Secreted phosphoprotein-24 kDa (Spp24) attenuates BMP-2-stimulated Smad 1/5 phosphorylation and alkaline phosphatase induction and was purified in a protective complex with alpha2-Macroglobulins From Serum†
The authors have no conflict of interest to declare.
Abstract
Secreted phosphoprotein-24 kDa (Spp24) binds cytokines of the bone morphogenetic protein/transforming growth factor-β (BMP/TGFβ) superfamily and is one of the most abundant serum phosphoproteins synthesized by the liver. Little is known about how Spp24 binding affects BMP signal transduction and osteoblastic differentiation or how this labile protein is transported from the liver to remote tissues, such as bone. When Spp24 was administered to W-20-17 mesenchymal stem cells with rhBMP-2, short-term Smad1/5 phosphorylation was inhibited, intermediate-term alkaline phosphatase (ALP) induction was blunted, and long-term mineralization was unaffected. This supports the hypothesis that Spp24 proteolysis restricts the duration of its regulatory effects, but offers no insight into how Spp24 is transported intact from the liver to bone. When Spp24 was immunopurified from serum and subjected to native PAGE and Western blotting, a high molecular weight band of >500 kDa was found. Under reducing SDS–PAGE, a 24 kDa band corresponding to monomeric Spp24 was liberated, suggesting that Spp24 is bound to a complex linked by disulfide bonds. However, such a complex cannot be disrupted by 60 mM EDTA under non-reducing condition or in purification buffers containing 600 mM NaCl and 0.1% Tween-20 at pH 2.7–8.5. LC–MS/MS analysis of affinity-purified, non-reducing SDS–PAGE separated, and trypsin digested bands showed that the Spp24 was present in a complex with three α2-macroglobulins (α2-macroglobulin [α2M], pregnancy zone protein [PZP] and complement C3 [C3]), as well as ceruloplasmin and the protease inhibitor anti-thrombin III (Serpin C1), which may protect Spp24 from proteolysis. J. Cell. Biochem. 114: 378–387, 2013. © 2012 Wiley Periodicals, Inc.
Abbreviations used:
α2M, α2-macroglobulin; α-MEM, α-minimum essential medium; ALP, alkaline phosphatase; DMEM, Dulbecco's modified Eagle's medium; FL-rb Spp24, full-length recombinant bovine secreted phosphoprotein-24 kDa; ITIH4, inter-α-trypsin inhibitor heavy chain H4; LRP1, low-density lipoprotein receptor-related protein 1; PZP, pregnancy zone protein; WST-1, (4-[3-(4-iodophenyl)-2-[4-nitrophenyl]-2H-5-tetrazolio]-1,3-benzene disulfonate).
Secreted phosphoprotein-24 kDa (Spp24), also known as secreted phosphoprotein-2 (SPP2: OMIM 602637), is a member of the cystatin (cysteine protease inhibitor) superfamily, but lacks the protease inhibitor sequence [Ochieng and Chaudhuri, 2010]. As a result, Spp24 does not inhibit cathepsins B and K [van den Bos et al., 2008]. Spp24 is found exclusively in vertebrates [Bennett et al., 2004], and its gene was originally cloned from dwarf chickens [Agarwal et al., 1995] and bovine cortical bone [Hu et al., 1995]. The major sources of Spp24 are the fetal and adult liver [Agarwal et al., 1995; Hu et al., 1995] and the kidney [Okazaki et al., 2002]. Spp24 is also found in cartilage [Meng et al., 2005], periosteum [Hu et al., 1995], and calvaria and long bones [van den Bos et al., 2008]. Like all secretory proteins, Spp24 contains a short amino (N)-terminal signal peptide that is cleaved by signal peptidase. Mature Spp24 consists of three major domains, including the single cystatin- or cathelicidin (neutrophil antimicrobial peptide precursor)-like domain with two internal disulfide bonds, a variable region rich in serine residues, and an arginine-rich carboxyl (C)-terminal domain [Agarwal et al., 1995; Hu et al., 1995; Bennett et al., 2004]. The polyserine domain of native bovine bone and serum Spp24 is heavily phosphorylated [Hu et al., 1995; Zhou et al., 2009], and Spp24 binds hydroxyapatite with high affinity [Zhou, 2007]. Apatite binding by phosphoserine and arginine residues in the C-terminus of Spp24 may account for the trace amounts of Spp24 found in the high molecular weight fetuin-matrix Gla protein-calcium phosphate mineral complex (FMC) obtained from the serum of rats treated with suprapharmacologic doses of the bisphosphonate etidronate [Price et al., 2003].
A short transforming growth factor-β type II receptor homology-1 (TRH1) subdomain is present within the larger cystatin- or cathelicidin-like domains of both Spp24 [Behnam et al., 2005] and fetuin [Demetriou et al., 1996]. These TRH1 subdomains mimic the ligand-binding domain of the receptor itself [Mace et al., 2006]. The TRH1 domain is a distinctive internal 18- or 19-amino acid residue β-pleated sheet/turn/β-pleated sheet (BTB) motif that is cross-linked at its N- and C-termini by a disulfide bond [Mace et al., 2006]. TRH1 domains bind cytokines in the BMP/TGFβ superfamily with high affinity and specificity [Demetriou et al., 1996]. The cyclic peptide corresponding to the TRH1 domain of Spp24, designated cyclic BMP-binding peptide (cBBP), binds rhBMP-2, BMP-7, and TGF-β2 with KD values ranging from 5 to 70 nM [Sun et al., 2010; Taghavi et al., 2010]. Transgenic over-expression of full-length (FL) Spp24 in vivo reduces bone mineral density, while FL-Spp24 inhibits BMP-2-stimulated heterotopic bone formation [Sintuu et al., 2008] and BMP-stimulated spinal fusion [Sintuu et al., 2011]. In contrast, cBBP enhances BMP-2-stimulated heterotopic bone formation [Behnam et al., 2005], spinal fusion [Alanay et al., 2008], and femoral bone defect healing [Morishita et al., 2010].
To date, the effects of Spp24 and its derivatives on intracellular signal transduction and differentiation in BMP-treated cells in vitro have not been described in detail. Spp24 acts as an extracellular pseudoreceptor for BMP [Brochmann et al., 2009] and prevents or attenuates post-receptor signaling [Hata et al., 2003; Gazzerro and Canalis, 2006; Keller et al., 2011]. The canonical pathway of BMP/TGF-β cytokine signaling is well-defined and begins with the binding of the ligand to type I and type II transmembrane serine/threonine kinase receptors. The cytokine initially binds to the type II receptor, which then heterodimerizes with and phosphorylates the type I receptor. Activated type I receptors subsequently activates the receptor-regulated Smads (R-Smads), including Smads1/5/8 in BMP-stimulated cells and Smads 2/3 in TGF-β-treated cells, by phosphorylating their carboxyl termini [Massague, 2000]. Phosphorylated R-Smads form a heterocomplex with the common partner Smad (Co-Smad) Smad4 [Gazzerro and Canalis, 2006]. The R-Smad/Co-Smad complex then translocates to the nucleus, where it binds to promoters of target genes directly, binds to and interact with other transcription factors, or binds to and displaces nuclear factors from their DNA binding sites, thus effecting either a positive or negative regulatory effect on gene transcription in target cells [Canalis et al., 2003; Massague et al., 2005; Miyazono et al., 2005]. The net result is the induction of the osteoblast phenotype in pluripotent mesenchymal precursor cells and the inhibition of differentiation into other cell types, such as myoblasts [Lee et al., 2000]. Noncanonical pathways of action include formation of secondary heteromeric receptor complexes with the ligand, activation of mitogen activated protein kinases (MAPK), extracellular regulated kinase (ERK)-1/2, c-JUN N-terminal kinases (JNK)-1 and -2/3, and p38 [Nohe et al., 2002]. Down-stream effects of MAPK activation include up-regulation of Fos/Jun transcription factors, as well as activating transcription factor-2 (ATF-2), which interacts with the activating protein-1 (AP-1) sequences present in genome [Gazzerro and Canalis, 2006]. Here we present evidence that while Spp24 attenuates the short- and intermediate-term effects on rhBMP-2-stimulated Smad 1/5 phosphorylation and induction of ALP activity in W-20-17 mesenchymal stem cells, it does not prevent longer-term BMP-mediated differentiation, assessed as Alizarin red staining of mineralizing nodules.
Recombinant [Murray et al., 2007] and native bone [Behnam et al., 2005] Spp24 are exquisitely sensitive to proteolysis, even in the presence of chaotropic agents and protease inhibitors. C-terminal truncation of FL-Spp24 reduces its inhibitory effects on BMP-2-mediated bone formation [Brochmann et al., 2010; Sintuu et al., 2011]. To date, the mechanisms by which FL-Spp24 is transported intact from the liver (the site of high-level synthesis) to remote tissues, such as bone, have not been elucidated. Here we present evidence that free or unbound Spp24 is not present in serum. Rather, Spp24 circulates as a high molecular complex with α2-macroglobulin (α2M), the major serum protease inhibitor and cytokine carrier [Borth, 1992; Hall et al., 1992], as well as pregnancy zone protein (PZP) and complement C3, both of which are also members of the α2-macroglobulin family. So, whereas α2M binds to a variety of cytokines with relatively high affinity (KD values range from 1 to 100 nM), is internalized by low density lipoprotein receptor related protein-1 (LRP1), and dissociates from its ligands at the cell surface or in an endocytic compartment [Borth, 1992; Lillis et al., 2008], transport of Spp24 by α2M provides a mechanism by which Spp24 is protected from proteolysis and delivered to LRP1-positive cells, such as osteoblasts [Grey et al., 2004], which are remote from the liver.
MATERIALS AND METHODS
Cell Culture, Proliferation, and Differentiation
W-20-17 mouse bone marrow cells were obtained from the American Type Culture Collection (Manassas, MD) and routinely cultured in growth media (Dulbecco's modified Eagle's medium [DMEM] supplemented with 10% calf serum, 2.5 mM glutamine, 100 U/ml penicillin G, and 100 µg/ml streptomycin) in a humid atmosphere of 95% air, 5% CO2, and 37°C. For WST-1 and ALP assays, the cells were plated in 96-well plates at 2,500 cells/well. Twenty-four hours later, low-serum (0.5%) DMEM containing the test agents indicated (vehicle, 10, 50, or 100 ng/ml rhBMP-2, 100 ng/ml full-length recombinant bovine Spp24 [FL-rb Spp24], or rhBMP-2 plus FL-rb Spp24) was added, and the cells were cultured for an additional 24 h before conducting the viability assay. ALP activity was assayed after culturing for 60 h under similar conditions, with the addition of 0, 50, or 100 ng/ml rhBMP-2 plus or minus 100 ng/ml FL-Spp24. Cell viability was assessed based on mitochondrial dehydrogenase activity by adding 10 µl of WST-1 reagent (Roche, Mannheim, Germany) to each well, incubating at 37°C for 30 min, and measuring the absorbance at 450 nm. Cellular differentiation was assessed based on ALP activity, measured as the rate of cleavage of p-nitrophenol from p-nitrophenylphosphate at alkaline pH as previously outlined in detail [Murray et al., 1993]. Mineralization was assessed by Alizarin red staining after culturing the cells in mineralization media (α-MEM supplemented with 2% calf serum, 10 nM dexamethasone, 50 µg/ml ascorbic acid, and 7 mM β-glycerolphosphate) plus or minus vehicle, rhBMP-2, FL-rb Spp24, or rhBMP-2 plus FL-rb Spp24 for 7 days and fixation in 10% neutral buffered formalin. For Smad1/5 and pSmad1/5 Western blotting, W-20-17 cells were plated in 6-well plates at 75,000 cells/well, cultured to near-confluence in growth media, and then maintained in low-serum test medium (DMEM plus 1% serum) over-night. The medium was replaced with DMEM containing 0.2% serum supplemented with vehicle, rhBMP-2, FL-rb Spp24, or rhBMP-2 plus FL-rb Spp24 as previously indicated. After a 30 min incubation at 37°C, the cells were lysed in Laemmli sample buffer, and aliquots corresponding to 16% (1/6th) of the total protein were subjected to SDS–PAGE and Western blotting as described in detail below.
Immunoaffinity Purification of Spp24 From Serum
A bovine C-terminal Spp24 peptide [(C)GEPLYEPSREMRRN] was immobilized to UltraLink iodoacetyl resin according to the manufacturer's (Thermo Scientific) instructions at a density of ∼100 nmol of peptide per ml. Rabbits were routinely immunized with the C-terminal Spp24 peptide linked to keyhole limpet hemocyanin (GenScript, Piscataway, NJ), and the specific IgG was then affinity purified using the C-peptide-Ultralink resin and coupled to UltraLink hydrazide according to the manufacturer's (Thermo Scientific) instructions at a density of ∼26 nmol/ml. Native Spp24 was subsequently isolated from bovine calf serum by affinity chromatography on anti-C-terminal Spp24 IgG-Ultralink resin. Calf serum (5 ml) was centrifuged at 16,000g for 20 min at 4°C and mixed with 2 ml IgG-Ultralink resin equilibrated with 20 mM phosphate buffer, pH 7.5, containing 150 mM NaCl and 1 µM leupeptin (PBS). The column was washed with eight bed volumes of PBS containing additional 450 mM NaCl and 0.1% Tween-20 followed by PBS. Bound proteins were eluted with 0.1 M glycine–HCl, pH 2.7, 150 mM NaCl, 0.02% NaN3 and 1 µM leupeptin, and collected as 1-ml fractions containing 40 µl of 1 M Tris–HCl, pH 9.5, to neutralize the pH. Protein concentrations were monitored by BCA assay, and the amount of the Spp24 complex present was monitored by ELISA.
Page and Immunoblotting
Samples were treated with Laemmli buffer containing no SDS (native PAGE), 2% SDS without a reducing agent (non-reducing), or 2% SDS with 1% β-mercaptoethanol (reducing). Reduced samples were boiled for 10 min prior to loading on 4–20% gradient gels. The gel was run in 0.1 M Tris–HEPES buffer containing no SDS (native PAGE) or 0.1% SDS, pH 8.0, followed by transblotting to polyvinylidene difluoride (PVDF) membrane in 0.025 M Tris–glycine, pH 8.0 containing 20% methanol. Proteins were visualized by Coomassie blue staining and the relative molecular masses (Mr) of which were determined by Spectra multicolor broad range protein ladder (Pierce) without heating. The membrane was blocked with 5% non-fat milk in 20 mM Tris–HCl, pH 7.5, 150 mM NaCl, 0.1% Tween-20 (TBST) for 1 h and then incubated in purified primary antibodies G4431 or antiphospho-Smad1/5 antibody (Cell Signaling, Danvers, MA) followed by HRP-conjugated goat anti-rabbit antibodies (1:2,000) in 1% BSA/TBST for 1 h. After washing 6 times in TBST, the membranes were incubated with SuperSignal West Pico ECL substrate (Thermo, Waltham, MA), and the bands were visualized on Kodak X-ray film. Images were scanned without any adjustment for presentation.
Protein Identification by LC–MS/MS
After comparing Western blots probed for Spp24 and Coomassie-blue stained SDS–PAGE gels, bands estimated to contain Spp24 were excised and sent to the UCLA W. M. Keck Proteomic Center (http://mic.ucla.edu/Prot/proteomicshome.htm) for definitive identification of the proteins present. The proteins within the band(s) were trypsin digested [Shevchenko et al., 1996], and the resulting peptides were separated with an Eksigent NanoLiquid chromatography-1D plus system (Dublin, CA) coupled to a Thermo LTQ-Orbitrap XL mass spectrometer (San Jose, CA). The resulting MS/MS spectra were identified by comparison to those in the Mascot database using the Matrix Science MASCOT Daemon search engine (Boston, MA). The criteria for the positive identification of a protein within a particular band are that it contains at least one unique peptide that has an ion score with a P < 0.05.
Statistical Analysis
All data from the WST-1 and ALP assays are expressed as the mean ± SEM (n = 8). Data were analyzed by ANOVA. Where the variation among column means was significantly greater than expected by chance, Tukey–Kramer multiple comparisons testing was performed to identify the source of the variation. Bartlett's test was performed to determine whether the differences among the standard deviations were statistically significant. Data analyses were conducted using GraphPad Instat (version 3.0, San Diego, CA).
RESULTS
Spp24 Does Not Have Cytotoxic Effects on Bmp-2-Treated Cells
The effects of Spp24 on W-20-17 murine mesenchymal stem cell metabolism were assessed under basal conditions and in the presence of rhBMP-2 in order to exclude nonspecific cytotoxic effects of Spp24. Cellular viability was assessed after 24 h of treatment with BMP-2 only (0, 10, 50, or 100 ng/ml) or BMP-2 plus Spp24 (100 ng/ml). Addition of Spp24 and BMP-2 to W-20-17 cells (open circles) had no significant adverse effects on WST-1 cleavage compared to the BMP-2 treatment alone at the same BMP-2 concentrations (filled circles) (Fig. 1). Similarly, Spp24 had no consistent statistically significant effects on the final amount of protein (µg/well) after 60 h of treatment (data not shown). From this, we conclude that Spp24 is not cytotoxic.
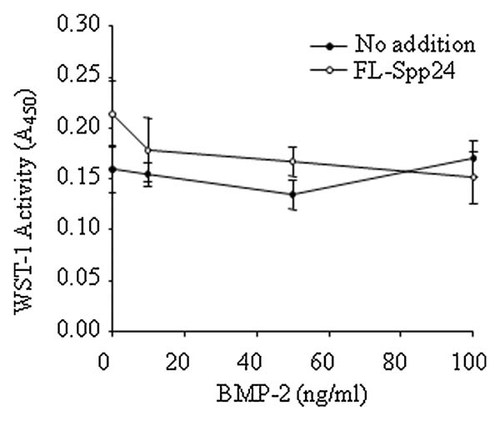
Spp24 is not cytotoxic to BMP-2-treated W-20-17 mesenchymal stem cells. Cells were cultured in low-serum (0.5%) DMEM containing vehicle, rhBMP-2 (10, 50, or 100 ng/ml), or rhBMP-2 plus FL-rb Spp24 (100 ng/ml) for 24 h. Viability, assessed as mitochondrial dehydrogenase activity, was assessed based on the conversion of WST-1 reagent to formazan at 450 nm. Data are reported as the mean ± SEM (n = 8). Addition of FL-Spp24 had no statistically significant effects on viability in BMP-2-treated cells.
Spp24 Attenuates Short-Term BMP-2-Stimulated SMAD1/5 Phosphorylation in W-20-17 Cells
We hypothesized that Spp24/BMP-2 binding will attenuate the down-stream signaling cascades stimulated by BMP-2 binding to its receptor. In order to test this hypothesis, we examined the effects of Spp24 on BMP-2-stimulated receptor-regulated Smad1/5 phosphorylation in W-20-17 cells by Western blotting. The effects of 30 min treatment with vehicle (Lane 1), 500 ng/ml Spp24 (Lane 2), 31–500 ng/ml BMP-2 plus 500 ng/ml SPP24 (Lanes 3–7), or 500 ng/ml BMP-2 alone (Lane 8) are shown in Figure 2. Under basal conditions (0 ng/ml rhBMP-2), there were very low levels of Smad1/5 phosphorylation (Lane 1). Addition of Spp24 alone (500 ng/ml) had little effect on Smad1/5 phosphorylation in the absence of rhBMP-2 (Lane 2). There was the expected dose-dependent increase in Smad1/5 phosphorylation following addition of 31–500 ng/ml rhBMP-2 that was not prevented by the presence of 500 ng/ml Spp24 (Lanes 3–7). However, cells treated with 500 ng/ml of BMP-2 only (Lane 8) had higher levels of Smad1/5 phosphorylation than those treated with 500 ng/ml of BMP-2 and Spp24 (Lane 7). Thus, FL-rb Spp24 attenuates the effects of BMP-2 treatment, in part, by reducing the amount of Smad1/5 phosphorylation that is observed 30 min after addition of these agents.
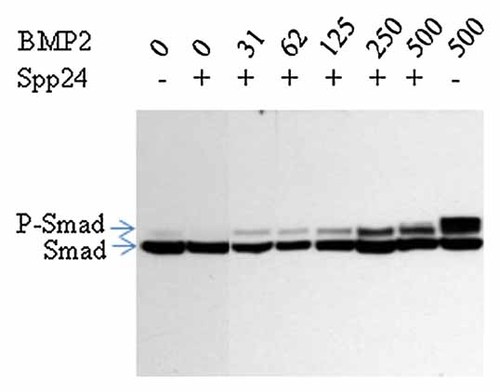
Spp24 inhibits BMP-2-stimulated Smad1/5 phosphorylation in W-20-17 cells. Cells were cultured to near confluence in DMEM supplemented with 10% serum, switched to low-serum (0.5%) test medium overnight, and refed with very low-serum (0.2%) DMEM containing vehicle, Spp24 alone (500 ng/ml), BMP-2 (31–500 ng/ml) plus Spp24 (500 ng/ml), or BMP-2 (500 ng/ml) alone for 20 min. After lysis in Laemmli sample buffer, identical aliquots were subjected to SDS–PAGE and Western blotting with HRP-conjugated anti-phospho-Smad 1/5/8 antibody that recognizes both the phosphorylated and unphosphorylated proteins.
Spp24 Attenuates BMP-2-Stimulated Alkaline Phosphatase Induction
Previous studies have demonstrated that recombinant human BMP-2 stimulates a dose-dependent increase in ALP activity in W-20-17 cells at days 2–7 of treatment [Thies et al., 1992]. In order to test the down-stream effects of Spp-24 binding to rhBMP-2 on osteoblast differentiation, we measured ALP activity in W-20-17 cells after 60 h of treatment with vehicle, rhBMP-2, Spp-24, or rhBMP-2 plus Spp24 in low-serum (0.5%) media. The results are expressed as nmol PNP produced/mg protein-hr (Fig. 3). In the absence of BMP, there was no significant difference in the ALP activity of cells cultured in low-serum media alone when compared to cells cultured in low-serum media plus 100 ng/ml Spp24. BMP-2 induced a dose-dependent increase in ALP activity, and addition of Spp24 (100 ng/ml) inhibited the BMP-2-stimulated rise in ALP activity by about 50% (P < 0.001 at 50 and 100 ng/ml BMP-2) (Fig. 3). Thus, the inhibitory effects of Spp24 on BMP-2-stimulated osteoblast differentiation, assessed as ALP activity, persist for at least 2.5 days after treatment (Fig. 3).
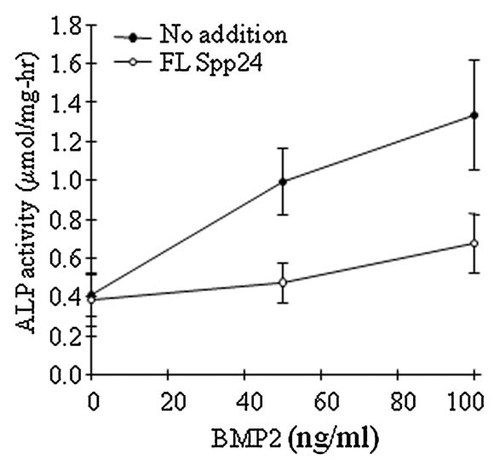
Spp24 inhibits BMP-2-stimulated alkaline phosphatase induction. W-20-17 cells were plated in 96-well dishes at 2,500 cells/well, then switched to low-serum (0.5%) DMEM supplemented with vehicle, rhBMP-2 (50 or 100 ng/ml), or rhBMP-2 plus FL-rb Spp24 (100 ng/ml) for 60 h. ALP activity was assessed based on cleavage of p-nitrophenol from p-nitrophenylphosphate at alkaline pH. ALP activity (mean ± SEM, n = 8) is expressed as µmol/mg protein-hr at 37°C. ALP activity was significantly inhibited by treatment with Spp24 plus rhBMP-2 (P < 0.001 at 50 and 100 ng/ml BMP-2) when compared to rhBMP-2 alone.
Spp24 Does Not Prevent BMP-2-Induced Mineralization
Based on the observations that Spp24 attenuated short-term BMP-2-stimulated Smad1/5 phosphorylation and intermediate-term ALP induction, we hypothesized that Spp24 would also inhibit the long-term effects of BMP-2 treatment, which were assessed based on the appearance of mineralized nodules in long-term cultures [Aubin and Herbertson, 1998]. W-20-17 cells reportedly do not mineralize when cultured in vitro in DMEM supplemented with 10% serum, ascorbic acid, β-glycerol phosphate, and 100 ng/ml rhBMP-2 for up to 10 days or in vivo in diffusion chambers in rats [Thies et al., 1992]. Therefore, mineralization was assessed after culturing W-20-17 cells in pro-osteogenic media (POM) consisting of α-MEM supplemented with 2% serum, dexamethasone, ascorbic acid, β-glycerolphosphate, and a relatively high level of rhBMP-2 (500 ng/ml). No mineralization, assessed based on Alizarin red staining, was observed when W-20-17 cells were cultured for 7 days in low-serum (2%) BMP-2-free POM, either alone (Fig. 4A) or in the presence of 100 ng/ml Spp-24 (Fig. 4B). However, strong staining was observed when 500 ng/ml rhBMP-2 was added to the POM (Fig. 4C). Addition of 1,000 ng/ml Spp24 to cultures treated with 500 ng/ml rhBMP-2 did not prevent mineralization (Fig. 4D). Instead, co-treatment with both Spp24 and BMP-2 appeared to result in less diffuse, more focal deposition of mineral (Fig. 4D). This demonstrates that the inhibitory effects of Spp24 treatment, even when administered at a higher dose than BMP-2, do not persist in long-term culture under pro-osteogenic conditions and is consistent with the observation that recombinant Spp24 is exquisitely labile to proteolysis in the presence of calcium [Murray et al., 2007]. Therefore, we hypothesized that Spp24, which is synthesized primarily in the liver and kidney, must be transported through serum to remote target tissues, like bone, in a manner that protects it from proteolysis.
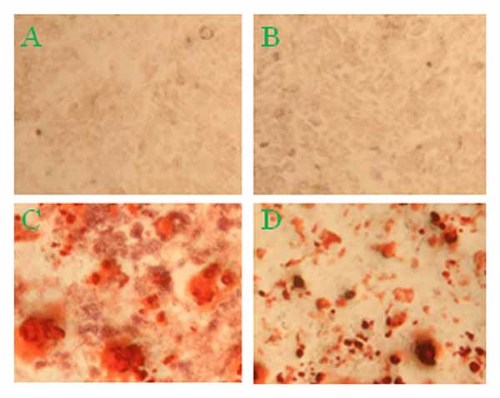
Spp24 does not prevent BMP-2-stimulated mineralization in W-20-17 cells. Cells were cultured in pro-osteogenic medium consisting of α-MEM supplemented with 2% serum, 1 nM dexamethasone, 50 µg/ml ascorbic acid, and 7 mM β-glycerophosphate plus or minus vehicle, 500 ng/ml rhBMP-2, 1,000 ng/ml Spp24, or rhBMP-2 plus Spp24 for 7 days, fixed in 10% formalin, and stained with Alizarin red to visualize mineral. Panel A: Vehicle control. Panel B: +Spp24. Panel C: +rhBMP-2. Panel D: +rhBMP-2, +Spp24. BMP-2 induced mineralization that was more focal and less diffuse in the presence of Spp24. Original magnification: 200×.
Native Spp24 Is Present in a High-Molecular Weight Complex Covalently Bound to α2-Macroglobulins in Serum
A scheme for immunoaffinity purification of Spp24 from serum was developed. Briefly, pure anti-Spp24 IgG was obtained by peptide antigen affinity chromatography, immobilized via its carbohydrate side chains, and used to purify Spp24 from calf serum. The results of a typical separation are shown in Figure 5. Spp24 bound tightly to the IgG column and remained bound even after extensive washes with buffers containing 600 mM NaCl and 0.1% Tween-20. The bound Spp24 was eluted with 0.1 M glycine–HCl buffer, pH 2.7, as shown in the chromatogram in Figure 5. The purified fractions were pooled, concentrated using centrifugal concentrators with a 10 kDa molecular cutoff, and subjected to an initial characterization by native PAGE or SDS–PAGE, followed by Coomassie blue staining and Western blotting, as outlined below.
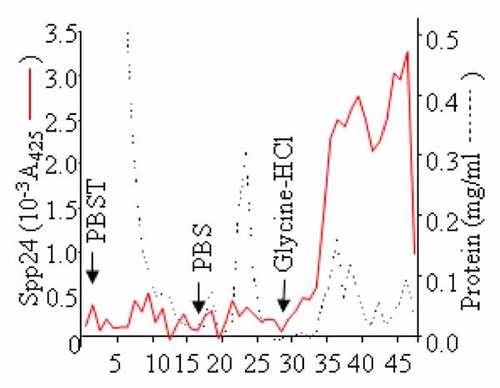
Affinity purification of Spp24 from calf serum. Calf serum was mixed anti-C-terminal Spp24 IgG-Ultralink resin, loaded into a column, extensively washed PBS, and eluted from the resin with 0.1 M glycine–HCl, pH 2.7, 150 mM NaCl in the presence of 1 µM leupeptin and 0.02% NaN3. Spp24 and protein concentrations are monitored by ELISA and BCA, respectively.
Purified Spp24 was separated by native PAGE, which was selected because it permits separation of proteins based on charge and mass, but avoids heat dissociation of non-covalently bound complexes or reduction of disulfide-bonded proteins within complexes. Coomassie blue staining revealed one band at the bottom of the loading well (>500 kDa) and a faint band at ∼80 kDa (Fig. 6A). In a duplicate PVDF probed with G4431, only a single heavy band can be seen also at the bottom of the loading well (Fig. 6B). This demonstrates that Spp24 in serum is present in a high molecular weight complex that cannot be separated by charge or size in native PAGE.
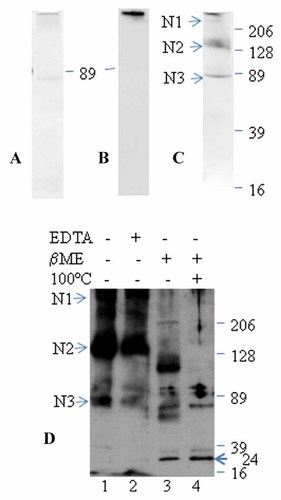
SDS–PAGE analysis of purified Spp24. Purified fractions from Figure 5 (4 µg) were pooled, concentrated, and analyzed by native PAGE (panels A and B) or SDS–PAGE (panels C and D). Proteins were stained with Coomassie Brilliant blue (panels A and C). Molecular weight markers are shown as kDa and the bands of interest are designated as N1, N2, or N3. The separated proteins were transferred to PVDF and immunoblotted with G4431 and visualized by goat anti-rabbit IgG-HRP (panels B and D). Monomeric Spp24 is marked by an arrow (panel D). Protein bands from panel C were excised, trypsin digested, and subjected to LC–MS/MS analysis.
SDS–PAGE of affinity-purified Spp24 was subsequently conducted under non-reducing and reducing conditions. Under non-reducing condition, the purified sample showed three bands (N1–N3) of molecular weights 80, 130, and >500 kDa, respectively by Coomassie blue staining (Fig. 6C). These three bands can be visualized by immunoblotting PVDF with G4431 (Fig. 6D, Lane 1). Surprisingly, incubation of the purified sample with additional 60 mM EDTA did not affect the stability and pattern of the complex (Fig. 6D, Lane 2). After treatment under 140 mM β-mercaptoethanol, a reducing agent, the purified sample revealed an expected band of approximately 24 kDa and the disappearance of major high molecular weight bands, especially when coupled with boiling at 100°C (Fig. 6D, Lanes 3 and 4). These results demonstrate that the affinity-purified Spp24 complex in serum can only be dissociated by addition of reducing agents and heat denaturation and suggest that disulfide bonds are involved in the stability of the complex. Unambiguous confirmation of the identity of Spp24 and characterization of the other proteins in the high molecular weight bands was achieved by LC–MS/MS analyses of Coomassie blue stained and trypsin digested bands (N1–N3) cut from non-reducing SDS–PAGE (Fig. 6C). A brief list of the proteins identified by LC–MS/MS is shown in Table I. Spp24, α2-macroglobulin (α2M), pregnancy zone protein (PZP), complement C3, and anti-thrombin III (Serpin C1) were all present in the rather faint band designated N1, which had an estimated mass of >500 kDa (Fig. 6C). Band N2 contained tryptic peptides consistent with those of α2M, complement C3, α1-antitrypsin (Serpin A1), and ceruloplasmin. A detailed list of the tryptic peptides identified in bands N1 and N2 is shown in Table II. Band N3 contained tryptic peptides consistent with the presence of lactoferrin, fetuin A (α2-HS-glycoprotein), inter-α-trypsin inhibitor heavy chain H4 (ITIH4), and α1 antitrypsin. Though no Spp24 was detected by LC–MS/MS in bands N2 and N3, Western blotting indicated the presence of Spp24 in band N2 and N3 (Fig. 6D, Lane 1).
| Proteins/cut bands | Mr (kDa) | Bands | ||
|---|---|---|---|---|
| N1 | N2 | N3 | ||
| α2-macroglobulin | 130 | + | + | |
| Pregnancy zone protein | Unknown | + | ||
| Complement C3 | 187 | + | + | |
| Bovine serum albumin | 67 | + | + | |
| Transferrin | 80 | + | + | |
| Anti-thrombin III | 62 | + | ||
| Spp24 | 30 | + | ||
| α1-antitrypsin | 52 | + | + | |
| Ceruloplasmin | 132 | + | ||
| ITIH4 | 120 | + | ||
| Lactoferrin | 72 | + | ||
| Fetuin A | 68 | + | ||
- Protein bands from SDS–PAGE were trypsin digested and subjected to LC–MS/MS analysis.
| Peptide sequence | Abundance (ppm) | m/za [M + nH]−n | Molecular weight | |
|---|---|---|---|---|
| Obs. | Calc. | |||
| Group I: Proteins containing a “bait” region and a thioester reactive center | ||||
| α2-Macroglubulin (α2M, NP_001103265) | ||||
| R.IQHHTLLASPV.R | 2.27 | 608.35 | 1214.68 | 1214.68 |
| R.IQHHTLLASPVR.A | 2.62 | 457.93 | 1370.78 | 1370.78 |
| T.DTAADAHDPARPGAK.V | 1.37 | 746.86 | 1491.71 | 1491.71 |
| K.DTIIKPLLVEPEGLEK.E | 2.27 | 897.52 | 1793.02 | 1793.02 |
| K.DTIIKPLLVEPEGLEK.E | 3.12 | 598.68 | 1793.02 | 1793.02 |
| R.DFVHFDDTSEPPTETVR.K | 2.79 | 996.45 | 1990.89 | 1990.89 |
| R.DFVHFDDTSEPPTETVR.K | 3.06 | 664.64 | 1990.90 | 1990.89 |
| R.NRDFVHFDDTSEPPTETVR.K | 3.74 | 754.69 | 2261.04 | 2261.03 |
| R.HFPPAAATDTAADAHDPARPGAK.V | 1.58 | 572.03 | 2284.10 | 2284.10 |
| R.HFPPAAATDTAADAHDPARPGAK.V | 1.66 | 762.37 | 2284.10 | 2284.10 |
| R.NRDFVHFDDTSEPPTETVRK.Y | 3.11 | 598.29 | 2389.14 | 2389.13 |
| R.AEMGRNRDFVHFDDTSEPPTETVR.K | 0.67 | 941.76 | 2822.26 | 2822.26 |
| R.AEMGRNRDFVHFDDTSEPPTETVR.K | 2.80 | 706.57 | 2822.26 | 2822.26 |
| Pregnancy zone protein (PZP, XP_583329.4) | ||||
| K.APGLASSR.S | 1.60 | 379.71 | 757.41 | 757.41 |
| L.PSNVVEGSAR.A | 1.22 | 508.26 | 1014.51 | 1014.51 |
| R.SGSLLNNAIK.G | 3.22 | 508.79 | 1015.57 | 1015.57 |
| K.LPSNVVEGSAR.A | 1.22 | 564.81 | 1127.60 | 1127.59 |
| R.KLQEMAQIQR.T | 2.60 | 630.84 | 1259.67 | 1259.67 |
| K.ITAAPYSLCGLR.A | 2.92 | 661.35 | 1320.69 | 1320.69 |
| R.SQGNTWLTAFVLK.S | 2.31 | 732.90 | 1463.78 | 1463.78 |
| R.SQGNTWLTAFVLK.S | 0.94 | 733.39 | 1464.76 | 1464.76 |
| K.HSDGSYSTFGDRDGR.S | 2.98 | 552.91 | 1655.70 | 1655.69 |
| Complement C3 (NP_001035559.2) | ||||
| K.VVPEGVRVNK.T | 6.94 | 548.83 | 1095.65 | 1095.64 |
| K.RQESLELIR.K | 2.38 | 572.33 | 1142.64 | 1142.64 |
| K.TSQGLETQQR.A | 4.83 | 574.29 | 1146.57 | 1146.56 |
| R.HQQTITIPAR.S | 0.64 | 582.83 | 1163.64 | 1163.64 |
| Group II: Protease/proteinase inhibitors | ||||
| Antithrombin-III (Serpin C1, NP_001029870) | ||||
| R.RVWELSK.A | 1.33 | 459.26 | 916.51 | 916.51 |
| K.SSELVSANR.L | 1.87 | 481.75 | 961.48 | 961.48 |
| K.SRLPGIVAEGR.S | 2.00 | 577.84 | 1153.66 | 1153.66 |
| K.EQLQDMGLEDLFSPEK.S | 1.82 | 947.94 | 1893.87 | 1893.87 |
| a1-antitrypsin (Serpin A1, NP_776307) | ||||
| K.LVDTFLEDVK.N | 3.55 | 589.82 | 1177.63 | 1177.62 |
| K.VLDPNTVFALVNYISFK.G | 3.38 | 970.53 | 1939.05 | 1939.05 |
| K.VLDPNTVFALVNYISFK.G | 4.68 | 647.36 | 1939.05 | 1939.05 |
| Group II: Metal transport proteins with oxidation/reduction activity | ||||
| Transferrin (TF, AAA96735) | ||||
| L.PDPQESIQR.A | 2.32 | 535.27 | 1068.52 | 1068.52 |
| R.YYGYTGAFR.C | 2.97 | 549.26 | 1096.50 | 1096.50 |
| K.KDTDFKLNELR.G | 3.24 | 460.25 | 1377.73 | 1377.73 |
| R.KPVTDAENCHLAR.G | 2.50 | 504.25 | 1509.74 | 1509.74 |
| K.DNPQTHYYAVAVVK.K | 2.99 | 802.91 | 1603.80 | 1603.80 |
| K.LYKELPDPQESIQR.A | 2.75 | 572.64 | 1714.89 | 1714.89 |
| K.HSTVFDNLPNPEDRK.N | 2.81 | 590.29 | 1767.86 | 1767.85 |
| K.GEADAMSLDGGYLYIAGK.C | 3.46 | 923.93 | 1845.85 | 1845.85 |
| R.TVGGKEDVIWELLNHAQEHFGK.D | 4.00 | 627.57 | 2506.27 | 2506.26 |
| Ceruloplasmin (CP, XP_604593.3) | ||||
| K.TESSTVTPTAPGETR.T | 1.05 | 767.37 | 1532.73 | 1532.73 |
| Group III: Target protein of the affinity column (G4431) | ||||
| Spp24 (NP_776613.2) | ||||
| K.VNSQSLSPYLFR.A | 2.23 | 705.87 | 1409.73 | 1409.73 |
- Only tryptic peptides unique to and representing respective proteins from non-reducing SDS–PAGE (bands N1 and N2) are listed, whereas peptides from bovine serum albumin are not listed due to their large numbers. Cleavage sites are shown by “.” between two residues, and modified amino acids due to oxidation or hydroxylation are indicated by underlining.
- a The observed mass over charge (m/z) represents singularly (n = 1), doubly (n = 2), or triply (n = 3) charged values, and their corresponding molecular weights (Mr) are converted by the formula: Mr = n (m/z − 1).
DISCUSSION
Taghavi et al. [2010] showed that Spp24 binds cytokines in the BMP/TGF-β superfamily with high affinity and specificity. Binding is mediated through a TRH1 (transforming growth factor receptor II homology-1) domain similar to those in fetuin and the type II receptor itself [Demetriou et al., 1996; Behnam et al., 2005]. Despite the clear demonstrations that Spp24 inhibits BMP-2-stimulated spinal fusion [Sintuu et al., 2011] and ectopic bone formation and reduces vertebral and femoral bone mineral density in transgenic mice over-expressing Spp24 [Sintuu et al., 2008], relatively little is known about the down-stream mechanisms by which Spp24 modulates bone metabolism after it has bound a specific BMP/TGF-β cytokine. This question was explored in W-20-17 mesenchymal stem cells, where BMP-2 induces osteoblastic differentiation, in part, by up-regulating ALP activity, osteocalcin production, and PTH sensitivity [Thies et al., 1992]. The results presented here for the first time show that Spp24 attenuates BMP-2-stimulated signal transduction and osteoblastic differentiation in W-20-17 cells, assessed as Smad1/5 phosphorylation and induction of ALP activity, respectively. Although Spp24 was administered at a higher dose than BMP-2 (1,000 ng/ml Spp24 vs. 500 ng/ml BMP-2), it did not inhibit BMP-2-stimulated mineralization. Thus, Spp24 seems to have greater effects on BMP-2-stimulated Smad signaling and the earlier phases of osteoblast differentiation (assessed as ALP induction) than it does on the later phases (assessed as the formation of mineralized nodules in the extracellular matrix). This is consistent with the previous observation that Spp24 is exquisitely labile to proteolysis [Murray et al., 2007]. Degradation of Spp24 would account for the reduction in its apparent effects on BMP-2-stimulated osteoblastic differentiation that were observed over the time-course of treatment. However, since the major sources of Spp24 are the liver and kidney, no mechanism has previously been proposed that would account for its transport, intact, from the liver or the kidney to its remote site of action in bone in vivo. We hypothesized that Spp24 circulates in such a manner as to be protected from proteolysis until it reaches its target tissues.
We report here for the first time the direct isolation of Spp24 covalently bound to α2-macroglobulins as a core complex (Spp24–α2M) from bovine calf serum. Due to the tetrameric nature of α2M, such a core complex might also include the protease inhibitor anti-thrombin III or Serpin C1. Two other members of the α2M family of proteins (PZP and complement C3) were also found to be associated with Spp24, and likely formed Spp24-PZP and Spp24-C3, respectively. This confirms decades of previous observations related to the biochemistry, physiological functions, and biology of α2M, and provides a novel insight into how Spp24 could be transported intact from the site of greatest biosynthesis (the liver) to its target tissues, such as bone. A simplified illustration of the process is summarized in Figure 7. Native α2M is a tetramer of identical subunits encoded by one gene and is composed of multiple domains homologous to complement C3 [Doan and Gettins, 2007]. Each monomer contains a “bait” region that is susceptible to cleavage by proteases, such as plasminogen activator, collagenase, trypsin, chymotrypsin, subtilisin, and matrix metalloproteinase I. Upon cleavage, the α2M monomer becomes two subunits that are connected by a disulfide bond. Cleavage results in a rapid conformational change in the protein that exposes a unique thioester whose glutamyl residue reacts with lysyl amino groups of the protease. The ε-lysyl-γ-glutamyl cross links effectively encapsulate or “trap” the protease in the complex [Sottrup-Jensen, 1989; Feinman, 1994]. In addition, the exposed reactive thiol can form a disulfide bond with cytokines (interleukin-1β, TGF-β1 and β2), insulin and IGFs, growth factors (PDGF, CSF-1, VEGF, and FGF), protease inhibitors and other proteins [Crookston et al., 1994]. Protease-activated α2M is capable of binding to the α2M receptor/LRP1 (low-density lipoprotein receptor-related protein-1) on target cells [Crookston et al., 1994]. The LRP1 receptor also acts as an independent TGF-β receptor and mediates the growth inhibitory response of TGF-β in smooth muscle cells [Lillis et al., 2008]. The binding of both the α2M: Spp24 complex and TGF-β by the LRP1 could provide a co-ordinated mechanism for delivering intact Spp24 to bone and co-localizing it with its cytokine binding partner. Finally, not all α2M molecules capture the protease that activated it, as immobilized trypsin can still cleave/activate α2M without being bound [Feinman, 1994].
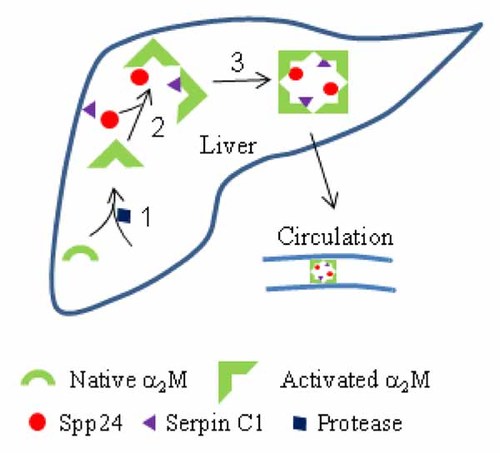
The proposed formation of an Spp24 complex with α2M via a disulfide bond in the liver. The α2-macroglobulin (α2M) monomer is digested by a protease at its “bait” region, triggering a conformational change, and capture of the protease by a reactive thioester (Reaction 1). The activated α2M can then accept a small ligand, cytokine, protease inhibitor, or Spp24 via its thiol group, forming disulfide bond (Reaction 2). Various ligand-loaded α2M monomers form dimers and tetramers in non-covalent manner (Reaction 3). For simplicity, only Spp24 and Serpin C1 are shown but the combination with other ligands in a tetrameric α2M molecule is more complicated.
Complex formation with α2M provides a mechanism for protecting Spp24 from proteolysis and transporting it to target tissues, such as bone. Since α2M is a tetramer, the Spp24–α2M complex could also contain other captured proteins that affect bone metabolism. For example, metal-binding transport proteins, such as ceruloplasmin (copper) and lactoferrin (iron), have been identified as components of activated α2M complexes. Lactoferrin is a novel bone growth factor, and it is bound to LRP1 [Naot et al., 2005]. The presence of proteinase/protease inhibitors, including Serpins C1 (antithrombin-III), A1 (α1-antitrypsin) and G1 (Factor XIIa inhibitor), and ITIH4 in the purified sample could provide additional protection for the Spp24–α2M complex. Due to the lower mass ratio of Spp24–α2M and other serum proteins, Spp24 peptide signal(s) may not be apparent in the HPLC chromatogram that precedes MS analysis. This might explain why ceruloplasmin, Serpins A1 (α1-antiproteinase), lactoferrin, and ITIH4 found in bands N2 and N3 of non-reducing SDS–PAGE, while the Spp24 peptide signal was missing. However, these proteins may associate with the Spp24–α2M complex non-covalently. Clearly, additional work will be required to identify other ligands in the Spp24–α2M complex and determine their stoichiometry.
Zorina et al. [2007] identified a spectrum of protein ligands, including IgG, IgA, IgM, and albumin, which are transported by α2M. The finding of immunoglobulin light chains in bands N1 and N2 in our study may confirm their observations. It is also possible that these polypeptides were leached from the immobilized G4431 IgG during the elution of the complex with pH 2.7 glycine buffer. Immunoglobulin heavy chain was also found in bands N2 and N3. It is likely that the presence of IgG chains is an experimental artifact, rather than a component of the complex, as our affinity column loses its binding capacity and cannot be used for more than nine purifications (data not shown). Such ligand leakage is common for all protein-based affinity resins used in a variety of purification applications. It is also clear that the presence of these IgG fragments gives rise in part, to a strong band at 50 kDa using the traditional two-step immunoblotting method. Thereafter, to avoid this potential artifact, HRP-coupled primary antibody should be used in future explorations. Bovine serum albumin and transferrin, two abundant serum proteins, may be co-purified with Spp24. In addition, a blast search of proteins homologous to the immunizing peptide used to generate the G4431 antibody in the current Bos taurus protein database resulted in about 180 hits. However, none of these proteins were found in our purified sample, indicating that our immuno-affinity resin was highly specific for Spp24 and that other proteins in the sample are likely either part of a complex or copurifying contaminants.
Spp24 has also been identified by Western blotting as a minor component of the serum FMC isolated from rats treated with etidronate 4- to 16-fold above the typical therapeutic level [Price et al., 2003]. The physiologic relevance of identifying trace amounts of the apatite-binding protein Spp24 [Hu et al., 1995; Behnam et al., 2005; Zhou, 2007] in the FMC is unclear. However, of interest is the observation that fetuin A can be found in Spp24–α2M complexes (Table I). There are a number of differences between our purified Spp24–α2M complex and FMC, most notably that: The Spp24–α2M complex exists in the absence of exogenous etidronate, the Spp24–α2M complex cannot be disrupted by EDTA treatment (Fig. 6D, Lane 2), and the Spp24–α2M complex does not contain any matrix Gla protein as does the FMC. To date, we have not determined if fetuin A is a contaminant or part of the Spp24–α2M complex. Many other additional questions remain to be explored. For example: Which of the two pairs of disulfide bonds in the Spp24 molecule are involved in forming a disulfide bond with activated α2M? What is the tissue distribution of the Spp24–α2M complex, and how is the complex processed by the osteoblast or other target cells in bone?
Acknowledgements
We wish to thank Mr. Robert Simon for his assistance in the initial characterization of serum Spp24 and Dr. Weibiao Huang for helpful discussions. This project was supported, in part, by the Geriatric Research, Education, and Clinical Centers, as well as grants from the Biomedical Laboratory Research (1I01BX000511) to S.M. and Development and Rehabilitation Research and Development Services (1I01RX000383) to E.B.M. of the Department of Veterans Affairs.




