Signal transduction pathways mediating the effect of adrenomedullin on osteoblast survival
Abstract
Adrenomedullin (ADM) plays an important role in the regulation of osteoblastic cells through both a proliferative and an anti-apoptotic effects. The present study investigated mechanisms involved in the effect of ADM on survival. We report that ADM can act in osteoblasts both through a non-transcriptional action, by phosphorylation of different kinases and components, and through a transcriptional effect by activation of CREB. So, we observed by Western blot analysis, modifications in the downstream targets of ERK, the pro-apoptotic protein Bad, which is inactivated by increase in Ser155 phosphorylation, and the transcription factor CREB, which is activated by phosphorylation at Ser133. CREB activation was confirmed by a CRE-dependent gene transcription assay and an immunocytochemical study. This increase in CREB phosphorylation could lead to its enhanced transcriptional activity, as indicated by the induced expression of the proliferation marker, PCNA. Moreover, ADM could also activate the tyrosine kinase Src and the PI3-Kinase, both of which are implicated in survival. The use of specific pharmacological inhibitors allowed to establish that ADM could activate a signaling cascade involving Src, MEK, ERK, p90RSK, and that the effect of ADM, in particular on the CREB protein, greatly depends on the regulatory control of interfering signaling pathways. Moreover, as Wnt signaling plays an important role in the control of osteoblast apoptosis, we explored a major component of this pathway, protein GSK3β. ADM-induced inactivation of GSK3β by phosphorylation at Ser9, highly suggests that ADM could also exert its survival effect in osteoblast via components of the Wnt pathway. J. Cell. Biochem. 112: 3807–3815, 2011. © 2011 Wiley Periodicals, Inc.
Adrenomedullin (ADM) is a 52-amino acid multi-functional regulatory peptide, originally isolated from human pheochromocytomas [Kitamura et al., 1993]. ADM belongs to the calcitonin/calcitonin gene-related peptide family [Muff et al., 1995]. ADM receptors, like CGRP receptors, are formed by the association of the calcitonin receptor-like receptor (CLR), a seven-transmembrane domain class B G protein-coupled receptor and a receptor activity modifying protein (RAMP). Co-expression of the CLR and RAMP-2 or RAMP-3 generates an ADM receptor, while co-expression of the CLR and RAMP1 generates a calcitonin gene-related peptide (CGRP) receptor [McLatchie et al., 1998]. Many of the biological actions of ADM are mediated by selective ADM receptors or the type-1 CGRP receptor [Hay and Smith, 2001]. ADM is expressed in many normal and tumoral tissues and cells [Ichiki et al., 1994; Hay et al., 2010]. In addition to its potent vasodilatory effect, many functions have been ascribed to adrenomedullin, such as proliferation, anti-apoptosis, differentiation, migration, and angiogenesis [Cuttitta et al., 2002; Shichiri and Hirata, 2003]. ADM exerts a growth-promoting action in many tissues and cells in an autocrine–paracrine manner [Shichiri et al., 2003].
In bone, osteoblastic cells express both ADM and the receptor components CLR and RAMPs and ADM exerts an autocrine–paracrine function in these cells [Naot et al., 2001; Uzan et al., 2004]. CGRP expression has also been observed in osteoblasts [Drissi et al., 1997] so as functional CGRP receptors [Vignery and McCarthy, 1996]. ADM has been reported to exert potent bone-stimulatory actions in vivo and to stimulate osteoblast proliferation in vitro [Cornish et al., 1997; Naot et al., 2001; Hamada et al., 2002]. In a previous study, we reported that in addition to its proliferative effect, the action of ADM in osteoblastic cells may also imply the inhibition of apoptosis [Uzan et al., 2008]. It was effectively observed that ADM treatment inhibited apoptosis induced by the withdrawal of growth factors in osteoblastic cells. This protective effect of ADM was partly antagonized by the (8–37) CGRP fragment, an antagonist of CGRP1 receptors, which suggests an action of ADM via CGRP receptors in osteoblastic cells. CGRP was also shown to be anti-apoptotic in these cells. Preliminary investigations of the signaling pathways implicated in the anti-apoptotic effect of ADM allowed to establish a role of the mitogen-activated protein kinase (MAPK), extracellular signal-regulated protein kinase (ERK1/2) signaling pathway, as already reported for its proliferative effect [Cornish et al., 2004], but did not show variations in the apoptotic factors Bax, Bcl-2, bcl-xL [Uzan et al., 2008]. The downstream MAPK signaling transduction cascade that mediates ADM-specific survival signals in osteoblasts therefore remains to be established.
Of the downstream targets of MAPK pathway effector proteins, the 90-kDa ribosomal S6 kinase (p90RSK) is a serine/threonine kinase activated by ERK1/2 phosphorylation [Dalby et al., 1998]. RSK is a major regulator of ERK-mediated cell survival [Shimamura et al., 2000] and one crucial target of RSK is the pro-apoptotic protein Bad. The phosphorylation of Bad by RSK is known to inhibit its pro-apoptotic function. Bad belongs to the Bcl-2 family of apoptotic factors, and it is a pro-apoptotic factor that is rendered inactive by phosphorylation. It can be phosphorylated at at least three serine residues, Ser112, Ser136, and Ser155 and possibly at a fourth Ser170 residue. Phosphorylation of one residue inactivates Bad by regulating its interaction either with Bcl-2 or Bcl-xL, or with the 14-3-3 protein, which induces its cytosolic sequestration [Downward, 1999; Masters et al., 2001]. It has been reported that activated p90RSK induces Ser155 Bad phosphorylation [Tan et al., 2000].
Moreover, after activation, RSK is imported into the nucleus where it phosphorylates a variety of substrates such as transcription factors, including the cAMP-responsive element-binding protein (CREB) [Frodin and Gammeltoft, 1999]. This transcriptional factor belongs to the CREB/ATF family, and binds to a specific sequence, known as cAMP-responsive element (CRE) [Meyer and Habener, 1993]. The CRE binding motif is known to be essential for the basal transcriptional activity of many promoters. CREB is known to regulate the expression of genes that are important in cell proliferation, differentiation, and survival in many cell types. The activation of CREB requires phosphorylation of the Ser-133 residue which increases its association with CREB-binding protein.
Up to now, the notable reported molecular mechanisms of ADM include cAMP, PI3K/Akt, and MAPK/ERK-mediated cascades [Hay and Smith, 2001; Shichiri and Hirata, 2003]. However, though, cAMP-PKA pathways are coupled to ADM receptors in various types of cells, in osteoblastic cells, changes in cAMP observed by Hamada et al. [2002] in the proliferative effect of ADM, had previously been reported to be limited by Grey et al. [1999], while for this author, MAPK appeared to be the major pathway mediating the mitogenic effect of ADM. PI3K/Akt signaling has been observed to be activated in some cell types, as in particular, endothelial cells and vascular smooth muscle cells [Iwasaki et al., 2001; Kim et al., 2003], but nothing has been reported about this signaling pathway in osteoblasts. Moreover, as the action of ADM can also be mediated through the CGRP receptor, as has been reported for its mitogenic and anti-apoptotic effects in osteoblasts [Cornish et al., 2003; Uzan et al., 2008], ADM could potentially activate the same signaling pathways as CGRP in osteoblasts. In this sense, it should be noted that it was recently observed [Mrak et al., 2010] that in osteoblasts, CGRP is able to modify the glycogen synthase kinase 3beta (GSK3β) protein and Wnt signaling, which both play an important role in the control of apoptosis [Bodine, 2008]. So, in view of these previous results it could be expected, that in osteoblasts, ADM, like CGRP, could activate this pathway in addition to the above pathways.
The present study was undertaken in order to explore the molecular mechanisms implicated in the survival actions of ADM in osteoblasts. Investigation of the MAPK signaling cascade components showed in particular that ADM inactivated the pro-apoptotic factor Bad by specific phosphorylation and importantly increased phosphorylation of the transcription factor CREB and increased CRE-dependent transcriptional activity. In addition to activating ERK1/2 signaling components, ADM promoted a major increase in the activation of the tyrosine kinase Src and of the PI3-Kinase. Using specific chemical pharmacological inhibitors, we established that ADM exerts an action involving Src-ERK1/2-p90RSK-CREB, and that the ADM-induced CREB downstream response will greatly depend on regulatory control of interfering signaling pathways. Moreover, we observed that ADM-induced inactivation of protein GSK3β, and this strongly suggests that ADM could also exert a survival effect in osteoblasts through an action on the Wnt pathway.
MATERIALS AND METHODS
Materials
Human ADM was purchased from Bachem (St. Helens, UK). The following antibodies, anti-phospho-ERK1/2, anti-phospho-CREB (Ser133), anti-CREB, anti-phopho-Src (Tyr416), anti-phospho-p90RSK (Ser380), anti-phospho-PI3Kp85(Tyr458)/p55(Tyr199), anti-phospho-Bad (Ser155), anti-phospho-Bad (Ser136), anti-Bad and anti-phospho-GSK3β (Ser9) were obtained from Cell Signaling Technology (Ozyme, Montigny le Bretonneux, France). Anti-PCNA antibody was from Santa Cruz Biotechnology (Heidelberg, Germany). Anti-GAPDH antibody was from Abcam (Paris, France), and anti-actin antibody and secondary antibodies conjugated with horseradish peroxidase were from Sigma–Aldrich (St. Quentin Fallavier, France). The chemical inhibitors, PD98059, UO156, H89, PP2, were purchased from Calbiochem (Merck, Darmstad, Germany). Fluorescent secondary antibodies were supplied by Jackson ImmunoResearch (Suffolk, England). Unless otherwise specified, all other products were of the highest purity.
Cell Culture and Treatments
The murine calvaria-derived MC3T3-E1 osteoblast-like cells were grown in α-MEM supplemented with 10% fetal calf serum (FCS), 2 mM L-glutamine, 1 mM sodium pyruvate, penicillin G (100 IU/ml) and streptomycin (100 µg/ml) at 37°C in a humidified atmosphere of 95% air-5% CO2.
To investigate components of the ADM-induced signaling pathway, cells were seeded in six-well plates. When they had reached subconfluence, the cells were rinsed twice with α-MEM and incubated in serum-free medium (0.1% BSA) for 30 min, and then stimulated by ADM (10−7 M) for the times indicated. For the antagonism studies, the cells were incubated in serum-free medium (0.1% BSA) for 30 min with or without specific inhibitors: the MEK inhibitor, UO126 (10 µM) or PD98059 (10 µM), the PKA inhibitor, H89 (10 µM) or the Src inhibitor, PP2 (20 µM), before being exposed to ADM (10−7 M) for 10 min, or for the time indicated. For the PCNA expression study, the cells were cultured in serum-free medium (0.1% BSA) for 24 h with or without ADM (10−7 M). At the end of the experiments, the cells were washed once with PBS and subsequently frozen and stored at −80°C until being extracted.
Measurement of Cell Survival
Cell survival was measured using WST-1 (Roche, Mannheim, Germany) assay. Cells were plated on 96-well plates and cultured under normal growth conditions. After 24 h, the media were replaced by serum-deprived medium for 24 h. The cells were then cultured with or without ADM (10−7 M) for a further 24 h. At the end of ADM incubation, WST-1 compound was added to each well and incubation continued for 3 h (n = 5). Absorbance was recorded according to the indicated recommendations. Cell survival assays were repeated at least twice to confirm the reproducibility of the results.
Immunoblotting
Cells were lysed in ice-cold extraction lysis buffer containing 20 mM Tris–HCl, 1 mM EDTA 16 mM Chaps, freshly added antiprotease cocktail and 1 mM PMSF (Sigma, St. Quentin Fallavier, France). Lysates were then centrifuged for 15 min at 4°C and 14,000g. The protein concentration was determined using the Micro BCA protein assay kit (Pierce, Rockford, IL). Equal amounts of protein were electrophoresed on precast 4–20% Tris-glycine polyacrylamide gel (Invitrogen, Cergy-Pontoise, France) under reducing conditions and electro-transferred to PVDF membrane. Membranes were blocked with 5% nonfat dry milk in TBST (10 mM Tris, pH 7.4, 150 mM NaCl, 0.1% Tween-20), they were then incubated with primary antibody in TBST at 4°C overnight, washed in TBST, and incubated for 1 h at room temperature with an appropriate HRP-conjugated anti-IgG antibody in TBST. Membranes were developed using an enhanced chemiluminescent Western blotting detection system, as recommended by the manufacturer (Biorad, Marnes la Coquette, France).
Transient Transfection and the Luciferase Reporter Assay
For the study of the regulation by ADM of CRE-dependent gene transcription, cells cultured in 24-well plates were transiently co-transfected with a CRE-luciferase reporter construct (pGL3-CRE-luc) (600 ng/well) or with the corresponding control vector (pGL3), and with a renilla construct (phRL-TK) as an internal control to normalize the observed values, using the Lipofectamine reagent (Invitrogen) according to the manufacturer's directions. Twenty-four hours later, cells were pretreated with the MEK1/2 kinase inhibitor PD98059 (10 µM) or UO126 (10 µM) inhibitor for 30 min in 0.5% FCS, before being treated with ADM for 6 h. At the end of the incubation, the cells were lysed and the luciferase activity analyzed according to the manufacturer's instructions using a light assay kit (Dual Luciferase Kit, Promega, Perkin-Elmer, USA). Each treatment was performed in quadruplicate wells along with appropriate controls. The experiments were repeated at least three times.
Immunocytochemistry
For the p-CREB immunofluorescence study, cells were seeded in 8-chamber slides (Labteck) (Fisher Scientific Bioblock, Illkirch, France). Subconfluent cells were rinsed twice with α-MEM, incubated in serum-deprived medium (0.1% BSA) for 30 min and then treated for 10 min with or without ADM (10−7 M). For the PCNA immunofluorescence study, cells were cultured in 8-chamber slides as above. After 48 h, the media were replaced by medium containing 0.5% FCS for an overnight culture of the cells. The cells were then treated for 4-h with or without ADM (10−7 M) in 2% FCS medium. At the end of the treatments, the cells were washed with PBS and fixed for 10 min in cold acetone (−20°C). After incubation in PBS/4% BSA for 30 min to block non-specific binding, the cells were incubated overnight at 4°C with specific antibody in PBS/4% BSA in a humidified chamber. Negative controls were carried out by omitting the primary antibody. The slides were then rinsed in PBS and incubated for 1 h at room temperature in a dark humid chamber with secondary antibodies conjugated to Cy2 (green) or to Cy3 (red) in PBS/4% BSA. After further washing steps, the slides were mounted and examined by fluorescence microscopy (Nikkon) the images being acquired using interface with the Microvision instrument software package.
Statistical Analysis
Data are expressed as mean ± SEM. Statistical analyses were carried out by using ANOVA followed by Fisher's test. Differences were considered as significant at P < 0.05%.
RESULTS
ADM Induced p90RSK Phosphorylation and Inhibited the Pro-Apoptotic Protein Bad
To explore MAPK downstream effector proteins, we examined the major proximal intracellular target for ERK1/2, protein p90RSK. Western blotting showed that ADM treatment of MC3T3-E1 cells increased p90RSK phosphorylation within 5 min, and that this had decreased after 20 min (Fig. 1A).
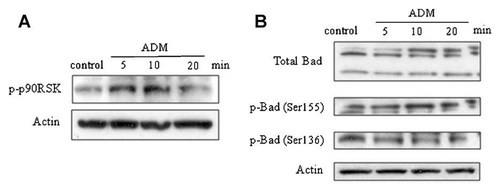
Increase in p90RSK and Bad phosphorylation by ADM in MC3T3-E1 cells. A: Representative Western blot showing that ADM treatment of the cells increased p90RSK phosphorylation within 5 min that decreased after 20 min. B: Representative Western blot showing that ADM modified the profile of the phosphorylation state of Bad. The upper panel shows that ADM induced a shift toward Bad isoforms with lower motility with the exposure time of the cells. Bad phosphorylation studies indicate that ADM elicited an increase in Bad phosphorylation at Ser155 whereas it did not affect Bad phosphorylation at Ser 136.
As protein p90RSK is a potential kinase of the pro-apoptotic protein Bad, which it renders inactive by phosphorylation at Ser155, Western blot analysis was performed to determine the Bad profile. We observed that ADM treatment induced a shift toward Bad isoforms with lower motility with the exposure time (Fig. 1B, upper blot), which indicated a modification in the phosphorylation state of the protein. Investigation of the Ser phosphorylation of Bad showed that ADM induced an increase in p-Bad Ser155 while no change was observed in p-Bad Ser136 (Fig. 1B).
ADM Induced CREB Phosphorylation and Transcriptional Activity
We next investigated the potential activation by ADM of the transcription factor CREB, downstream of p90RSK, by phosphorylation at Ser133. Western blot analysis of a time course study showed that CREB phosphorylation was evident as soon as 5 min of treatment, was sustained at 10 min and decreased after 20 min (Fig. 2A). To further confirm this observation, immunofluorescence staining for p-CREB (Ser133) on cells cultured on chamber slides was performed after ADM stimulation for 10 min. Accordingly, a higher immunofluorescence staining was observed with ADM treatment as compared to the control cells with, in particular, a nuclear localization (Fig. 2B) which suggests a potential action of ADM on gene transcription through CREB in MC3T3-E1 cells.
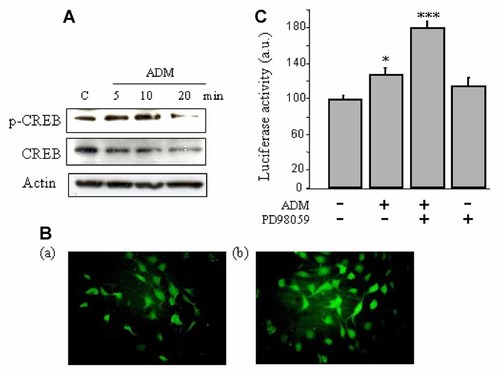
ADM elicited CREB phosphorylation and activated transcriptional activity in MC3T3-E1 cells. A: Representative Western blot showing that an increase in the phosphorylation of p-CREB Ser133 was observed after ADM treatment of the cells. B: ADM-induced CREB phosphorylation was also confirmed by fluorescence immunostaining of the cells. In particular a nuclear localization was observed with ADM, (a) control cells, (b) ADM-treated cells. C: CRE-luciferase assay showing that ADM triggered an increase in CRE-luciferase activity, which was maintained in the presence of the MEK inhibitor, PD98059. Data are shown as the mean ± SEM (n = 4). *P < 0.05; and **P < 0.001 versus control.
To investigate whether the increase in p-CREB induced by ADM results in a change in CRE-dependent gene transcription, CRE-luciferase assays were performed after CRE-luc plasmid transfection. We observed that ADM increased CRE-luciferase activity, and this result established that ADM could effectively increase CRE-dependent gene expression in MC3T3-E1 cells. To find out whether MEK is a potential upstream effector of CREB-induced transcriptional activity, a MEK inhibitor was tested. We observed that the increase was not abolished, but unexpectedly increased in the presence of PD98059 (Fig. 2C).
ADM Induced Expression of the PCNA Protein
In order to determine that ADM could potentially up-regulate gene expression in osteoblasts, we therefore investigated expression of the proliferative PCNA protein, because ADM is known to stimulate osteoblast proliferation and the PCNA promoter could potentially bind the transcription factor CREB. Western blot analysis showed that a higher PCNA level was observed after treatment of MC3T3-E1 cells with ADM for 24 h (Fig. 3A). Similarly, immunofluorescence staining of PCNA proteins showed higher staining in ADM-treated MC3T3-E1 cells than in the control cells (Fig. 3B), which confirmed the previous observation. Though we were not able to state for a role of CREB activation in the up-regulation of PCNA expression, this increase in the amount of PCNA with ADM treatment substantiated the mitogenic action of ADM in osteoblasts.
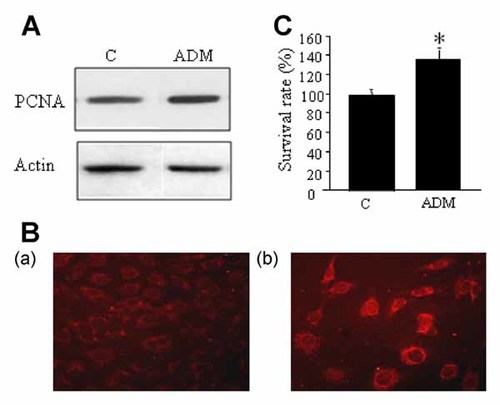
ADM increased the expression of the proliferative PCNA protein. A: Representative Western blot showing an increase in PCNA level after treatment of MC3T3-E1 cells with ADM for 24 h. B: ADM-induced increase in the PCNA amount in the cells was confirmed by fluorescence immunostaining: (a) control cells, (b) ADM-treated cells. C: Survival analysis of MC3T3-E1 cells using the WST-1 assay. MC3T3-E1 cells cultured for 24 h in serum-deprived medium in the presence of ADM showed a higher survival rate than control cells. Data are shown as the mean ± SEM (n = 5). *P < 0.05 versus control.
Consistent with the above results and previous reports [Cornish et al., 2003; Uzan et al., 2008], the survival-promoting action of ADM was confirmed in MC3T3-E1 cells using the WST-1 assay, as ADM treatment protected MC3T3-E1 cells cultured in low serum medium as compared to the control cells, as soon as 24 h (Fig. 3C).
Interaction of PKA With the MEK/ERK Pathway
Upper results indicating that the MEK inhibitor did not abolish ADM-induced CREB transcriptional activity, the effect of MEK inhibition on CREB phosphorylation was further explored, and variations in ERK1/2 and p90RSK phosphorylation levels were investigated. Pretreatment of the cells with the MEK inhibitor UO126 (10 µM) for 30 min before stimulation with ADM (10−7 M) for 10 min, did not inhibit CREB phosphorylation, but in contrast increased CREB phosphorylation level (Fig. 4A), suggesting that MEK inhibition led to feedback up-regulation of other pathways upstream of CREB. Similar results were observed with the MEK inhibitor PD98059 (data not shown). Anyway, the concomitant decrease in ERK1/2 and p90RSK phosphorylation induced allows to establish the effectiveness of the MEK inhibiting treatment (Fig. 4B), and also indicates that a MEK1/2-ERK1/2-p90RSK signaling module is activated by ADM.
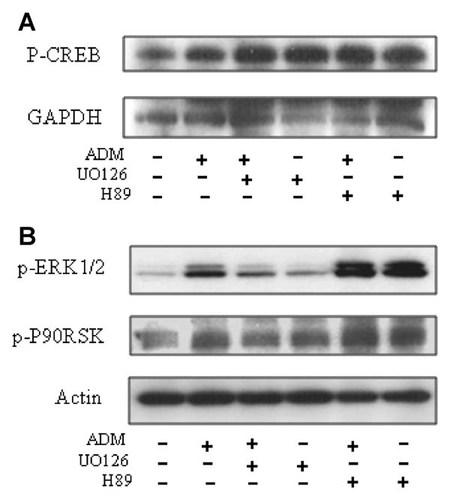
Interaction of PKA with the MEK1/2-ERK1/2-p90RSK signaling module. A: Representative Western blot showing that pretreatment of the cells with the MEK inhibitor, UO126, for 30 min and stimulation by ADM for 10 min did not prevent ADM-induced CREB phosphorylation, but elicited a major increase. A similar observation was made after pretreatment with the PKA inhibitor, H89. B: Representative Western blot showing that pretreatment with the MEK inhibitor, UO126 was effective to prevent ADM-induced phosphorylation of ERK1/2 and of its downstream target, p90RSK, whereas pretreatment with H89 increased the phosphorylation of both ERK1/2 and p90RSK.
This observation of an increase in CREB phosphorylation in the presence of a MEK inhibitor suggests the existence of a constitutive MEK inhibitory control of other CREB phophorylation-induced pathways. We therefore investigated the potential implication of PKA, which is another major molecule involved in CREB activation and which has also been suggested as playing a role in the action of ADM in osteoblasts [Hamada et al., 2002]. As shown in Figure 4, pretreatment of the cells with the PKA inhibitor H89 induced a potent increase in ERK1/2 activation along with increases in both p90RSK and CREB. This strongly suggested that in MC3T3-E1 cells, PKA exerts a major constitutive inhibitory control on the MEK/ERK/p90RSK/CREB cascade. Taken together, these results indicate that CREB is regulated by the concerted action of different kinases in osteoblasts.
ADM Induced PI3-K and Src Activation
To obtain further information about the molecular mechanisms induced by ADM, potential activation of the proteins PI3K and Src was also investigated. Data shown in Figure 5A indicate that after ADM treatment a rapid and transient increase in tyrosine phosphorylation of PI3K was observed that establish activation of this protein in cells treated by ADM. We then looked at protein Src which can directly activate PI3K and is upstream of the Ras/Raf/MEK kinases. We studied c-Src phosphorylation at the level of tyrosine 416, which corresponds to the site of c-Src autophosphorylation, and reflects Src kinase activity. We observed that ADM treatment induced a transient increase in Src kinase activation as shown by Western blot analysis for phosphorylated Src-Tyr416 (Fig. 5B) as the level increased within 5 min and returned to basal level after 10 min.
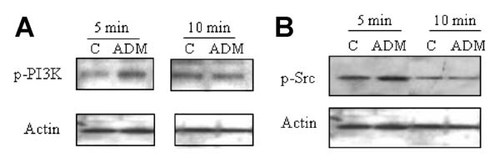
ADM induced activation of the tyrosine kinases PI3K and Src. A: Immunoblotting with an anti-p-PI3K antibody indicates that ADM induced a transient activation of PI3K. B: Investigation of the Src kinase activity using anti-phospho-Src Tyr416 antibody shows that ADM treatment also triggered a transient increase in Src kinase activation. Representative Western blots are shown.
Effect of PP2 Treatment on ADM-Induced ERK1/2 Activation
In order to address the importance of Src in ADM-induced MEK/ERK1/2 signaling, a selective Src-like tyrosine kinase inhibitor, PP2 (20 µM), was used to pre-treat cells for 30 min before stimulation by ADM for 10 min, and the effect on ERK activation studied by immunoblotting. We observed that in the presence of PP2, the effect of ADM on ERK1/2 phosphorylation was notably reduced, as was the basal ERK1/2 phosphorylation level (Fig. 6). This suggests that ADM activates a signaling pathway involving Src as a link upstream of MEK/ERK1/2/p90RSK.

Effect of the Src inhibitor, PP2, on ADM-induced ERK1/2 phosphorylation. Western blot analysis showing that pretreatment of serum-deprived MC3T3-E1 cells with the Src inhibitor, PP2, for 30 min, before stimulation with ADM for 10 min, prevented ADM-induced ERK1/2 phosphorylation. A representative Western blot is shown.
ADM Inhibited GSK-3β Activity
We then investigated the phosphorylation of GSK-3β at Ser9, as this phosphorylation led to reduced GSK-3β activity and cell apoptosis. Western blot analysis showed an increase in phospho-Ser9-GSK3-β after exposure to ADM for 10 min (Fig. 7). Moreover, PP2 pretreatment before ADM stimulation prevented this increase in p-GSK3β, which suggests the involvement of an upstream Src mediator.
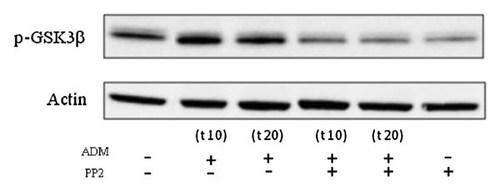
ADM-induced GSK-3β phosphorylation. Representative Western blot showing an increase in the phosphorylation of GSK-3β at Ser9 of serum-deprived cells stimulated with ADM for 10 min which is prevented by the Src inhibitor, PP2.
DISCUSSION
In this study, we demonstrated that the action of ADM in osteoblastic cells could be triggered by multiple signaling pathways. We establish for the first time, that ADM could exert its survival effect via the MAPK pathway by transcription-dependent and transcription-independent mechanisms: the activation of the transcription factor CREB and the phosphorylation of Bad, respectively.
Up to now, the MAPK/ERK pathway has been reported to be implicated in osteoblast proliferation after ADM treatment by Cornish et al. [2004], and we previously showed that this signaling pathway was also implicated in the anti-apoptotic action of ADM in osteoblastic cells [Uzan et al., 2008]. However, the downstream effectors mediating ADM survival remained to be identified.
It is well established that, once activated, the MAPKs phosphorylate their respective substrates including several nuclear and cytoplasmic targets thereby controlling proliferation, differentiation, and survival [Chang and Karin, 2001]. We demonstrated that, in osteoblasts, ADM signaling via the MAPK/ERK pathway converges on p90RSK which is one of the major proximal intracellular targets for ERKs [Dalby et al., 1998]. The p90RSK protein is thought to stimulate cell survival through the phosphorylation and inactivation of the pro-apoptotic Bcl-2 family member, Bad [Shimamura et al., 2000; Tan et al., 2000], and we effectively observed an increase in Bad phosphorylation at Ser155 in response to ADM stimulation (Fig. 1). Bad phosphorylation could prevent apoptosis by homodimerization with Bcl-2 and or Bcl-xL. We have previously reported that ADM does not modify the level of the Bcl-2 family proteins, Bcl-2, Bcl-xL, Bax in MC3T3-E1 cells [Uzan et al., 2008]. Our present results suggest that ADM-induced apoptosis protection could be mediated by Bad phosphorylation. The ability of ADM to phosphorylate Bad has not previously been addressed. It has been reported that rescue from apoptosis by ADM through up-regulation of Max, a heterodimeric partner of cMyc, may occur in endothelial cells [Shichiri et al., 1999].
Phospho-p90RSK could also phosphorylate a variety of substrates after nuclear translocation, such as the transcription factor CREB, and our results demonstrate that ADM induced phosphorylation of CREB in osteoblasts and stimulated CREB transcriptional activity using CRE-Luciferase assay (Fig. 2). The transcription factor CREB regulates genes involved in various cellular processes by binding to CRE sequences present in their promoter regions.
Thus, CREB activation could be involved in cell survival by increasing the expression of proliferative proteins, and our study indicates that the increase in p-CREB is likely to activate CRE-dependent gene transcription of PCNA (Fig. 3). PCNA is synthesized in early G1 and S phase of the cycle, and serves as an excellent marker of proliferating cells [Takahashi and Caviness, 1993]. This result strengthened the previously established mitogenic action of ADM in osteoblasts. Up to now, such an activation of CREB and consequently a potential increase in gene transcription has not been reported after ADM treatment. Recently it has been established that the proliferative action of ADM in glioblastoma cells involves activation of AP-1 sites and an increase in the cyclin D1 protein [Ouafik et al., 2009].
We have previously reported that the anti-apoptotic effect of ADM in MC3T3-E1 cells involves the CGRP receptor, and that CGRP is also anti-apoptotic in these cells [Uzan et al., 2008]. CGRP also exerts a mitogenic effect in osteoblasts [Cornish et al., 1999], and the CGRP antagonist can still antagonize the mitogenic activity of ADM in osteoblasts [Cornish et al., 2003]. Taken together, these data highly suggest that the same receptor is involved in the actions of ADM and CGRP in osteoblasts, and it can be hypothesized that common signaling pathways could be induced by these peptides. In this sense, though notable effects of CGRP are mediated by cAMP/PKA, many CGRP receptors are also known to be coupled to the ERK signaling pathway [Schaeffer et al., 2003]. Moreover, an effect of CGRP on the phosphorylation of CREB and on CRE-dependent gene transcription has previously been reported in neuronal cells [Seybold et al., 2003; Anderson and Seybold, 2004], as modification in the expression of PCNA, which has also been identified as an effector molecules in CGRP downstream signaling in the neuroblastoma cell line SK-N-MC [Pluder et al., 2007].
It should also be noted that CREs have been reported in the promoter of the ADM gene [Minamino et al., 1998] and in that of CGRP [Freeland et al., 2000], which strongly suggests the existence of an autocrine activation loop for these peptides.
In response to MEK inhibitors we would expect the down-regulation of CREB activation as CREB is a reliable effector of ERK1/2 pathway. However, in fact we observed that treatment with MEK inhibitors did not impair ADM-stimulated phosphorylation of CREB, but on the contrary, increased CREB phosphorylation and CREB-transcriptional activity, suggesting that MEK inhibition leads to a feedback up-regulation of other CREB-activating pathways. As CREB is a common substrate for both PKA and MAPK/ERK1/2 signaling, we investigated the effect of PKA inhibition on the activation of CREB, so as to determine potential interactions between the PKA and the MEK pathways. Treatment with H89, a PKA inhibitor, induced an increase in CREB activation along with major increases in p-ERK1/2 and p-p90RSK (Fig. 4), which highly suggests that PKA exerts a constitutive potent inhibition on the MEK-ERK1/2-CREB cascade or on an upstream regulator of MEK in the MC3T3-E1 cells. Such an inhibiting effect of PKA had previously been observed with PTH in osteoblasts, and PKA was shown to interfere with MAPK activation at the level of Raf-1 [Lai and Mitchell, 2009]. Though we were not able to establish the pathway constitutively inhibited by MEK, and in particular, whether PKA activity was modified in presence of a MEK inhibitor, the possibility of an interaction can be suggested, as multiple levels of cross-talk between cAMP/PKA pathway and the MAPK cascades have been described [Gerits et al., 2008].
We also observed that in osteoblasts, ADM induced the up-regulation of PI3K and Src (Fig. 5). The activation of Src leads to the activation of a variety of downstream signaling pathways and, in particular, Src is a potential upstream kinase of the Ras/Raf/MEK kinases and of PI3K [Pleiman et al., 1994]. Src is a non-receptor protein tyrosine kinase that transduces signals that are involved in many fundamental cellular processes, including cell proliferation, migration, invasion, and survival [Thomas and Brugge, 1997]. The importance of Src on bone metabolism is well established [Zambuzzi et al., 2010]. Using a Src inhibitor we were able to establish the existence of an ADM signaling pathway involving the Src kinase upstream to MEK signaling (Fig. 6). Such an effect of ADM on Src kinase has been previously reported in vascular smooth muscle cells by Iwasaki et al. [2001]. ADM also activates the kinase, PI3K. PI3-kinases are important regulators of cell growth, adhesion, apoptosis, survival and motility [Cantley, 2002; Díaz-Montero et al., 2006]. The activation of PI3K by ADM has also been observed previously in endothelial cells and vascular smooth muscle cells [Iwasaki et al., 2001; Kim et al., 2003].
Anyway, up to now, many of the effects of adrenomedullin on cell growth have been reported to be mediated by members of the MAP kinase family or by tyrosine kinases [Shichiri and Hirata, 2003]. However, it should be noted that a proliferative action of ADM in osteoblasts via a PKA/cAMP has also been reported, but the mechanism remains unclear, and the authors suggest that another intracellular signal transduction or feedback system may be involved [Hamada et al., 2002].
Our results indicated that ADM could also increase the phosphorylation level of Ser9 in GSK-3β (Fig. 7). The activity of GSK-3β is regulated by the phosphorylation status of both serine and tyrosine residues: it is decreased or increased by the phosphorylation of Ser9 or Tyr 216, respectively [Cohen and Frame, 2001]. Thus, in osteoblasts ADM decreased the activity of GSK3β. By inactivation of the kinase, ADM could inhibit apoptosis in osteoblasts through another mechanism, as reduced GSK3β activity leads to reduced apoptosis. It is well established that growth factors can control the activity of GSK3β by the most downstream kinase of MAPK, p90RSK. Although we did not study the involvement of the MAPK/p90RSK pathway in this effect, our results establish that the Src molecule is an upstream component of GSK3β. This effect of ADM on GSK3β had previously been reported in neuronal cells, as ADM could promote astrocytes survival by inactivating GSK3β [Xia et al., 2004]. It should be noted that a similar effect on GSK3β was recently reported for CGRP in osteoblasts [Mrak et al., 2010]. Moreover, the authors established that CGRP had an effect on Wnt signaling by increasing β-catenin stabilization. It can be hypothesized that similarly, by modification of GSK3β activity, ADM could play a role in the canonical Wnt/β-catenin signaling pathway, which exerts an important role in the control of apoptosis in osteoblast [Bodine, 2008]. Future studies must be conducted to explore if such a signaling pathway is activated by ADM in osteoblasts.
In conclusion, our results establish that ADM can induce osteoblastic cell survival by multiple molecular mechanisms. The action of ADM can be promoted (1) by activation of the Src-MAPK/p90RSK signaling pathway and inhibition of Bad, (2) by a transcription-dependent mechanism, as ADM is able to induce the transcriptional activity of CREB, (3) by an increase in the mitogenic PCNA protein, (4) by activation of the tyrosine kinase Src and the protein PI3K, and also (5) by inactivation of the protein GSK3β.
Acknowledgements
The authors want to thank IBAIC (Université Paris Sud, Orsay, France) for acquisition of immunofluorescence images. This work was supported by Inserm and by a grant from the Rhumatismes et Travail Association.




