Human Wharton's Jelly stem cell conditioned medium and cell-free lysate inhibit human osteosarcoma and mammary carcinoma cell growth in vitro and in xenograft mice†
Conflicts of interest statement: All authors have no conflict of interests.
Abstract
Human Wharton's jelly stem cells (hWJSCs) were shown to inhibit the growth of human mammary carcinomas. It is not known whether cell-free secretions or lysates of hWJSCs do the same on different cancers. They may be less controversial than cells to regulatory bodies for clinical application. We examined the influence of hWJSC conditioned medium (hWJSC-CM) and cell-free lysate (hWJSC-CL) on two osteosarcoma cell lines (MG-63, SKES-1) in vitro and on human mammary carcinomas in immunodeficient mice. When exposed to hWJSC-CL, increased vacuolations in MG-63 and increased membrane fragmentation in SKES-1 cells were observed, with greater cell death in SKES-1. Exposure of SKES-1 and MG-63 cells to hWJSC-CL showed significant decreases in cell proliferation of 46.48 ± 6.66% and 24.32 ± 5.67% respectively compared to controls. MG-63 and SKES-1 cells were annexin V-FITC positive and SKES-1 TUNEL positive following treatment with hWJSC-CM and hWJSC-CL. MG-63 cells were positive and SKES-1 cells negative for anti-BECLIN-1 and anti-LC3B following treatment with hWJSC-CM and hWJSC-CL. RT-PCR showed that the pro-apoptotic BAX gene and the autophagy-related ATG-5 and BECLIN-1 genes were up-regulated while the anti-apoptotic BCL2 and SURVIVIN genes were down-regulated in MG-63 and SKES-1 cells treated with hWJSC-CM and hWJSC-CL. Injections of hWJSCs and hWJSC-CM into mammary carcinomas in immunodeficient mice resulted in decreased tumor sizes and weights of 24.86 ± 6.05% to 37.03 ± 5.91% and 47.14 ± 7.36% to 55.09 ± 5.87% respectively at 6 weeks compared to controls. hWJSC-CM and hWJSC-CL inhibit mammary carcinoma and osteosarcoma cells via apoptosis and autophagy. J. Cell. Biochem. 114: 366–377, 2013. © 2012 Wiley Periodicals, Inc.
Novel anticancer therapies targeted at cell function and signaling pathways are gaining tremendous interest today. These new agents involve protein kinase and angiogenesis inhibitors, monoclonal antibodies, the small molecule methyl transferase inhibitors, small-interfering RNAs, and mesenchymal stem cells (MSCs) [Ma and Adjei, 2009; Bhardwaj et al., 2010; Burstein, 2011; Yuan et al., 2011]. MSCs were shown to home to sites of tissue malfunction [Chamberlain et al., 2007] and because of this property these cells have been used as vehicles to deliver genes and anti-cancer agents to tumor sites to attenuate their growth [Fritz and Jorgensen, 2008; Prockop, 2009]. When bone marrow MSCs (BMMSCs) were injected intravenously in mice suffering from Kaposi's sarcoma, they migrated to the tumor site and attenuated its growth [Khakoo et al., 2006]. MSCs also have the property of being able to attenuate or abrogate certain tumors when injected directly into the tumor. Engineered BMMSCs that expressed interleukin-12 suppressed melanoma and cervical cancer growth by inducing a tumor-specific T-cell response in mouse models [Seo et al., 2011] and BMMSCs expressing suicide genes inhibited prostate cancer cell growth in an athymic murine model [Cavarretta et al., 2010].
Besides the bone marrow, MSCs from other sources also attenuate or abrogate tumor cell growth both in vitro and in vivo. Primitive MSCs isolated from the umbilical cord Wharton's jelly (hWJSCs) were shown to abrogate certain solid tumors [Rachakatla et al., 2007; Ayuzawa et al., 2009; Sun et al., 2010; Chao et al., 2011]. Human WJSCs (hWJSCs) have the distinct advantages over human BMMSCs (hBMMSCs) in that they can be harvested painlessly in large numbers from discarded umbilical cords, multiply rapidly in vitro, maintain their stemness characteristics for many passages in serial culture, are hypoimmunogenic and do not induce tumors in vivo [Bongso et al., 2008; Fong et al., 2010; Gauthaman et al., 2012b]. When hWJSCs were injected intravenously into severely combined immunodeficient (SCID) mice with breast cancer tumors, the hWJSCs homed to metastatic tumor sites in the lungs of the animals confirming the homing abilities of hWJSCs [Rachakatla et al., 2007]. Also, when rat WJSCs were injected intravenously or intra-tumorally 4 days after the administration of rat breast cancer tumor cells into orthotopic sites in rats with an intact immune system (early tumor protocol), the rat WJSCs caused complete cessation of breast tumor growth in 34–38 days compared to controls [Ganta et al., 2009]. Two other independent studies confirmed these findings in the human by showing that when hWJSCs were administered intravenously 8 days after tumor transplantation in a human breast cancer xenograft rat model they migrated to metastatic tumor sites in the lungs and reduced tumor burden [Ayuzawa et al., 2009; Ganta et al., 2009; Maurya et al., 2010]. Engineered hWJSCs expressing human interferon-β also abrogated breast tumor growth in animal models [Chamberlain et al., 2007]. Recently, populations of cancer stem cells (CSCs) were isolated from the commercial breast cancer cell line MDA-MB-231 and when these CSCs were exposed to hWJSCs in culture their cell growth was inhibited. The authors postulated that the inhibitory effects were probably via the inhibition of the phosphoinositide 3-kinase and AKT signaling mechanisms [Ma et al., 2012]. Inhibition of breast cancer cells by hWJSCs in vitro was reported to be by an entosis mechanism where the hWJSC was first engulfed by the breast cancer cell followed by disintegration of the hWJSC within the cancer cell and release of its contents resulting in apoptosis of the breast cancer cell [Chao et al., 2011]. Other workers claimed that the extracellular matrix of WJSCs also inhibited breast cancer cell proliferation by secretion of dickkopf-1 and suppression of the Wnt signaling pathway [Sun et al., 2010].
The conditioned medium and cell-free lysate of MSCs also appear to have anticancer properties. Immortalized BMMSC conditioned medium inhibited two hepatoma cell lines in vitro and also induced tumor regression in a hepatoma SCID mouse xenograft model by regulating the Wnt signaling pathway [Qiao et al., 2008]. We recently showed in in vitro studies that the conditioned medium and cell-free lysate of hWJSCs (hWJSC-CM and hWJSC-CL) not only inhibited breast (MDA-MB-231) and ovarian cancer (TOV-112D) but also osteosarcoma cells (MG-63). Interestingly the anticancer effect was most pronounced on the MG-63 osteosarcoma cell line [Gauthaman et al., 2012a]. We suggested that the anticancer effects were probably mediated by key soluble molecules secreted by hWJSCs such as interleukins, cell adhesion molecules, hyaluronic acid, and glycosoaminoglycans [Fong et al., 2011b].
We have now extended these studies to find out (1) whether the pronounced anticancer effects of hWJSC-CM and hWJSC-CL are specific to the osteosarcoma cell line MG-63 only or inhibit other osteosarcoma cell lines such as SKES-1 as well and (2) whether hWJSCs and hWJSC-CM attenuate or abrogate breast cancer cells in vivo in a xenograft immunodeficient mouse model.
MATERIALS AND METHODS
Cells
The hWJSCs were derived according to our published protocols [Fong et al., 2007, 2010] from umbilical cords donated by patients after Ministry of Health, Domain Specific Review Board (DSRB), Singapore ethical approval. Commercial human cancer cell lines namely osteosarcoma (MG-63), Ewing's sarcoma (SKES-1), mammary carcinoma (MDA-MB-231) and foreskin fibroblasts (CCD-1112sk) were purchased from the American Type Culture Collection (ATCC, Rockville, MD) after ethical approval for their use was given by the National University of Singapore (NUS) Institutional Review Board (IRB).
Cell Culture
The hWJSCs were cultured in sterile tissue culture flasks [Becton–Dickinson (BD), Franklin Lanes, NJ] using hWJSC culture medium comprising of 80% DMEM medium supplemented with 20% fetal bovine serum (Invitrogen Life Technologies, Carlsbad, CA), 16 ng/ml basic fibroblast growth factor (Millipore Bioscience research agents, Temecula, CA), 1% non-essential amino acids, 2 mM L-glutamine, 0.1 mM β-mercaptoethanol, 1% insulin-transferrin-selenium and antibiotic mixture of penicillin (5 IU) and streptomycin (50 µg/ml) (Invitrogen). Frozen human ostesarcoma cells (MG-63, SKES-1) and human foreskin fibroblast cells (CCD1112sk) were thawed and initially cultured in their respective culture media specified by the supplier (ATCC) and then weaned and cultured in hWJSC medium for a minimum of three passages before being used for all experiments.
Conditioned Media and Cell-Free Lysates
Conditioned media and cell-free lysates were prepared from hWJSCs and CCD1112sk cells. Both cell types were grown to 80% confluence and their cell-free lysates prepared using a mammalian cell extraction kit that contained a protease inhibitor cocktail and dithiothreitol (BioVision, Mountain View, CA). Briefly, the cultured cells were washed once with phosphate buffered saline that contained no calcium and magnesium (PBS(−)), dissociated with trypsin-EDTA (TrypLE™ express, Invitrogen) and centrifuged at 500g × 5 min to obtain a cell pellet. The cell pellet was mixed with 100 µl of the cell lysis buffer and resuspended several times and then incubated on ice for 15 min. The tubes were then centrifuged at 12,000g × 5 min (Eppendorf, Germany) and the clear supernatant (cell lysate) was separated and stored at −80°C until further use. The total protein content of the cell-lysate was measured using a Nanodrop™ spectrophotometer (Nanodrop technologies, Wilmington, DW). The hWJSC medium in which the hWJSCs and CCD1112sk cells were grown was collected after 48 h as conditioned media (hWJSC-CM; CCD-CM). Both conditioned media were filter-sterilized using 0.22 µm Millex-GP syringe filters (Millipore, Billerica, MA). The pH and osmolality of the hWJSC-CM and CCD-CM were determined before use in all experiments.
Cell Morphology
The human osteosarcoma cell lines MG-63 and SKES-1 were cultured at a seeding density of 2 × 104 cells/well in 24-well tissue culture plates (BD, Franklin Lakes, NJ). The two osteosarcoma cell lines were cultured separately in (a) hWJSC-CM (50%), (b) CCD-CM (50%), (c) hWJSC-CL (15 µg/ml protein) in hWJSC medium, and (d) CCD-CL (15 µg/ml protein) in hWJSC medium for 72 h with fresh changes of the respective conditioned media and cell lysates at 48 h. Changes in cell morphology of the osteosarcoma cells were monitored daily and photographed using inverted phase contrast optics (Nikon Instruments, Tokyo, Japan).
Cell Proliferation (MTT Assay)
The cell proliferation rates of the human osteosarcoma cell lines MG-63 and SKES-1 were evaluated by culture of the individual cell lines at 2 × 104 cells/well in 24-well tissue culture plates (BD, Franklin Lakes, NJ) in (a) hWJSC-CM (50%), (b) CCD-CM (50%), (c) hWJSC-CL (15 µg/ml protein) in hWJSC medium, and (d) CCD-CL (15 µg/ml protein) in hWJSC medium for 72 h with fresh changes of the respective conditioned media and cell lysates at 48 h. The cell proliferation assay was performed using a MTT reagent kit [3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide] (Sigma, St. Louis, MO) according to the manufacturer's instructions. Briefly, 10 µl MTT reagent (final concentration of 0.5 mg/ml) was added to the culture dishes and incubated for 4 h until a purple precipitate was visible. The supernatant was then aspirated and 100 µl of the detergent reagent was added and the plates incubated in the dark for 2 h. Absorbance at 570 nm was spectrophotometrically measured using a microplate ELISA reader (µQuant-BioTek, Winooski, VT) with a reference wavelength of 630 nm. Following deduction of the blank cell absorbance, the treated cell absorbance over the control cell absorbance was expressed as a percentage of cell survival or proliferation.
Annexin V-FITC Assay
The human osteosarcoma cell lines MG-63 and SKES-1 were cultured at a seeding density of 2 × 105 cells in T25 tissue culture flasks (BD, Franklin Lakes, NJ) in (a) hWJSC-CM (50%), (b) CCD-CM (50%), (c) hWJSC-CL (15 µg/ml protein) in hWJSC medium, and (d) CCD-CL (15 µg/ml protein) in hWJSC medium for 72 h with fresh changes of the respective conditioned media and cell lysates at 48 h. The cells were dissociated with trypsin, washed once with PBS (−) and then with Annexin V binding buffer (1×). The cells were stained with 5 µl Annexin V-FITC at room temperature for 15 min and then counterstained with propidium iodide (PI) (1 µg/ml) and analyzed using a CyAn™ ADP Analyzer.
Tunel Assay
The Dead-End Fluorometric System (Promega) was used according to the manufacturer's instructions to detect TUNEL positive cells. The human osteosarcoma cell lines MG-63 and SKES-1 were cultured at a seeding density of 2 × 103 cells/well in 96-well tissue culture plates (NUNC, Rochester, NY) in (a) hWJSC-CM (50%), (b) CCD-CM (50%), (c) hWJSC-CL (15 µg/ml protein) in hWJSC medium, and (d) CCD-CL (15 µg/ml protein) in hWJSC medium for 48 h. The cells were then washed and fixed with 4% methanol-free formaldehyde solution for 25 min at 4°C followed by addition of Triton X-100 solution for 5 min at 4°C. Labeling of the DNA fragments was carried out by treating the cells with fluorescin-12dUTP and terminal deoxynucleotidyltransferase for 1 h at 37°C in a humidified chamber in the dark. The reaction was stopped by the addition of sodium chloride-sodium citrate solution and the cells washed thrice and then stained with PI (1 µg/ml) in PBS containing DNase-free RNase 250 µg/ml for 15 min at room temperature in the dark. The TdT (terminal deoxynucleotidyl transferase)-mediated dUDP nick-end labeling (TUNEL) positive cells were analyzed under a fluorescence microscope.
Immunohistochemistry
The human osteosarcoma cell lines MG-63 and SKES-1 were cultured at a seeding density of 2 × 104 cells/well in 24-well tissue culture plates (BD, Franklin Lakes, NJ) in (a) hWJSC-CM (50%), (b) CCD-CM (50%), (c) hWJSC-CL (15 µg/ml protein) in hWJSC medium, and (d) CCD-CL (15 µg/ml protein) in hWJSC medium for 72 h and then analyzed for BECLIN-1 and LC3B by immunohistochemistry. Briefly, the cells were fixed with ice cold ethanol, washed with PBS and treated with 10% normal goat serum (NGS). The cells were then incubated with rabbit polyclonal anti-BECLIN-1 and anti-LC3B primary antibodies (1:100; Cell Signaling, Boston, MA) for 1 h and goat anti-rabbit secondary antibody (Alexa Fluor 588; 1:500 dilution) for 30 min. Following several washes with PBS the cells were incubated with 4′-6-Diamidino-2-phenylindole (DAPI; 0.5 µg/ml) (Molecular probes, Invitrogen Life Technologies) for 5 min at room temperature, washed with PBS again and then analyzed using fluorescence microscopy.
Quantitative Real Time Polymerase Chain Reaction (QRT-PCR)
The human osteosarcoma cell lines MG-63 and SKES-1 were cultured at a seeding density of 2 × 105 cells in T25 tissue culture flasks (BD, Franklin Lakes, NJ) in (a) hWJSC-CM (50%), (b) CCD-CM (50%), (c) hWJSC-CL (15 µg/ml protein) in hWJSC medium, and (d) CCD-CL (15 µg/ml protein) in hWJSC medium for 72 h with fresh changes of the respective conditioned media and cell lysates at 48 h. Total RNA was then extracted from the osteosarcoma cells using TRIzol™ reagent (Invitrogen). RNA quality and quantity were measured using a Nanodrop™ spectrophotometer (Nanodrop technologies, Wilmington, DW) and all samples were treated with DNase-I prior to first strand cDNA synthesis with random hexamers using the SuperScript™ first strand synthesis system (Invitrogen). Primer sequences were taken from earlier published studies [Gauthaman et al., 2012a]. qRT-PCR analysis was performed with the ABI PRISM 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA) using SYBR green and relative quantification was performed using the comparative CT (2−ΔΔCT) method.
Injection of hWJSCs and hWJSC-CM Into Mammary Carcinomas in Xenograft Mice
The anti-cancer effects of hWJSCs and hWJSC-CM were evaluated in vivo in severely combined immunodeficient (SCID) mice on previously injected mammary carcinoma cells (MDA-MB-231) using an early and a late tumor protocol. Briefly, 1 × 106 mammary adenocarcinoma cells in 100 µl of PBS were injected subcutaneously over the shoulder region in 6–8-week old SCID mice (n = 48) to induce ectopic breast tumors. The SCID mice were then divided into two main groups of 24 in each group (Group A: Early tumor protocol; Group B: Late tumor protocol). Groups A and B were each subdivided into four subgroups of six mice per subgroup as follows for intra-tumoural injections of PBS (controls) and hWJSCs (treatment). [Group A: Early tumor protocol: Sub-Group I: Control: 100 µl of PBS; Sub-Group II: 1 × 106 hWJSCs in 100 µl of PBS; Sub-Group III: 5 × 106 hWJSCs in 100 µl of PBS; Sub-Group IV: 100 µl of hWJSC-CM (50%): Group B: Late tumor protocol: Sub-Group I: Control: 100 µl of PBS; Sub-Group II: 1 × 106 hWJSCs in 100 µl of PBS; Sub-Group III: 5 × 106 hWJSCs in 100 µl of PBS; Sub-Group IV: 100 µl of hWJSC-CM (50%)].
For Group A the hWJSCs or hWJSC-CM were administered intra-tumorally 4 days after mammary carcinoma cell transplantation and for Group B they were administered intra-tumorally 5 weeks after mammary carcinoma cell transplantation. The animals were monitored for 6 weeks for tumor attenuation or abrogation. At the end of 6 weeks the animals were sacrificed and tissues from injection sites and any tumor remnants were removed, fixed in formalin and submitted for histopathology.
Statistical Analysis
The differences observed between treatment and control groups were compared and analyzed by the Student's t-test using the statistical package for Social Sciences (SPSS 13). The results were expressed as mean ± SEM from three different replicates for individual assays and a value of P < 0.05 was considered statistically significant.
RESULTS
In Vitro Effects of hWJSC-CM and hWJSC-CL on Osteosarcoma Cells
Cell morphology
Both osteosarcoma cell lines MG-63 and SKES-1 after weaning to the hWJSC medium maintained their characteristic morphology (fibroblast and epithelial respectively) in culture. Both MG-63 and SKES-1 cells did not show any changes in morphology when exposed to hWJSC-CM (50%) and CCD-CM (50%) compared to controls (Fig. 1). Exposure to CCD-CL (15 µg/ml protein) also did not show any morphologic changes in culture for both MG-63 and SKES1 cells (Fig. 1). However, exposure of MG-63 and SKES-1 to hWJSC-CM (50%) led to reduction in cell numbers at 72 h and treatment with hWJSC-CL (15 µg/ml protein) led to varying changes in cell morphology and cell death compared to untreated controls. MG-63 showed increased vacuolation (Fig. 1g) while SKES-1 showed increased membrane fragmentation (Fig. 1n) resulting in greater cell death and less of viable cells in culture compared to MG-63.
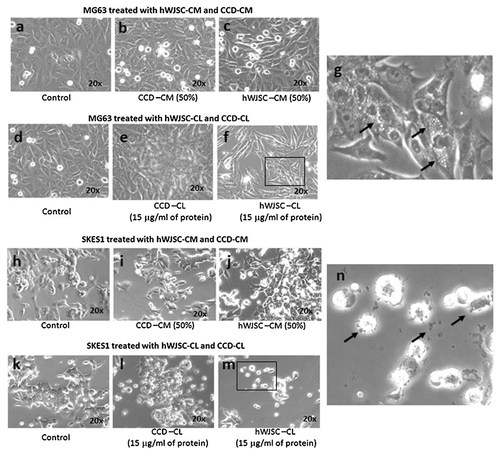
Morphological changes in osteosarcoma cell lines treated with hWJSC-CM and hWJSC-CL in vitro at 72 h. a–c: MG-63 cells following treatment with hWJSC-CM (50%) and CCD-CM (50%). Note slight reduction in MG-63 cell numbers with hWJSC-CM (50%). d–f: MG-63 cells following treatment with hWJSC-CL (15 µg/ml of protein) and CCD-CL (15 µg/ml of protein). Note greater reduction in MG-63 cell numbers with hWJSC-CL (15 µg/ml of protein). g: Enlarged image of boxed areas in f showing numerous vacuolations in MG-63 cells (indicated by arrows). CCD-CM and CCDCL did not induce any morphological changes or have any inhibitory growth effect on MG-63 cells. h–j: SKES-1 cells following treatment with hWJSC-CM (50%) and CCD-CM (50%). k–m: SKES-1 cells following treatment with hWJSC-CL (15 µg/ml of protein) and CCD-CL (15 µg/ml of protein). Note drastic reduction in SKES-1 cell numbers with hWJSC-CL (15 µg/ml of protein). n: Enlarged image of boxed areas in m showing cell fragmentation (indicated by arrows). CCD-CM and CCD-CL did not induce any morphological changes or have any inhibitory growth effect on SKES-1 cells (Magnification 20×; Scale bar: 100 µM).
Cell Proliferation (MTT Assay)
The osteosarcoma cell line MG-63 showed decreases in cell proliferation when exposed to hWJSC-CM (50%) at 72 h compared to controls but these decreases were not statistically significant (Fig. 2A). However, treatment of MG-63 cells with hWJSC-CL (15 µg/ml protein) showed statistically significant decreases in cell proliferation of 24.32 ± 5.67% compared to controls (Fig. 2B).
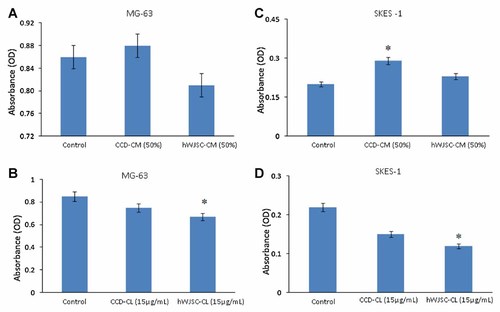
A–C: Cell proliferation (MTT assay) of MG-63 and SKES-1 cells cultured in vitro for 72 h following treatment with hWJSC-CM (50%), CCD-CM (50%), hWJSC-CL (15 µg/ml of protein), and CCD-CL (15 µg/ml of protein) for 72 h with fresh changes of hWJSC-CM and hWJSC-CL at 48 h. All values are expressed as mean ± SEM from three different replicates. Asterisks (*) indicate statistical significance at P < 0.05 compared to respective controls.
No significant changes in cell proliferation were observed when SKES-1 cells were exposed to hWJSC-CM (50%) at 72 h (Fig. 2C). However, treatment of SKES-1cells with hWJSC-CL (15 µg/ml protein) showed decreases in cell proliferation of 46.48 ± 6.66% compared to controls and these decreases were statistically significant (Fig. 2D).
Annexin V-FITC Assay
The osteosarcoma cell lines MG-63 and SKES-1 showed positive staining for annexin V-FITC following treatment with hWJSC-CM (50%) and hWJSC-CL (15 µg/ml of protein). MG-63 cells exposed to hWJSC-CM showed increases in positively stained annexin V-FITC cells of 9.75% compared to controls while exposure to hWJSC-CL showed increases of 12.39% compared to controls (Fig. 3A). SKES-1 cells treated with hWJSC-CM showed increases in positively stained annexin V-FITC cells of 5.15% compared to controls while SKES-1 cells treated with hWJSC-CL showed increases of 4.40% compared to controls (Fig. 3B).
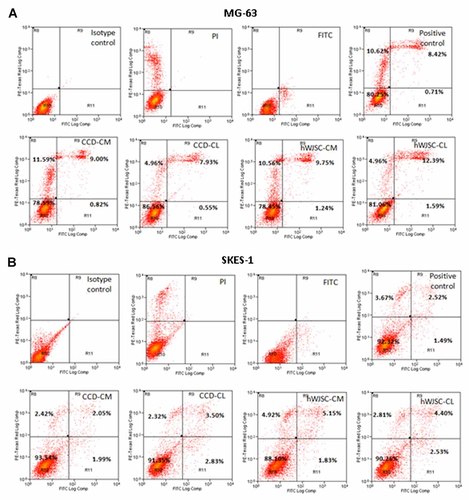
Representative contour plots (annexinV-FITC flow cytometry) of (A) MG-63 and (B) SKES-1 cells cultured in vitro for 72 h following treatment with hWJSC-CM (50%), CCD-CM (50%), hWJSC-CL (15 µg/ml of protein) and CCD-CL (15 µg/ml of protein). AnnexinV-FITC positive cells were expressed as mean ± SEM from three replicates.
TUNEL Assay
Both MG-63 and SKES-1 cells when treated alone with DNase (positive control) showed TUNEL positive cells (Fig. 4Aa and Bg). However MG-63 cells were TUNEL negative when treated with CCD-CM (50%) (Fig. 4Ac), CCD-CL (15 µg/ml of protein) (Fig. 4Ae), hWJSC-CM (50%) (Fig. 4Ad) and hWJSC-CL (15 µg/ml of protein) (Fig. 4Af) for 48 h. SKES-1 cells were TUNEL negative when treated with CCD-CM (50%) (Fig. 4Bi) and CCD-CL (15 µg/ml of protein) (Fig. 4Bk), but showed TUNEL positive cells when treated with hWJSC-CM (50%) (Fig. 4Bj) and hWJSC-CL (15 µg/ml of protein) (Fig. 4Bl) for 48 h.
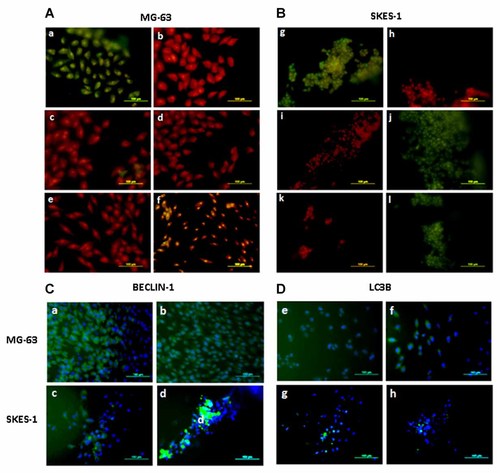
TUNEL images of (A) MG-63 and (B) SKES-1 cells cultured in vitro for 48 h following treatment with hWJSC-CM (50%), CCD-CM (50%), hWJSC-CL (15 µg/ml of protein), and CCD-CL (15 µg/ml of protein). a and g: DNAse treated positive controls; (b,h) Negative controls; (j,l) TUNEL positive SKES-1 cells treated with hWJSC-CM (50%) and hWJSC lysate (15 µg/ml of protein). Scale bar: 100 mm. Immunohistochemistry images of MG-63 and SKES-1 cells treated with (C) BECLIN-1 and (D) LC3B primary antibodies following treatment with hWJSC-CM (50%) and hWJSC-CL (15 µg/ml of protein). BECLIN-1 was positive in MG-63 cells (a,b) and negative in SKES-1 cells (c,d) and the green fluoresence in SKES-1 cells is due to the remnant unbound secondary antibody. LC3B was positive in MG-63 cells (e,f) and negative in SKES-1 cells (g,h). Scale bar: 100 mm.
Immunohistochemistry
MG-63 cells treated with hWJSC-CM (50%) and hWJSC-CL (15 µg/ml of protein) were positive for BECLIN-1 (Fig. 4Ca and Cb), whereas SKES-1 cells treated with hWJSC-CM (50%) and hWJSC-CL (15 µg/ml of protein) were negative (Fig. 4Cc and Cd). MG-63 cells treated with hWJSC-CM (50%) and hWJSC-CL (15 µg/ml of protein) were also positive for LC3B (Fig. 4De and Df) whereas SKES-1 cells treated with hWJSC-CM (50%) and hWJSC-CL (15 µg/ml of protein) were negative (Fig. 4Dg and Dh).
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Treatment of MG-63 (Fig. 5A) and SKES-1 (Fig. 5C) with hWJSC-CM (50%) for 72 h showed increased expression of the pro-apoptotic BAX gene and decreased expression of the anti-apoptotic BCL2 and SURVIVIN genes (Fig. 5A and C). Treatment with hWJSC-CM (50%) showed increases in BAX by 2.76- and 0.86-fold for MG-63 and SKES-1 respectively (Fig. 5A and C). The expression of the anti-apoptotic gene BCL2 was decreased by 0.94- and 0.87-fold for MG-63 and SKES-1 respectively and SURVIVIN was decreased by 0.81- and 0.63-fold for MG-63 and SKES-1 respectively. Exposure to hWJSC-CL (15 µg/ml protein) showed increases in pro-apoptotic BAX gene expression of 1.39- and 1.32-fold for MG-63 and SKES-1 respectively while BCL2 expression was decreased by 0.95- and 0.91-fold and SURVIVIN decreased by 0.9- and 0.89-fold for MG-63 and SKES-1 respectively (Fig. 5B and D).
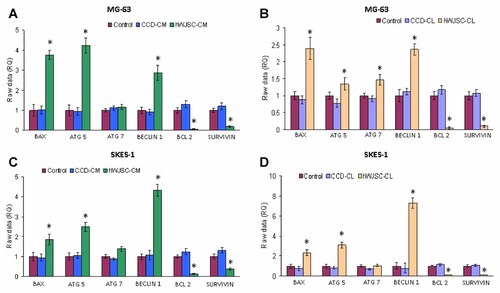
Gene expression profiles (qRT-PCR) of the pro-apoptotic related gene (BAX), autophagyrelated genes (ATG5, ATG7, and BECLIN-1), and anti-apoptotic related genes (BCL2 and SURVIVIN) in (A) MG-63 cells treated with hWJSC-CM (50%) and CCD-CM (50%) for 72 h; (B) MG-63 cells treated with hWJSC-CL (15 mg/ml protein) and CCD-CL (15 mg/ml protein) for 72 h (C) SKES-1 cells treated with hWJSC-CM (50%) and CCD-CM (50%) for 72 h (D) SKES-1 cells treated with hWJSC-CL (15 mg/ml protein) and CCD-CL (15 mg/ml protein) for 72 h. The results are expressed as means ± SEM from three replicates. Data analysis and relative quantitation was done using the comparative Ct (DDCt) method.
The autophagy-related genes ATG-5 and BECLIN-1 had increased expression by 2.09- and 6.28-fold for SKES-1 following treatment with hWJSC-CM (50%) (Fig. 5C) while exposure of MG-63 cells to hWJSC-CL (15 µg/ml of protein) showed increased expression of the autophagy-related gene ATG 7 by 0.47-fold (Fig. 5B).
Attenuation of Mammary Carcinomas In Vivo by hWJSCs and hWJSC-CM
Ectopic mammary adenocarcinomas developed in 90% of the SCID mice transplanted with MDA-MD-231 mammary carcinoma cells. After intra-tumoral injections of hWJSCs and hWJSC-CM, tumor sizes and tumor weights decreased by 37.03 ± 5.9% and 47.14 ± 7.36% respectively by 6 weeks in all the sub-groups of mice of Group A (early tumor protocol) compared to the controls (Fig. 6A–C). For the late tumor protocol (Group B), tumor sizes and tumor weights decreased by 24.86 ± 6.05% and 55.09 ± 5.87% respectively by 6 weeks in all sub-groups of mice. Tumor attenuation was greater in the late tumor protocol compared to the early tumor protocol (Fig. 6A–C).
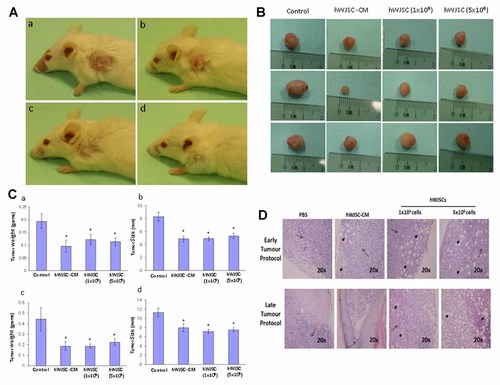
Inhibition of mammary adenocarcinomas in xenograft immunodeficient mice. A: Representative images of four groups of mice (a) control; (b) hWJSC-CM (50%); (c) hWJSCs (1 × 106); and (d) hWJSCs (5 × 106) following treatment with hWJSCs and hWJSC-CM showing gradual attenuation of subcutaneous mammary adenocarcinomas after 6 weeks. B: Representative images of dissected tumours from the same four groups of mice at 6 weeks. Gross tumour sizes became smaller with time in the treated groups compared to controls. C: Histograms showing dissected tumour sizes and wet weights after 6 weeks. Wet weights tumours showed decreases of 47.14 ± 7.36% in the treated groups [hWJSCs and hWJSC-CM (50%)] compared controls. Tumour sizes reduced by 37.03 ± 5.9% in the treated groups [hWJSCs and hWJSC-CM (50%)] compared controls (n = 6 per group). D: Haematoxylin and eosin (H&E) stained images of tumour sections from treated and control animals. Lymphocytic infiltrations (arrows) and vacuolations (arrow-heads) were observed in cells in the treatment group. Magnification: 200×.
Tumor attenuation effects were greatest in sub-groups III (Groups A and B) (hWJSCs: 5 × 106) followed by sub-group II (Groups A and B) (hWJSCs: 1 × 106) and sub-group IV (Groups A and B) (50% hWJSC-CM) compared to the control sub-groups I (Groups A and B) (PBS) (Fig. 6A–C). Histopathology of tumor tissues showed increased lymphocytic infiltration and vacuolation of the tumor cells in the hWJSC and hWJSC-CM (50%) treated sub-groups compared to the controls (Fig. 6D). Evaluation of complete abrogation of the tumors after 6 weeks could not be assessed as approval for the study protocol was given for only 6 weeks so as to avoid undue distress to the animals.
DISCUSSION
During early human development, stem cells originating from the yolk sac (YS) and aorta-gonad-mesonephros (AGM) undergo two waves of migration [Wang et al., 2008; Taghizadeh et al., 2011]. They first migrate from the YS and AGM through the umbilical cord to the placenta and then reverse-migrate from the placenta via the umbilical cord to home in the fetal liver and bone marrow. En route these stem cells get trapped in the gelatinous Wharton's jelly of the umbilical cord where they reside, multiply and undergo transformation in their new environment. Even though these hWJSCs and the homed bone marrow MSCs have a common origin they bear unique differences [Huang et al., 2012].
hWJSCs are an uncontroversial source and unlike bone marrow MSCs can be harvested painlessly in abundance from human umbilical cords. They grow as adherent cells with a mesenchymal morphology, are self-renewing, express high levels of MSC markers (CD10+, CD13+, CD29+, CD44+, CD90+, CD14−, CD33−, CD56−, CD31−, CD34−, CD45−), express low levels of some hESC markers (OCT4, NANOG, SOX2) and can be differentiated into a wide spectrum of desirable tissues [Fong et al., 2007, 2010; Karahuseyinglu et al., 2007; Chao et al., 2008; Troyer and Weiss, 2008; Weiss et al., 2008; La Rocca et al., 2009; Fan et al., 2011]. They are tolerated in preclinical xenograft models without the need for immunosuppression therapy [Weiss et al., 2003; Medicetty et al., 2004] express HLA-G, MHC class I antigens and possess immunosuppressive properties in splenocyte proliferation and mixed lymphocyte reaction assays [Weiss et al., 2008]. They can thus be used in both allogeneic and autologous settings without the need for tissue typing and without induction of graft versus host disease [Weiss et al., 2008].
We undertook a series of studies recently to examine the role of human umbilical cord Wharton's jelly stem cells (hWJSCs) as an anticancer agent after it was shown by several independent groups [Rachakatla et al., 2007; Ayuzawa et al., 2009; Sun et al., 2010; Chao et al., 2011] that these cells attenuate or abolish mammary carcinoma cells in vitro and in vivo in animal models. We first established that hWJSCs do not generate tumours in immunodeficient mice unlike human embryonic stem cells (hESCs) when transplanted at high doses and at various injection sites [Gauthaman et al., 2012b]. Unlabelled hESCs + matrigel, labelled hWJSCs (green fluorescent protein, GFP), and unlabelled hWJSCs + matrigel were injected via three routes [subcutaneous (SC); intramuscular (IM); intraperitoneal (IP)] into SCID mice. The animals that received hESCs + matrigel developed teratomas in 6 weeks (75–100%) while none of the animals that received hWJSCs developed tumors or inflammatory reactions at the injection sites when monitored for periods for as long as 20 weeks. Positive staining for the anti-human nuclei antibody (green) indicated the presence of viable hWJSCs at the injection sites at 20 weeks [Gauthaman et al., 2012b]. We also confirmed that hWJSCs unlike bone marrow mesenchymal stem cells (MSCs) do not transform to tumor-associated fibroblasts (TAFs) that are involved in enhanced tumor growth and metastasis [Subramanian et al., 2012].
We then confirmed that hWJSCs and their extracts [conditioned medium (hWJSC-CM) and cell lysate (hWJSC-CL)] inhibited the growth of mammary carcinoma (MDA-MB-231), osteosarcoma (OS) (MG-63) and ovarian carcinoma (TOV-112D) cells in vitro and of these three tumors the anticancer effect was most pronounced on OS cells [Gauthaman et al., 2012a]. All three cancer cell lines showed cell shrinkage, blebbing, and vacuolations when exposed to hWJSC-CL and hWJSC-CM compared to controls. MTT and BrdU assays showed inhibition of cell growth by 2–6% and 30–60% while the Transwell migration assay showed inhibition by 20–26% and 31–46% for hWJSC-CM and hWJSC-CL respectively for all three cancer cell lines. Increases in sub-G1 and G2/M phases of the cell cycle were noted for all three cancer cell lines suggestive of apoptosis and metaphase arrest. Annexin V-FITC and TUNEL positive cells were observed in TOV-112D and MDA-MB-231 suggesting that inhibition was via apoptosis while the presence of anti-BECLIN1 and anti-LC3B antibodies seen with MG-63 indicated that inhibition was via autophagy. Upregulation of the pro-apoptotic BAX gene and downregulation of anti-apoptotic BCL2 and SURVIVIN genes were observed in all three cancer cell lines and additionally the autophagy genes (ATG5, ATG7, BECLIN1) were upregulated in MG-63 cells. We concluded that hWJSCs possess tumour inhibitory properties that are not specific to breast cancer cells alone and these effects are possibly mediated via agents in its extracts.
The results of the present study which is an extension of our previous work are consistent with the reports of other workers who showed that hWJSCs attenuate or abolish mammary carcinomas. Based on our previous reports [Gauthaman et al., 2012a] and the present study it appears that these anticancer effects of hWJSCs, hWJSC-CM, and hWJSC-CL can be induced both in vitro and in vivo. It is interesting to note that the hWJSC-CM and hWJSC-CL anticancer effects occurred on both OS cell lines with the effects on SKES-1 being more pronounced than MG-63. As SKES-1 cells demonstrated more fragmentation and underwent rapid cell death, autophagy-related vacuolations or protein expression were not clearly identified. However, the gene expression profiles showed that both autophagy and apoptotic-related genes were increased in both MG-63 and SKES-1 indicating that there were overlapping mechanisms of autophagy and apoptosis resulting in final cell death. Unfortunately, we were unable to induce OS tumors as xenografts in animals to confirm the hWJSC in vivo anticancer effects. The inhibitory effects on OS cells seen by the secretions and cell-free lysates of hWJSCs in the present study suggest that certain factors released by the hWJSCs have anticancer effects. Some of these factors are interleukins, cell adhesion molecules, hyaluronic acid, glycosoaminoglycans, and growth factors [Friedman et al., 2007; Angelucci et al., 2010; Fong et al., 2011b].
Using DNA microarrays and GO Biological Process analysis, we compared a detailed transcriptome profiling of hWJSCs with that of a panel of known hESCs, hMSCs, and stromal cells [Fong et al., 2011a]. We showed that several pro-apoptotic and tumor suppressor genes were upregulated in hWJSCs. These genes may be responsible for the non-tumorigenecity and anti-tumorigenecity seen with hWJSCs. Our transcriptome studies also confirmed that several cytokines were upregulated in the hWJSCs including IL12A which is associated with the induction of apoptosis and thus may be one of the important molecules involved in the anticancer effects of hWJSCs, hWJSC-CM, and hWJSC-CL. IL12A and IL12B code for the p35 and p40 subunit of the IL12 heterodimer respectively [Kobayashi et al., 1989; Wolf et al., 1991] and IL12 has been shown to be a potent anti-tumour agent [Colombo and Trinchieri, 2002; Weiss et al., 2007]. IL8, IL1B, and IL6 which were also highly expressed in the hWJSCs act as mediators in the inflammatory response [Moser et al., 2004] and the secretion of IL8 by hWJSCs at the tumour site may attract immune cells to kill the cancer cells.
Hyaluronan oligosaccharides were reported to inhibit the tumorigenicity of the osteosarcoma cell lines (MG-63 and LM-8) [Hosono et al., 2007] and GAGs was shown to inhibit the cell proliferation of osteoblasts and osteosarcoma cells [Nikitovic et al., 2005]. Thus the anticancer effects of hWJSCs, hWJSC-CM, and hWJSC-CL on MG-63 and SKES-1 in the present study may also be related to changes in the dynamics of HA and GAGs.
Osteosarcomas (OS) are tumours of MSC origin, contain cancer stem cells (CSCs) and have been claimed to be the ideal model for future CSC targeted therapies [Siclari and Qin, 2010]. They are the most common primary malignant tumor in children and young adults, is locally very destructive and has a high metastatic potential. Despite the improvement in the 5-year survival rate of this high grade malignancy using a combination of surgery, adjuvant and neo-adjuvant therapy, the cure rate is poor due to pulmonary metastasis [Guise et al., 2009; Tan et al., 2009]. It was postulated that this may due to the presence of quiescent and slow cycling CSCs which remain refractory to therapy. It was therefore suggested that treatment should be targeted at the CSCs in OS tumors [Guise et al., 2009; Suva et al., 2009; Tan et al., 2009; Siclari and Qin, 2010]. Since osteosarcomas have MSC properties and contain CSCs, future studies on rational therapeutic targeting of these tumors with hWJSCs, hWJSC-CM, or hWJSC-CL are warranted.
When all these studies are taken together, it can be concluded that hWJSCs and their products (hWJSC-CM and hWJSC-CL) are both non-tumorigenic and anti-tumorigenic and may therefore be safe and novel candidates for future cancer therapy. Most importantly, the products of hWJSCs viz., hWJSC-CM and hWJSC-CL have paradoxical anti-tumorigenic properties and may be less controversial than cells to regulatory bodies for clinical application.




