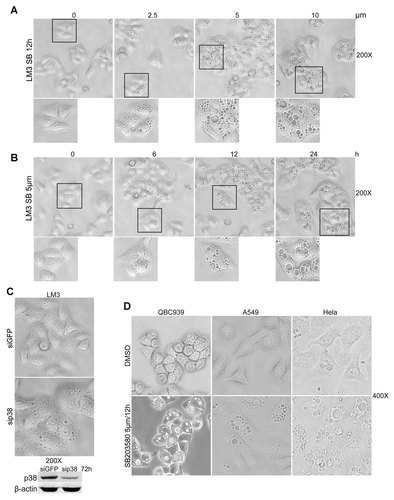
p38 blocking initiates cytoplasmic vacuoles formation. A: After treated with various doses of SB203580 (SB) for 12 h, images of LM3 cells were recorded by light microscopy. Images were taken at a magnification of 200×. B: After treated with SB203580 (SB, 5 µM) for the indicated time periods, images of LM3 cells were recorded by light microscopy. Images were taken at a magnification of 200×. C: After transfected with p38 siRNA for 72 h, images of LM3 cells were recorded by light microscopy. Images were taken at a magnification of 200×. D: p38 blocking initiates cytoplasmic vacuoles formation in various cancer cell lines. After treated with SB203580 (SB, 5 µM) for 12 h, images of QBC939, A549, and HeLa cells were recorded by light microscopy. Images were taken at a magnification of 400×.
p38 Blocking-Induced Cytoplasmic Vacuoles Disrupt the Integrity of Endoplasmic Reticulum
To investigate the association between p38 blocking-induced cytoplasmic vacuoles formation and ER, ER-tracker red was used to visualize ER apparatus. In confocal micrographs of untreated cells, the ER appeared as normal (Fig. 4A). In contrast, SB203580-induced cytoplasmic vacuoles disrupted the ER structure and integrity in LM3 and A549 cells (Fig. 4A).
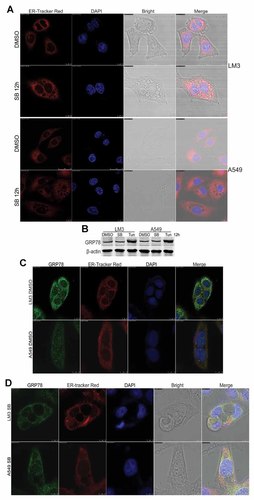
p38 blocking-induced cytoplasmic vacuoles disrupt the integrity of ER. A: After treated with dimethyl sulfoxide (DMSO) or SB203580 (SB, 5 µM) for 12 h, LM3 and A549 cells were stained with the ER-tracker red and DAPI, and then subjected to confocal microscopy analysis. B: Localization of ER marker protein GRP78. After treated with dimethyl sulfoxide (DMSO), SB203580 (SB, 5 µM) or tunicamycin (Tun, 2.5 µg/ml) for 12 h, LM3 and A549 cells were subjected to Western blot analysis. B and C: After treated with dimethyl sulfoxide (DMSO) (B) or SB203580 (SB, 5 µM) (C) for 12 h, LM3 and A549 cells were subjected to immunofluorescence staining and confocal microscopy analysis.
To make sure whether p38 blocking-induced cytoplasmic vacuoles formation is derived from ER, we investigated the distribution of ER marker protein glucose regulated protein 78 (GRP78) in LM3 and A549 cells. We first examined the protein levels of GRP78 in LM3 and A549 cells. As shown in Figure 4B, both basal expression and ER stress induced expression of GRP78 were detected in LM3 and A549 cells. Further results showed that GRP78 mainly located in the ER in DMSO-treated LM3 and A549 cells (Fig. 4C). It is notable that, GRP78 still mainly located in the ER, but not in cytoplasmic vacuoles in SB203580-treated LM3 and A549 cells (Fig. 4D). These data not only indicate that p38 blocking-induced cytoplasmic vacuoles formation is not derived from ER, but also suggest that the cytoplasmic vacuoles disrupt the ER structure and integrity.
Intravacuolar pH and Cytoplasmic Components of p38 Blocking-Induced Cytoplasmic Vacuoles
As an acidic intravacuolar pH has been reported in some kinds of cytoplasmic vacuoles, it is interesting to investigate whether an acidic intravacuolar pH is existed in p38 blocking-induced cytoplasmic vacuoles. The observations were made by confocal fluorescence imaging of Lyso-tracker red-stained LM3 and A549 cells. As shown in Figure 5A, only a minor fraction of p38 blocking-induced cytoplasmic vacuoles were labeled with Lyso-tracker red, indicating that only a minor fraction of p38 blocking-induced cytoplasmic vacuoles have an acidic intravacuolar pH. To investigate whether p38 blocking-induced cytoplasmic vacuoles formation represents artifacts of an immature autophagic response, we investigated the distribution of lysosomal membrane protein LAMP2 and autophagy marker LC3-II. As shown in Supplementary Figure 2A,B, both LAMP2 and LC3-II cannot be stained in p38 blocking-induced cytoplasmic vacuoles. Moreover, the Western blot results showed that p38 inhibition had no demonstrable effect on LC3-II induction (Supplementary Fig. 2C). These data indicate that p38 blocking-induced cytoplasmic vacuoles formation does not represent artifacts of an immature autophagic response.
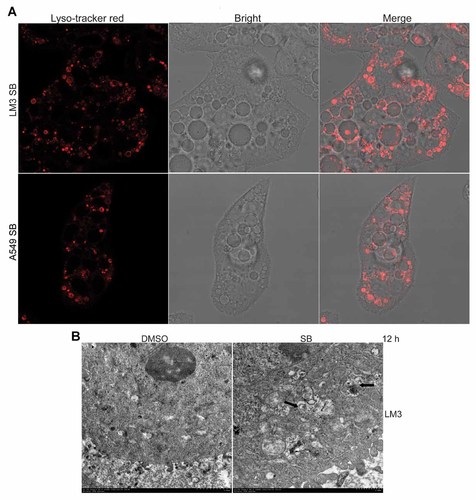
Intravacuolar pH and cytoplasmic components of cytoplasmic vacuoles. A: After treated with SB203580 (SB, 5 µM) for 12 h, LM3 and A549 cells were stained with the Lyso-tracker red and subjected to confocal microscopy analysis. B: Representative electron micrographs of LM3 cells treated with dimethyl sulfoxide (DMSO) or SB203580 (SB, 5 µM) for 12 h. Cytoplasmic components in cytoplasmic vacuoles are indicated by black arrow.
To investigate whether p38 blocking-induced cytoplasmic vacuoles contain cytoplasmic components, transmission electron micrographs was used to exam the ultrastructure of vacuolated cells. In contrast to untreated control cells (Fig. 5B), SB203580 treatment induced intracytoplasmic vacuoles formation in LM3 cells (Fig. 5B). Cytoplasmic components were apparent within the vacuoles (arrows, Fig. 5B), which were enclosed within a membrane (Fig. 5B). These data indicate that the presence of a bounding membrane with contents consisting of degenerate cytoplasmic components in p38 blocking-induced cytoplasmic vacuoles.
Cytoplasmic Vacuoles Formation Induced by Blocking of p38 Is Reversible
Since SB203580 is a reversible inhibitor of p38 kinase activity, we investigate the effect of SB203580 withdraw in cytoplasmic vacuoles formation. The data showed that vacuoles induced by p38 blocking regressed in LM3 and A549 cells by light microscopy 6 h after SB203580 withdraw (Fig. 6A). Furthermore, the ER structure and integrity were restored after SB203580 withdraw 6 h in LM3 and A549 cells (Fig. 6B). These data indicate that cytoplasmic vacuoles formation induced by blocking of p38 MAPK is reversible.
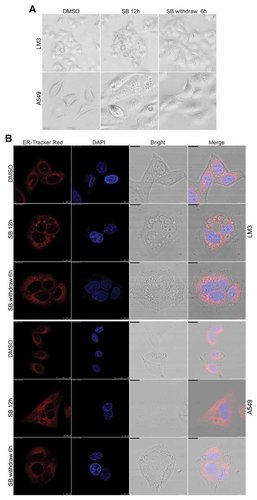
Cytoplasmic vacuole formation induced by blocking of p38 MAPK is reversible. A: After treated with SB203580 (SB, 5 µM) for 12 h, LM3 and A549 cells were incubated in SB203580 (SB) free medium for another 6 h and then subjected to light microscopy analysis. B: After treated with SB203580 (SB, 5 µM) for 12 h, LM3 and A549 cells were incubated in SB203580 (SB) free medium for another 6 h, then LM3 and A549 cells were stained with the ER-tracker red and DAPI, and then subjected to confocal microscopy analysis.
p38 Blocking-Induced Cytoplasmic Vacuoles Are Originated From the Perinuclear Region
To investigate the origin of p38 blocking-induced cytoplasmic vacuoles formation, SB203580-treated LM3 and A549 cells were imaged by confocal microscopy. The representative images in Figure 7A demonstrated that nascent vacuoles initially appeared to concentrate in the perinuclear region. After 3 h, the vacuoles had increased in number and size, occupying much of the cytoplasm (Fig. 7A). Moreover, time-lapse confocal microscopy of SB203580-treated LM3 cells confirmed that p38 blocking-induced cytoplasmic vacuoles were originated from the perinuclear region (Video 1). Interesting, cytoplasmic vacuoles disappeared in the perinuclear region after SB203580 withdraw in LM3 and A549 cells (Fig. 7B, Video 2). Thus, p38 blocking-induced cytoplasmic vacuoles formation is originated from the perinuclear region.
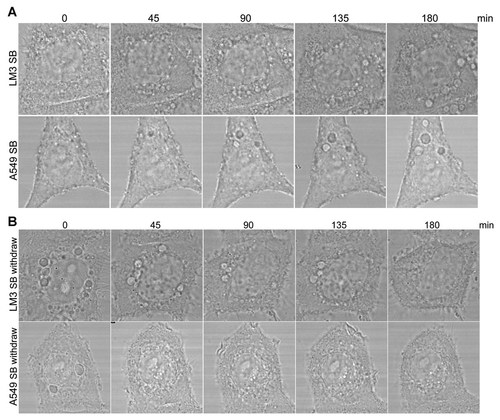
p38 blocking-induced cytoplasmic vacuoles are originated from the perinuclear region. A: After treated with SB203580 (SB, 5 µM) for the indicated time periods, images of LM3 and A549 cells were captured by confocal microscopy. B: After treated with SB203580 (SB, 5 µM) for 12 h, LM3 and A549 cells were incubated in SB203580 (SB) free medium for the indicated time periods, images of LM3 and A549 cells were captured by confocal microscopy.
DISCUSSION
Cytoplasmic vacuolation is a widely observed morphological phenomenon found in cultured mammalian cells [Belkin et al., 1962; Ohkuma and Poole, 1981; Henics and Wheatley, 1999; Morissette et al., 2004; Ropolo et al., 2007; Kar et al., 2009]. Cytoplasmic vacuolation occurs spontaneously or via a wide range of stimuli in most cell types [Chatellard-Causse et al., 2002; Suzuki et al., 2003; Al-Abbadi et al., 2010; Wang and Chen, 2012]. The disturbance of local environment is an underlying mechanism for cytoplasmic vacuolation formation. However, the cellular signaling pathways responsible for cytoplasmic vacuolation formation remain largely unknown. In this study, we investigated the effect of the p38 and JNK pathways on cytoplasmic vacuolation formation. The present work reveals that the p38 pathway, but not the JNK pathway plays a pivotal role in regulating the formation of cytoplasmic vacuolation.
Cytoplasmic vacuoles were abolished by p38 and JNK agonist anisomycin treatment in HepG2 cells, indicating that p38 and JNK signaling might be involved in regulating the formation of cytoplasmic vacuolation. As p38 blocking, but not JNK blocking inhibited anisomycin-mediated cytoplasmic vacuoles abolishment, it is reasonable to suggest that anisomycin mediated p38 activation, but not JNK activation responsible for the abolishment of cytoplasmic vacuoles formation of HepG2 cells upon anisomycin treatment. Based on the data that p38 blocking, but not JNK blocking initiated cytoplasmic vacuoles formation, we suggest that p38 inhibition plays an important role in cytoplasmic vacuoles formation. The role of p38 inhibition in cytoplasmic vacuoles formation was confirmed by suppression of p38 expression. A recent publication suggested that cytoplasmic vacuolization induced by SB202190, another p38 MAPK inhibitor, was not dependent on p38 pathway [Menon et al., 2011], this discrepancy may be due to different cell types used. Vacuolization occurs in HepG2 cells, but not LM3 or A549 cells, spontaneously, this discrepancy may be due to the basal activity of p38 are different in different cell types. Moreover, the function of p38 pathway vary considerably among different cell types, and p38 is not the only pathway involved in cytoplasmic vacuolization regulation. Thus, the effects of p38 on cytoplasmic vacuolization might be quite different in various cell types, and the degree of p38 activating or blocking-induced cytoplasmic vacuolization depends to a considerable degree on the cell type.
It has been reported that some types of cytoplasmic vacuoles contain an acidic intravacuolar pH [Valdeira and Geraldes, 1985; Alcami et al., 1989; Kobayashi et al., 2002; Siebert et al., 2004; Hiruma and Kawakami, 2011; Funakoshi et al., 2012]. Thus, we tested whether p38 blocking-induced cytoplasmic vacuoles contain an acidic intravacuolar pH. As our data showed that only a minor fraction of p38 blocking-induced cytoplasmic vacuoles were labeled with Lyso-tracker red, staining lysosomes, and other acidic vesicles, we suggest that only a minor fraction of p38 blocking-induced cytoplasmic vacuoles have an acidic intravacuolar pH. Based on the data that p38 blocking-induced cytoplasmic vacuoles regressed promptly when p38 inhibitor was removed, we suggest that cytoplasmic vacuole formation induced by blocking of p38 MAPK is reversible.
An important question before us is the origin of p38 blocking-induced cytoplasmic vacuoles. Recent reports have shown that cytoplasmic vacuoles can be derived from ER, mitochondria, lysosomes, and nuclear [Henics and Wheatley, 1999; Hirokawa et al., 2000; Kobayashi et al., 2002; Nakajima et al., 2008; Wasik et al., 2011; Funakoshi et al., 2012; Komiya et al., 2012]. Since p38 blocking-induced cytoplasmic vacuoles were not labeled with ER-tracker red and ER marker protein GRP78, we suggest that the cytoplasmic vacuoles induced by p38 blocking are not derived from ER. As p38 blocking-induced cytoplasmic vacuoles cannot be labeled with Lyso-tracker red, it is reasonable to suggest that p38 blocking-induced cytoplasmic vacuoles are not derived from lysosomes. Importantly, the prime subcellular region for early vacuole formation and accumulation induced by p38 blocking tends to be perinuclear. Then, vacuoles moved towards the periphery, as they increase in both number and size. Interestingly, cytoplasmic vacuoles moved back to perinuclear and regressed after p38 inhibitor withdrawing. Further studies are needed to investigate whether p38 blocking-induced cytoplasmic vacuoles formation is derived from nuclear.
In brief, the present work reveals that blocking the activity of p38 plays an important role in cytoplasmic vacuoles induction. More detailed studies on the mechanism of p38 blocking-induced cytoplasmic vacuoles formation will contribute to the understanding of molecular mechanisms of cytoplasmic vacuolization.




