MURF1 deficiency suppresses unloading-induced effects on osteoblasts and osteoclasts to lead to bone loss
Abstract
Loss of mechanical stress or unloading causes disuse osteoporosis that leads to fractures and deteriorates body function and affects mortality rate in aged population. This bone loss is due to reduction in osteoblastic bone formation and increase in osteoclastic bone resorption. MuRF1 is a muscle RING finger protein which is involved in muscle wasting and its expression is enhanced in the muscle of mice subjected to disuse condition such as hind limb unloading (HU). However, whether MuRF1 is involved in bone loss due to unloading is not known. We therefore examined the effects of MuRF1 deficiency on unloading-induced bone loss. We conducted hind limb unloading of MuRF1 KO mice and wild-type control mice. Unloading induced about 60% reduction in cancellous bone volume (BV/TV) in WT mice. In contrast, MuRF1 deficiency suppressed unloading-induced cancellous bone loss. The cortical bone mass was also reduced by unloading in WT mice. In contrast, MuRF1 deficiency suppressed this reduction in cortical bone mass. To understand whether the effects of MuRF1 deficiency suppress bone loss is on the side of bone formation or bone resorption, histomorphometry was conducted. Unloading reduced bone osteoblastic formation rate (BFR) in WT. In contrast, MuRF1 deficiency suppressed this reduction. Regarding bone resorption, unloading increased osteoclast number in WT. In contrast, MURF1 deficiency suppressed this osteoclast increase. These data indicated that the ring finger protein, MURF1 is involved in disuse-induced bone loss in both of the two major bone remodeling activities, osteoblastic bone formation and osteoclastic bone resorption. J. Cell. Biochem. 112: 3525–3530, 2011. © 2011 Wiley Periodicals, Inc.
Loss of mechanical stress or unloading causes disuse osteoporosis leading to fractures and deteriorates body function [Sambrook et al., 1987] and these fractures increase mortality rate in aged population [Lyles et al., 2007]. Bone mass is under the influence of mechanical stress that affects not only growing bone but also adult bone metabolism [Judex et al., 2009]. Therefore, when patients are aged, bone mass is further reduced and fracture risk is highly increased. In addition, cardiovascular diseases and brain diseases are additional major causes for bedridden situation and hence bone loss associated with unloading. Even in general physical point of view, reduced physical activity per se in aged patients also significantly reduces bone mass.
This disuse-induced bone loss and osteoporosis is not limited in aged population but also is seen in young adults when these patients are suffering from pain-induced reduction of joint problems or immobilized. Disuse-induced bone loss occurs even in healthy astronauts clearly. This phenomenon is acutely seen in the case of bone loss in space flight but also lasts long even after the astronauts return to earth. Thus, the link between disuse and bone loss is well established. The cells involved in such bone loss are both osteoblasts and osteoclasts. Unloading reduces osteoblastic bone formation and increases osteoclastic bone resorption. However, the molecular mechanism underlying the changes of these cellular activity and disuse-induced bone loss is incompletely understood.
MURF1 is thought to be a muscle specific ubiquitin ligase containing ring finger domain, B box, and coiled-coil domain. In disuse atrophy, muscle protein is ubiquitinated by ubiquitin ligase and degraded by proteasome [Spencer et al., 2000; McElhinny et al., 2002]. MURF1 KO mice is shown to suppress denervation-induced muscle atrophy [Bodine et al., 2001]. However, the role of MuRF1 in disuse-induced bone loss in not known. Therefore, we examined whether MURF1 is involved in osteoblastic bone formation and osteoclastic bone resorption that lead to bone loss due to unloading based on hind limb suspension.
MATERIALS AND METHODS
Animals
Female wild type (WT) or muscle-specific RING finger-1 (MURF1) deficient mice (20 weeks old) were used in the experiments. WT and MURF1 deficient mice were randomized regarding body weight into two groups. The two groups were subjected either to hind limb unloading (HU) or normal housing (loading) (NL) [Ishijima et al., 2001; Ono et al., 2007; Mizoguchi et al., 2008]. Mice were housed for at least 1 week prior to the study. All the mice were injected intraperitoneally with calcein at 4 mg/kg at 4 and 2 days before sacrifice. All animal experiments were approved by the Animal Welfare Committee of our institute.
Hind Limb Unloading (HU) Model
HU was conducted by applying a tape to the surface of the hind limb to set a metal clip [Ishijima et al., 2001; Ono et al., 2007; Mizoguchi et al., 2008]. The other end of the clip was fixed to an overhead bar. The height of the bar was adjusted to maintain the mice at 30° head down tilt with the hind limbs elevated above the floor of the cage. The mice were subjected to hind limb unloading for 14 days. Loaded control mice were also housed under the same conditions except for hind limb unloading for the same duration.
Body Weight
The body weight of the mice was monitored during the experimental period. There were no significant changes in body weight in any of the groups during the course of the study. This confirmed that stress could be considered minimal in our experiments as previously described [Ishijima et al., 2001; Ono et al., 2007; Mizoguchi et al., 2008].
Two-Dimensional (2D) and Three-Dimensional (3D) Micro-Computed Tomography (CT) Analysis and DEXA Analysis of Bone
Bone volume/tissue volume (BV/TV) was measured by both 2D and 3D micro-CT. For 2D micro-CT analysis we used Musashi (Nittetsu-ELEX Co., Kita-Kyushu City, Japan). The threshold level for the measurements was set at 110 for the analyses [Ishijima et al., 2001; Ono et al., 2007; Mizoguchi et al., 2008]. To evaluate the thickness of cortical envelope of distal femur, cross-sections at the midshaft of the femora were analyzed by 2D micro-CT, and the thickness was measured at defined five points. For 3D micro-CT analysis we used Scan-Xmate-E090 (Comscan Techno Co., Ltd, Sagamihara, Japan) and TRI/3D-Bon (Ratoc System Engineering Co., Ltd, Tokyo, Japan) as computer software. BV/TV were analyzed in the secondary trabecular regions from around 0.35 to 0.7 mm away from the chondro-osseous junction. Pixi was used to measure BMD.
Histomorphometric Analysis of Bone
At the end of the experiments, the femora of each mouse were removed and fixed in 70% ethanol. Serial 4-µm-thick sagittal sections were made as undecalcified sections. For bone formation rate (BFR), metaphyseal cancellous bone was used to obtain bone fraction in a rectangular area of 0.34 mm2 (0.5 mm × 0.67 mm) with its closest and furthest edges at 0.3 and 0.8 mm distal to the growth plate, respectively. For decalcified sections, the left tibiae of the mice were removed at the end of the experiments and fixed in 4% paraformaldehyde and then decalcified in 20% EDTA. Serial 5-µm-thick sagittal sections were made using a microtome and stained for tartrate-resistant acid phosphatase (TRAP). TRAP-positive multinucleated cells attached to bone were counted as osteoclasts. Measurements were made within an area of 0.24 mm2 (0.6 mm × 0.4 mm), with its closest and furthest edges at 0.3 and 0.7 mm distal to the growth plate of the proximal ends of the tibiae. Number of osteoclasts (Oc.N/BS) and osteoclast surface (Oc.S/BS) was analyzed to quantify the bone resorbing activity as before [Ishijima et al., 2001; Ono et al., 2007; Mizoguchi et al., 2008].
Statistical Analysis
All data were shown as means and standard deviation (n = 6 per group), and statistical evaluation was performed based on 2-way ANOVA using a statistical software package for Windows, Statview version 5.0 (SAS Institute). A P-value less than 0.05 was considered to be statistically significant.
RESULTS
MURF1 Deficiency Suppresses Unloading-Induced Reduction in Cancellous Bone Mass
We examined the effects of MURF1 deficiency on unloading-induced bone loss in trabecular regions. The bone volume/tissue volume (BV/TV) was analyzed by three-dimensional micro-computed tomography (3D-micro-CT). The metaphyseal region of micro-CT analysis is shown in Figure 1A. In WT mice, unloading-induced sparse pattern of cancellous bone compared to denser trabecular bone patterning (Fig. 1A, left two panels). MURF1 deficiency by itself did not alter the pattern of trabecular bone. However, MURF1 deficiency reduced the unloading-induced changes in the pattern of trabecular bone (Fig. 1A, right two panels).
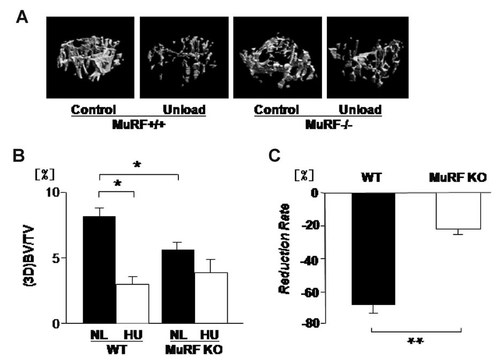
MuRF1 deficiency decreases cancellous bone mass and prevents unloading-induced reduction in femoral cancellous bone (3D micro-CT). Mice were hind limb unloaded (HU) or normally loaded (NL) for 14 days. Bone volume/tissue volume (BV/TV) of femur was analyzed by three-dimensional micro-CT (A). In WT, HU reduced BV/TV, but in MuRF1 deficiency (MURF1 KO) were no effect by HU. MURF1 deficiency by itself decreased the BV/TV (B). In MURF1 deficiency mice, HU induced BV/TV reduction was suppressed (B) and reduction rate which is calculated as “Reduction Rate = {(Control − Unload)/Control} × 100(%)” is lower than WT (C). Data shown are mean ± SD and were analyzed statistically by 2-factor ANOVA, with MURF1 deficiency and loading condition as factors. *P ≤ 0.05 and **P ≤ 0.01 were accepted as significant.
Quantification of cancellous bone mass based on three-dimensional micro-CT indicated that unloading significantly reduced bone mass (by over 50%) in wild type (Fig. 1B). MURF1 deficiency per se slightly reduced the cancellous bone mass in normal loaded condition (Fig. 1B, lane 1 vs. lane 3). In contrast to wild type, MURF1 deficiency suppressed the unloading-induced reduction in bone mass (Fig. 1B). In MURF1 deficient mice, the reduction rate is suppressed compared to WT (Fig. 1C).
MURF1 Deficiency Suppresses Unloading-Induced Reduction in Cortical Bone Mass
In addition to cancellous bone, effects of MURF1 deficiency on cortical bone mass was also examined based on two-dimensional micro-CT. In WT, unloading reduced cortical bone mass (Fig. 2A, lane 1 vs. lane 2). In contrast, MURF1 deficiency suppressed such unloading-induced cortical bone loss (Fig. 2A, lane 3 vs. lane 4). Another parameter of cortical bone quantification is the cortical thickness of the midshaft region of the bone. Unloading reduced cortical thickness in wild type (Fig. 2B, lane 1 vs. lane 2). In contrast, MURF1 deficiency suppressed such unloading-induced reduction in cortical thickness (Fig. 2B, lane 3 vs. lane 4). Interestingly, MURF1 deficiency slightly enhanced the cortical bone mass (Fig. 3A, lane 1, lane 3) and cortical bone thickness (Fig. 3B, lane 1 vs. lane 3) compared to WT mice under normal loaded condition. These data indicated that MURF1 deficiency suppresses not only unloading-induced reduction in cancellous bone mass but also that of cortical bone mass.
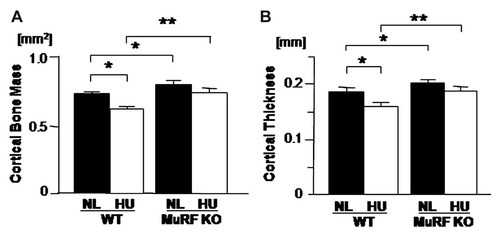
MURF1 deficiency prevents unloading-induced reduction in cortical envelope. Mice were hind limb unloaded (HU) or normally loaded (NL) for 14 days. The long axis of the femora at the levels one-half were analyzed by two-dimensional-micro-computed tomography (CT). In WT, unloading reduced cortical bone mass (A) and thickness (B). MuRF1 deficiency by itself increased the cortical bone mass (A) and thickness (B). In MuRF1 deficiency mice, HU induced cortical bone mass (A) and thickness (B) reduction was suppressed. Data shown are mean ± SD and were analyzed statistically by 2-factor ANOVA, with MURF1 deficiency and loading condition as factors. P ≤ 0.05 and **P ≤ 0.01 were accepted as significant.

MURF1 deficiency suppresses unloading-induced reduction and bone mineral density (BMD). Mice were hind limb unloaded (HU) or normally loaded (NL) for 14 days. For BMD analysis, whole femur was subjected to dual energy X-ray absorptiometry (DEXA). HU reduced BMD in WT, but in MURF1 deficiency (MURF1 KO) were no effect by HU both femur (A). MURF1 deficiency by itself increased the femoral BMD (A). Reduction rate of BMD was calculated as “Reduction Rate = {(Control − Unload)/Control} × 100(%)” (B). Data shown are mean ± SD and were analyzed statistically by 2-factor ANOVA, with MURF deficiency and loading condition as factors. P ≤ 0.05 and **P ≤ 0.01 were accepted as significant.
MURF1 Deficiency Suppresses Unloading-Induced Reduction in Bone Mineral Density (BMD)
In terms of BMD, whole femora were analyzed by dual X-ray absorptiometry (DEXA). Unloading (HU) reduced the BMD in WT compared to normally loaded (NL) mice (Fig. 3A). In contrast, MURF1 deficiency suppressed such unloading-induced reduction in bone mineral density (Fig. 3A). MURF1 deficiency suppressed the reduction rate of BMD compared to wild type (Fig. 3B). Thus, MURF1 deficiency suppresses unloading-induced loss of bone mineral density in bone in addition to its suppression on the unloading-induced structural deteriorations (BV/TV).
MURF1 Deficiency Suppresses Unloading-Induced Reduction in Osteoblastic Bone Formation
To elucidate whether MURF1 deficiency affects bone mass via altering cellular activity underlying bone formation or bone resorption or both, the dynamic parameters for bone formation was examined. Unloading reduced osteoblastic BFR (Fig. 4A,B). In contrast, MURF1 deficiency suppressed unloading-induced reduction in BFR (Fig. 4B). Mineralizing surface levels (MS/BS) were reduced by unloading (Fig. 4C). In contrast, MURF1 deficiency suppressed unloading-induced reduction in mineralizing surface (Fig. 4C). Thus, MURF1 deficiency suppresses unloading-induced reduction of bone formation.
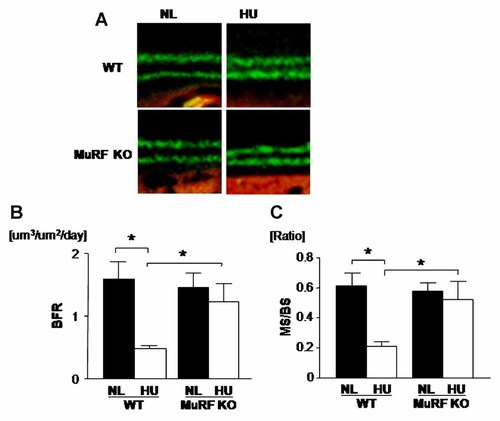
MURF1 deficiency prevents unloading-induced reduction in BFR. Mice were hind limb unloaded (HU) or normally loaded (NL) for 14 days and were injected intraperitoneally with calcein 24 and 48 h prior sacrifice (A). In WT, HU reduced bone formation rate (BFR) (B) and mineralized surface/bone surface (MS/BS), but in MURF1 deficiency (MuRF1 KO) were no effect by HU. Unloading reduced distance of calcein double label in both WT and MURF KO (C) which mean HU reduced mineral apposition rate in both WT and MURF KO (data not shown). Data shown are mean ± SD and were analyzed statistically by 2-factor ANOVA, with MURF deficiency and loading condition as factors. *P ≤ 0.05 was accepted as significant.
MURF1 Deficiency Suppresses Unloading-Induced Increase of Osteoclast Number
Osteoclastic bone resorption is a critical catabolic side of the bone metabolism. Therefore, we investigated the role of MURF1 to obtain osteoclast number and osteoclast surface. In the WT, unloading increased the appearance of osteoclasts in vivo as reported previously (Fig. 5A, WT). Quantification indicated that unloading significantly increased the number of osteoclasts per bone surface (Fig. 5B). Interestingly, MURF1 deficiency by itself enhanced base line osteoclast number in loaded mice compared to WT normal loaded animals (Fig. 5B, lane 1 vs. lane 3). In contrast to WT, MURF1 deficiency suppressed unloading-induced increase in osteoclast number (Fig. 5B, lane 3, lane 4). Unloading also enhanced the levels of osteoclast surface per bone surface (indicator of osteoclast spreading and activity) in WT (Fig. 5C, lane 1 vs. lane 2). MURF1 deficiency per se enhanced osteoclast surface levels under loaded condition (Fig. 5C, lane 1 vs. lane 3). In contrast to WT, MURF1 deficiency suppressed unloading-induced increase in osteoclast surface (Fig. 5C, lane 3 vs. lane 4). Thus, MURF1 deficiency suppresses unloading-induced increase in bone resorption in addition to its suppression of the unloading-induced reduction in bone formation.
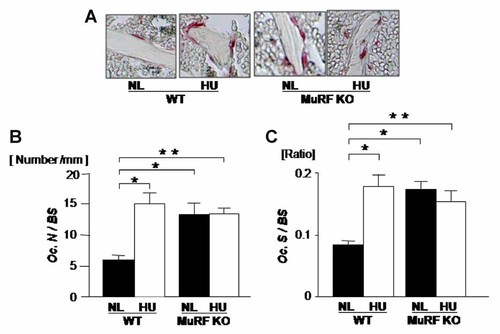
MURF1 deficiency increased osteoclast number and no further increase was not observed by unloading. Mice were hind limb unloaded (HU) or normally loaded (NL) for 14 days. Decalcified sections were made from proximal tibia and stained with tartrate-resistant acid phosphatase (TRAP) for osteoclast analysis. In WT, HU increased osteoclast number/bone surface (Oc.N/BS) (B) and osteoclast surface/bone surface (Oc.S/BS), but in MuRF1 deficiency (MuRF1 KO) were no effect by HU. MuRF1 deficiency by itself increased the Oc.N/BS (B) and Oc.S/BS (C). Data shown are mean ± SD and were analyzed statistically by 2-factor ANOVA, with MURF deficiency and loading condition as factors. *P ≤ 0.05 and **P ≤ 0.01 were accepted as significant.
DISCUSSION
Unloading leads to rapid loss of bone to result in disuse osteoporosis, but the molecular basis for the cellular activities underlying this phenomena is still incompletely understood. We discovered that MURF1 is a novel molecule involved in osteoblastic side as well as osteoclastic side of disuse-induced bone loss.
We conducted unloading experiment to setup disuse condition using MURF1 KO mice and wild-type (WT) control mice. Trabecular bone volume (BV/TV) analysis indicated that unloading significantly reduced trabecular bone volume in WT mice. In contrast, MURF1 deficiency suppressed such reduction in bone mass. Moreover, MURF1 deficiency suppresses both of the two characteristic features of disuse osteoporosis, namely unloading-induced reduction in osteoblastic bone formation and unloading-induced enhancement of osteoclastic bone resorption. Thus, MURF1 is involved in the double features of rapid reduction of bone mass induced in disuse osteoporosis.
Since MURF1 is known to function in protein degradation, it is of interest if this molecule is playing its role in bone in a way to alter protein degradation. We have previously observed that osteopontin is playing a role in unloading-induced bone loss. Similar to MURF1 deficiency, osteopontin deficiency suppresses both unloading-induced reduction in osteoblastic bone formation as well as unloading-induced increase in osteoclastic bone resorption. It was reported that some of the actions of osteopontin could be taking place intracellularly [Shinohara et al., 2006; Ono et al., 2008; Shinohara et al., 2008; Cantor and Shinohara, 2009]. If MURF1 is targeting some proteins related to osteopontin function is still to be elucidated [Ishijima et al., 2007; Chung et al., 2008; Morishita et al., 2011].
Cortical bone mass is the important component of bone to support the physical activity and thus it is notable that MURF1 deficiency reduces unloading-induced loss in cortical bone envelope in the midshaft of femur. Especially, MURF1 deficiency suppresses unloading-induced loss of BMD and this is corresponding to its effects on structural deterioration induced by unloading. There was a small but statistically significant increase in cortical bone mass in MURF1 deficient mice. This is an interesting observation, however the reason for the increase of cortical bone mass by MURF1 deficiency is not known at this point.
MURF1 deficiency per se also increased osteoclast number. Such increase may be an indication of cell type dependent effect of MURF1 MURF1X deficiency alone reduced the bone mass levels and the increase in osteoclasts may be related to this observation.
In conclusion, we found that MURF1 is a new molecule that involved in unloading and disuse-induced changes in osteoblasts and osteoclasts that lead to bone loss.




