ERα17p, an ERα P295-T311 fragment, modifies the migration of breast cancer cells, through actin cytoskeleton rearrangements†
Marilena Kampa and Vassiliki Pelekanou have equally contributed.
Abstract
Recently, our knowledge on estrogen receptor alpha (ERα) functions and fate has progressed: ERα enters in repeated transcription-modulating cycles (nucleus/cytoplasm/membrane trafficking processes and proteasomal degradation) that are governed by specific protein–protein interactions. Receptor fragments, especially those resulting from the proteolysis of its ligand binding domain, as well as corresponding synthetic peptides, have been studied with respect to their estrogenic/antiestrogenic potency. A peptide, corresponding to the human ERα P295-T311 sequence (ERα17p) has been shown to alter breast cancer cell fate, triggering proliferation, or apoptosis. The aim of this work was to explore the effect of ERα17p on breast cancer cell migration and actin cytoskeleton dynamics and further analyze the mechanism of its membrane action. We show that ERα17p increases (MCF-7 and SK-BR-3 cells) or decreases (T47D and MDA-MB-231 cells) migration of breast cancer cells, in an ERα-independent manner, by mechanism(s) depending on Rho/ROCK and PI3K/Akt signaling pathways. Moreover, the peptide enhances the association of both estrogens and androgens to membranes and modifies cell migration, induced by E2-BSA. Additionally, initial evidence of a possible agonistic action of the peptide on GPR30 is also provided. ERα17p can be considered as a cell migration-modulator and could therefore constitute a therapeutic challenge, even in anti-estrogen-resistant tumors. J. Cell. Biochem. 112: 3786–3796, 2011. © 2011 Wiley Periodicals, Inc.
Tumor progression and metastasis are directed by highly complex processes, with cell migration constituting one of the most important initial steps [Brooks et al., 2010]. Cell migration is controlled by a number of key intracellular events, among which the assembly of polarized actin shares a prominent place. Indeed, it regulates lamellipodia and filopodia protrusions that propel the leading edge of malignant cells through tissues, leading to tumor invasiveness [Insall and Machesky, 2009]. Likewise, a number of GTPase-activating proteins (GAPs), required for the transmission of membrane receptor effects, trigger the nucleation of new actin filaments, via specific downstream pathways (e.g., Rho, Rac, and Cdc42), to generate either branched or linear filament arrays [reviewed in Schmitz et al., 2000; Heasman and Ridley, 2008]. In this regard, the control of actin-based processes appears pleiotropic [Insall and Machesky, 2009]. Steroids, and particularly estradiol, hold a key position among actin cytoskeleton modulators, displaying either a direct effect on actin reorganization and/or associated small GTPases activity. Indeed, estradiol modifies cell motility in thyroid [Rajoria et al., 2010], melanoma [Matsuoka et al., 2009], ovarian [Hua et al., 2008], endometrial [Acconcia et al., 2006] and breast cancer cells [Malek et al., 2006].
In breast cancer models, the role of estrogen receptors (ERs) remains ambiguous. They have been associated with reduced invasiveness and migratory potential [Platet et al., 2000], while recent investigations report that ERs facilitate migration and invasion through extranuclear processes [Giretti et al., 2008; Sanchez and Simoncini, 2010]. The extranuclear action of steroids and especially estradiol, has been primarily observed in the 60's [Szego and Davis, 1967], while a more exhaustive approach was conducted during the last two decades [see Kampa et al., 2008 and Levin, 2009; for reviews]. Despite the lack of consensus regarding the exact nature of the membrane estrogen binding counterparts, it has been proposed that they may include, in a non-exclusive way, the classical estrogen receptor alpha (ERα), or some alternatively spliced variants, anchored at the plasma membrane [Acconcia et al., 2005], a G-coupled membrane receptor, such as GPR30 (although this site is rather considered as a co-regulator than a real estrogen binding site), or a non-yet identified membrane protein [Thomas et al., 2005; Levin, 2009; Wendler et al., 2010]. Interestingly, GPR30 activation has been recently reported to increase the migration of breast cancer cells [Pandey et al., 2009], or breast cancer associated fibroblasts [Madeo and Maggiolini, 2010].
Undoubtedly, targeting of ER by selective estrogen receptor modulators (SERMs) is the hallmark of breast cancer treatment [see Jordan, 2002; Peng et al., 2009, for reviews]. Despite of the undeniable significant progress in survival and quality of life of breast cancer patients, resistance to endocrine regimens persists and necessitates ameliorated understanding of ER-related functions in view of novel therapeutic and clinical strategies. In this goal, the chase of alternative interaction sites at the structure of ER appears promising and may open new therapeutic avenues [Leclercq et al., 2006; Sengupta and Jordan, 2008].
An ERα motif located between the D- (hinge) and the E- (Ligand Binding Domain; LBD) regions of the autonomous activation function 2a (AF-2a), serves as a platform for key post-translational modifications (acetylation, phosphorylation, ubiquitination), as well as calmodulin and Hsp70 recruitment (P295-T311 sequence: H-PLMIKRSKKNSLALSLT-OH) [Gallo et al., 2008a; Gallo et al., 2008c]. Remarkably, a synthetic 17-mer (ERα17p) corresponding to this sequence has been shown to display pseudo-estrogenic effects, such as induction of estrogen-regulated reporter gene expression (pS2 and PR), down-regulation of ERα and stimulation of ERα-positive breast cancer cells growth, under steroid starvation [Gallo et al., 2007b]. Furthermore, ERα17p may antagonize the interaction of the LxxLL binding motif of p160 co-activators with purified ERα, as does a control synthetic peptide containing this motif [Gallo et al., 2008c]. These features are relevant to the concept that ERα17p might be a new PERM (peptide estrogen receptor modulator). Nevertheless, its mode of action remains obscure, even if a repressive function, exerted by the endogenous P295-T311 motif on the inactivated receptor, is strongly suspected [Gallo et al., 2007b]. In this context it has been proposed that an ERα17p analog could be produced by the proteasome, to maintain constant basal estrogenic effects in the cell, through completely blocking this repressive function [Gallo et al., 2008b].
Recent investigation revealed a complementary involvement of ERα17p in breast cancer lines' apoptotic potential, under full (serum-supplemented) culture medium [Pelekanou et al., 2011]. Hence, one may hypothesize that ERα17p could elicit a large spectrum of cellular responses, by interfering with various signaling pathways, depending on the culture conditions. The present work has been focused on the action of ERα17p on rapid membrane-initiated estrogenic signaling pathways and on resulting effects on the migration of breast cancer cells. We show that ERα17p triggers specific signaling cascades in ERα-positive (MCF-7, T47D) and -negative breast cancer cells (MDA-MB-231 and SK-BR-3). It also modifies cell migration, in an ERα-independent manner, through specific modifications of actin cytoskeleton, involving small GTPases.
MATERIALS AND METHODS
Cell Cultures and Chemicals
MCF-7 (ERα+, ERβ+, GPR30+), T47D (ERα+, ERβ+, GPR30+), MDA-MB-231 (ERα−, ERβ+, GPR30+), and SK-BR-3 (ERα−, ERβ−, GPR30+) breast cancer cells (DSMZ, Braunschweig, DE and ATCC, LGC Standards GmbH, Wesel, Germany) were cultured in RPMI 1640, DMEM/F12, and McCoys 5A medium (Invitrogen, Life Technologies, Paisley, UK) respectively, supplemented with 10% fetal bovine serum, at 37°C and with 5% CO2.
ERα17p (sequence: H-PLMIKRSKKNSLALSLT-OH) was synthesized by solid phase peptide synthesis (Eurogentec, Seraing, Belgium), as previously described [Gallo et al., 2007a]. ERα17p-FITC, was prepared by grafting fluorescein–COOH on the N-terminal proline residue.
Estradiol, β-Estradiol 6-(O-carboxy-methyl)oxime-BSA (Estradiol-BSA, E2-BSA, 30 steroid molecules per molecule BSA), β-Estradiol 6-(O-carboxy-methyl)oxime-BSA-FITC (10 steroid molecules per molecule BSA, E2-BSA-FITC), Testosterone 3-(O-carboxy-methyl)oxime-BSA (Testosterone-BSA, Testo -BSA, 28 steroid molecules per molecule BSA), Testosterone-BSA-FITC (11 molecules Testosterone per molecule BSA, Testo-BSA-FITC), and BSA-FITC were purchased from Sigma (St Louis, MI). Prior to experiments, the BSA-conjugated steroids were charcoal-treated (3% charcoal and 0.3% dextran, 30 min at 4°C), to eliminate any non-complexed steroids. Wortmannin (PI3K/Akt inhibitor) and SB203580 (p38 kinase inhibitor) were from Calbiochem (San Diego, CA), while Y27632 (ROCK inhibitor) was purchased from Sigma. All other chemicals were obtained from Sigma, unless specifically stated. The specific GPR30 antagonist G15 was a kind gift of Dr. ER Prossnitz (University of New Mexico, Albuquerque, NM).
Detection of Membrane Binding Sites
One million cells/ml in PBS were incubated with 10−6 M ERα17p-FITC, E2-BSA-FITC, or Testosterone-BSA-FITC conjugates, for 10 min, in absence or presence of unlabeled ERα17p (10−5 M). BSA-FITC was used to determine non-specific binding. At least, 10,000 gated cells were analyzed. Fluorescence was measured with a Beckton–Dickinson FACSArray apparatus (Beckton–Dickinson, Franklin Lakes, NJ) and then analyzed with the CELLQuest® (Beckton–Dickinson) software.
Actin Cytoskeleton Visualization
Cells growing on poly-L-lysine-coated coverslips were treated with ERα17p for the indicated time (10–60 min), fixed with 4% paraformaldehyde (PFA) for 10 min, washed with PBS, and permeabilized by 0.5% Triton for 10 min. Afterwards, they were incubated with 2% BSA (15 min), followed by rhodamine-labeled phalloidin (Molecular Probes) staining (45 min), visualized in a confocal laser scanning microscope (CLSM, Leica TCS-NT) and analyzed with the Image J® program (Research Services Branch, NIMH, NIH, Bethesda, MD). Actin fibers remodeling and morphological modulation of the membrane were quantified by assessment of the intensity of actin fluorescence, as previously described [Giretti et al., 2008]. Briefly, we selected random boxes across a cell and spatially recorded the intensity of the fluorescent signal. Similar measurements, when appropriate, were obtained with random boxes across the membrane (including the extra- and intra-cellular space), which allowed us to identify the apparent “thickness” of the membrane section (representing sub-membrane accumulating polymerized actin). In each case, five areas were sampled per cell and we repeated this on 10 different cells per experimental condition. Therefore, membrane intensity, cytosol intensity, their ratio and membrane thickness have been calculated and presented as a mean ± SD.
Assay of the Polymerization State of Actin (F/G Ratio)
The ratio of filamentous actin (F) to monomeric globular (G) was obtained by electrophoresis in PAGE gels, of the Triton soluble and insoluble cellular protein fractions, incubated for 20 min with or without ERα17p (10−6 M), supplemented or not with E2- or Testosterone-BSA and analyzed by immunoblotting, using a monoclonal anti-actin (Amersham-Pharmacia, Bukinghamshire, UK) and a second horseradish peroxidase-coupled antibody (Chemicon, Temecula, CA).
Migration Assays
Wound healing assay
Cells were plated in six-well plates until they formed a monolayer and incubated for 1 h with 10 µg/ml mitomycin C (Sigma, St Louis, MI, a selective inhibitor of DNA synthesis), in order to inhibit mitotic activity. Cell viability (not modified) and absence of cell proliferation were checked in preliminary experiments with MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, not shown). Afterwards, a 200–500 µm width linear scratch was made with a micropipette tip. Thereafter, cells were washed with PBS to remove cellular debris and incubated in the presence or in the absence of ERα17p, estradiol-BSA (10−6 M each), the pure ER antagonist ICI 182,780 (10−5 M), the GPR30 antagonist G15 (10−5 M) or the different kinase inhibitors (SB203580 25 µM, Wortmannin 0.1 µM, or Y27632 1 µM). The subsequent colonization of the denuded area was quantified with an inverted microscope (DM IRE2, Leica) at different time-intervals (12–48 h). Normalized differences in migration (as % of control, non-treated, cells) were estimated by subtracting the size of the denuded area from its size on time 0 and by dividing by size on t = 0. As might be expected, when the wounded area is not precisely controlled, the method may be encumbered with problems of quantification and reproducibility. In order to limit the variance of wound extent and resulting bias in data interpretation, three different points were measured in each well. All conditions were assayed in triplicates.
Trans-migration assay
Cell trans-migration was evaluated using the Corning Transwell assay (Cambridge, UK). Cells (2−5 × 104/100 µl) were seeded in the upper side of an 8 µm pore size polycarbonate membrane, with 300 µl medium at the lower chamber and incubated for 24 h at 37°C. Afterwards, cells were removed from the top surface, while underside (migrated) cells were stained with 0.2% crystal violet for 10 min, solubilized in 30% acetic acid and absorbance was measured at 600 nm.
Immunoblotting
Cells were lysed in 1% SDS 10 mM Tris buffer pH 7.4 (containing 1 mM sodium orthovanadate, complete protease inhibitor cocktail (Roche Applied Science, Basel, Switzerland) and benzonase, Sigma, 1.25 kU/ml) and were incubated at 95°C for 3 min. Eighty micrograms of proteins were run in 12% polyacrylamide gels and then transferred onto nitrocellulose membrane. Membranes were blocked for 2 h at room temperature with 5% non-fat dry milk or BSA in TBS-T (50 mM Tris; 150 mM NaCl; 0.05% Tween 20, pH 7.6) and incubated overnight with specific antibodies: Rabbit polyclonal anti-serum against phospho-Akt (Ser473) (Cell Signaling Technology, 1:200), or the monoclonal anti-actin antibody (Amersham-Pharmacia, 1:400). Afterwards they were washed twice for 10 min with TBS-T and incubated for 1 h at room temperature with anti-rabbit HRP conjugated secondary antibody (Thermo Fisher Scientific Pierce, UK, 1:5,000). The ECL system (Pierce, SuperSignal West Pico Chemiluminescent Substrate) was used to reveal specific bands. For presentation, results were normalized as per actin expression. Preliminary experiments and data shown here ensured that actin cell concentration remained unaltered under either treatment of cells.
RT-PCR
Total RNA was isolated from cell cultures with the Miniprep RNA isolation kit (Machinery-Nagel EURL, Fr). RNA was treated with DNase I (Invitrogen, Carlsbad, CA) to remove genomic DNA, and was subjected to reverse transcription (RT): 1 µg RNA was added to a mixture incubated at 42°C for 60 min and containing 5 µM oligo d(T)12–18 primers, 0.5 mM dNTPs, 1× RT buffer, 5 mM dithiothreitol (DTT), 2.5 units RNasin Plus (Promega, Madison, WI) and 10 units SuperScript II RT (Invitrogen) in a total volume of 20 µl. RT was inactivated at 95°C for 5 min. Afterwards the PCR reaction was performed. The PCR reaction mixture included 1 µl cDNA, 1 unit Dynazyme II (Finnzymes, Espoo, Finland), and 200 µM dNTP mix, 2 mM MgCl2, and 500 nM gene-specific primers in a final volume of 25 µl. The conditions for the PCR reaction (Primus 96® Advanced Gradient, PeqLab-Biotechnologie, Erlangen, Germany) were as follows: (1) Denaturation at 94°C for 5 min, (2) 35 cycles of 0.5 min at 94°C, (3) annealing at 60°C for 0.5 min, and (4) extension at 74°C for 0.5 min. The primers used were: For GPR30 sense: 5′- TGA GCT TGT CCC TGA AGG TC -3′ and antisense: 5′- TGG TGG TGA ACA TCA GCT TC -3′, product-size 765 bp and for the housekeeping G3PDH sense: 5′-TCC ACC ACC CTG TTG CTG TA-3′ and antisense 5′-ACC ACA GTC CAT GCC ATC AC-3′, product-size 449 bp. The latter was used as a positive control, while mRNA samples that were not subjected to RT served as negative controls. The amplified products were run on 2% agarose gels in parallel with a Gene RulerTM 100 bp DNA ladder (Fermentas International Inc. CA) and were stained with ethidium bromide.
Statistical analysis
Statistical analysis was performed with the PASW V18 (SPSS Inc, Chicago, IL) program. A significant level of P < 0.05 was retained.
RESULTS
ERα17p Binds on Breast Cancer Cell Membranes
In connection with its amphipathic character, ERα17p could associate to cell membranes to exert, at least partially, its actions [Pelekanou et al., 2011]. Indeed, we observed that a FITC-labeled peptide binds specifically (albeit weakly) to breast cancer cell membranes (Supplemental Fig. 1A), providing evidence that ERα17p may interact with a cell membrane partner. Whether it interacts with the phospholipidic bilayer or with a membrane-engulfed protein, such as membrane forms of ER, remains questionable. Thus, we examined in both ER(+) and ER(−) breast cancer cells the ability of ERα17p to compete with E2-BSA-FITC, a non-permeable fluorescent estradiol analog, that associates with plasma membrane-associated estrogen binding sites. We observed an unexpected increase of fluorescence at the membrane of ER-positive T47D breast cancer cells (Supplemental Fig. 1B), deducing a unlike competition between ERα17p and E2-BSA-FITC at a membrane E2-binding site. A similar effect was also recorded in ERα− (MDA-MB-231, SK-BR-3) cells, excluding a direct association of ERα17p with membrane-bound form of ERα (data not shown). Moreover, an analogous action was seen with Testosterone-BSA-FITC (Supplemental Fig. 1C). We therefore concluded that ERα17p might participate in indirect steroid membrane-mediated mechanisms. In addition, one should evoke the possibility that ERα17p may also modify the composition and fluidity of the membrane in a non-specific manner, which could interfere with the affinity of E2-BSA binding.
ERα17p Modifies the Migratory Activity of Breast Cancer Cells
ERα17p modifies the rate of migration in ERα (+) (T47D, MCF-7) and ERα (−) (MDA-MB-231, SK-BR-3) breast cancer cells (Fig. 1). As an example T47D and SKBR3 cell photos are given in Figure 1A and B, while the effect of ERα17p on the migration of all studied cell lines at 48 h is presented in Figure 1C. The peptide decreased the migration of T47D and MDA-MB-231 cells by 45 and 20%, respectively, while it enhanced the migratory activity of MCF-7 and SK-BR-3 cells by ∼40–50%, excluding again a direct interaction of ERα17p with ERα. The effect of ERα17p on cell migration was also verified by a trans-migration assay (Supplemental Fig. 3A). Accordingly, the addition of the pure ER antagonist ICI 182,780 did not affect the motility of breast cancer cell lines (data not shown), confirming our findings that ERα17p exert its action on cell motility, in an ERα-independent manner.
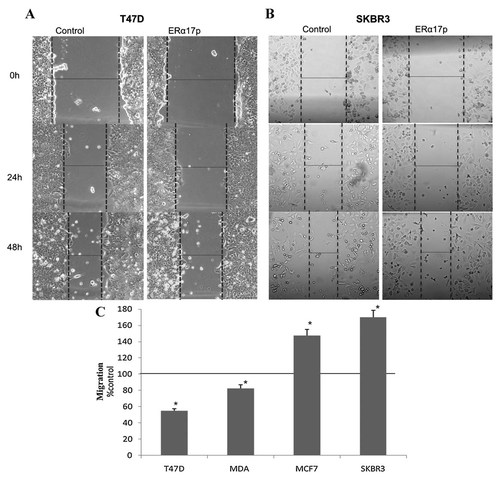
The effects of ERα17p on breast cancer cells migration were tested in four different breast cancer cell lines, with a different estrogen receptor-spectrum. A linear scratch was performed on a cell monolayer and cells were incubated with or without 10−6 M ERα17p (control cells were incubated only with the vehicle). The healing progress of the denuded area was photographed at different time intervals up to 48 h. Representative pictures at 0, 24, and 48 h are presented, showing the anti-migratory effect of ERα17p on T47D (A) and SK-BR-3 cells (B). C: The effect of ERα17p on the migration of the four cell lines was assayed after 48 h and compared with the migration rate of the vehicle treated (control) cells. The results (% difference in migration over control, non-treated, cells) are the Mean ± SEM of three experiments performed in triplicate. *p < 0.05. Three different point measurements were taken for each well (see Material and Methods for further details).
Apart from ERα or β (which were excluded as potential partners, as the peptide exerted its actions also in SK-BR-3 cells, negative for both ER isoforms), another potential membrane candidate for such an action could be the G-protein-coupled 7-loop transmembrane estrogen receptor (known as GPR30 or GPER1), which is associated with migration of breast cancer cells [Pandey et al., 2009; Madeo and Maggiolini, 2010]. Initially, we explored whether GPR30 was expressed in our cell lines. RT-PCR assays confirmed the expression of this site in all cell lines, with, however, a lower level in MDA-MB-231, as reported previously [Thomas et al., 2005; Lin et al., 2009] (Supplemental Fig. 2A). Then, we investigated the implication of GPR30 in ERα17p-induced migration, by means of G15, a specific GPR30 antagonist [Dennis et al., 2009]. In this aim, we used MCF-7 and SK-BR-3 cells, which express a constitutively high level of GPR30 and on which the effect of ERα17p was more prominent. Remarkably, G15 reversed the action of ERα17p (Supplemental Fig. 2B), while alone had no apparent effect on cell migration. Thus, we concluded on an implication of GPR30 to ERα17p migratory action, in ER-positive and -negative breast cancer cells, supporting a potential mechanism by which ERα17p might operate at the membrane level.
ERα17p Effect on Motility Relays on Specific Signaling Pathways
A major consequence of the membrane-initiated action of steroids is the triggering of specific signaling cascades [Kampa and Castanas, 2006]. To explore this hypothesis on ERα17p, we tested its effects on cell migration, after the incubation of the different cell lines with specific signaling inhibitors (Y27632 for Rho/ROCK, wortmannin for PI3K/Akt, and SB203580 for p38 MAPK). In T47D and MDA-MB-231 breast cancer cells, the Rho kinase inhibitor Y27632 reversed the anti-migratory effects of ERα17p, suggesting that the peptide might interact with the Rho/ROCK pathway (Fig. 2A). In contrast, the inhibition of PI3K/Akt by wortmannin enhanced the anti-migratory effect of ERα17p, evoking an additional role of PI3K in the action of ERα17p. Finally, SB203580 had no effect, excluding the involvement of p38 MAPK in these cell lines.
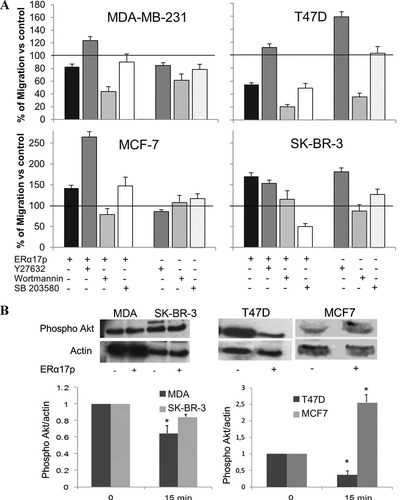
A: Effects of kinase inhibitors on the action of ERα17p on cell migration. A linear scratch was performed on a cell monolayer. The cells were treated with 10−6 M ERα17p, alone or in the presence of the kinase inhibitors wortmannin (W, 0.1 µM) for PI3K, SB203580 (SB, 25 µM) for p38/MAPK, Y27632 (Y, 10 µM) for ROCK or vehicle. The denuded area was photographed before treatment and after 48 h. The migration was calculated in presence of the kinase inhibitors and normalized compared to vehicle treated cells (100%). Migration corresponds to the Mean ± SEM of three independent experiments, performed in triplicates. *P < 0.05. Three different point measurements were taken for each well. B: ERα17p effect on Akt phosphorylation. Cells were treated with 10−6 M ERα17p or vehicle for 15 min. Whole cell lysates were analyzed by immunoblotting for the phosphorylated Akt (Ser473) and actin. Band intensities were quantified and the ratio phospho-Akt/actin was recovered for normalization purposes. Representative blots and plots of phospho-Akt/actin representing the mean ± SEM of three independent experiments are presented. *p < 0.05.
In MCF-7 cells (Fig. 2A), where ERα17p increased cell migration, Y27632 enhanced the peptide action, while wortmannin had an opposite effect. The inhibition of p38 MAPK by SB203580 did not change the ability of ERα17p to modify cell migration, as also recorded for T47D and MDA-MB-231 cells. Interestingly, SB203580 inhibited the migration of SK-BR-3 cells (Fig. 2A). Hence, in this cell line, p38 MAPK could be preferentially targeted, while in MCF-7, T47D, and MDA-MB-231 cells Rho/ROCK and PI3K/Akt pathways may play a pivotal role in the ERα17p-induced migration-modifying effects (Fig. 2A).
In support of the above conclusion, ERα17p was found to directly affect Akt phosphorylation. It decreased phospho-Akt after 15 min of incubation (Fig. 2B) in T47D and MDA-MB-231 cells, while it induced it in MCF7 and not significantly modified it in SK-BR-3 cells, accounting for the differential effect of the peptide on the migration, of these cell lines.
Effect of ERα17p on Actin Cytoskeleton Polymerization and Cellular Distribution
The functional interaction of ERα17p with the afore-mentioned signaling pathways and its effect on cell motility could be associated with a rearrangement of actin network and cytoskeleton dynamics. Indeed, the incubation of breast cancer cells with 10−6 M ERα17p induced rapid changes in actin organization (Figs. 3 and 4). In support of the above differential effects of ERα17p on cell motility, the observed actin changes were not uniform among the investigated cell lines.
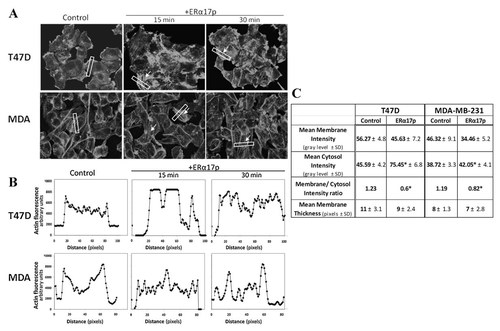
Actin cytoskeleton changes, induced by ERα17p in T47D and MDA-MB-231 cells. A: Cells were treated with 10−6 M ERα17p or with vehicle for short time intervals (15 min, 30 min) and actin cytoskeleton was visualized by rhodamine–phalloidin staining, revealing an intense network of stress fibers (arrows) after ERα17p treatment. B and C: The intensity of actin staining at different cell compartments was derived by image analysis after sampling an area (white box) across a cell as previously described [Giretti et al., 2008]. Ten different random cells were assayed in each experimental condition and in three independent experiments. Representative results are presented as plots of fluorescence intensity versus path in the box in (B), while all results (Mean± SD) and a membrane/cytosol ratio are shown in (C). *p < 0.05 versus control.
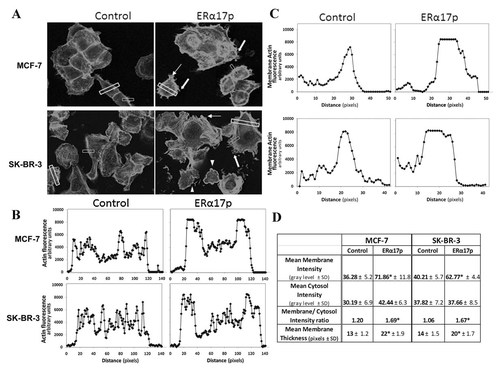
Actin cytoskeleton changes, induced by ERα17p in MCF7 and SK-BR-3 cells. A: Cells were treated with 10−6 M ERα17p or with the vehicle for 15 and 30 min. Actin cytoskeleton was visualized by rhodamine–phalloidin staining showing the modifications of the actin and the formation of specialized cell membrane structures. White arrows indicate pseudopodia, white block arrows fillopodia and arrow heads membrane ruffles. B–D: The intensity of actin staining at different cell compartments was derived by image analysis after sampling an area (block white box) across a cell. In each case, five areas were sampled per each cell and 10 different random cells were assayed in each experimental condition and in three independent experiments. Cell membrane thickness changes were also assayed by using a sample area (five per cell) across the cell membrane (small white box) of 10 different random cells. Representative results are presented as plots of fluorescence intensity versus distance across a cell in (B) and across a cell membrane in (C). All results (Mean± SD) and a membrane/cytosol ratio are shown in (D). *p < 0.05 versus control.
In T47D and MDA-MB-231 cells, in which a decreased cell migration was observed under ERα17p, the thin actin fibers, which were uniformly and randomly located within the cytoplasm in untreated cells, increased and orientated longitudinally (Fig. 3). This phenomenon was transient and time-dependent, with a maximum after 15–30 min. After 60 min, a basal level (similar to untreated cells) was recovered (data not shown). The apparition of an intense network of stress fibers was also shown by the analysis of the fluorescent actin signal intensity, in sample boxes across a cell, that revealed an intracellular accumulation of polymerized actin. As it is shown in Figure 3B, in control cells the actin signal is higher at the membrane, while, in ERα17p treated cells, the signal intensity is preferentially intracellularly distributed. This was also evident by calculating the membrane to cytosol intensity ratio, as shown in Figure 3C.
In MCF-7 and SK-BR-3 cells, in which ERα17p increased cell motility, a different actin cytoskeletal remodeling was observed (Fig. 4): ERα17p induced a shift of actin, principally toward the membrane (Fig. 4A,B,D), characterized by the development of filopodia (finger-like protrusions, block arrow), pseudopodia (identified as weakly adherent longitudinal protrusions, arrow), and membrane ruffles (visible as sheet-like membrane protrusions, arrowheads). Due to the accumulation of sub-membrane polymerized actin, these morphological modifications were accompanied by an increase of the apparent thickness of the cell membrane (Fig. 4C) and an increased membrane to cytosol intensity ratio (Fig. 4D).
The effects of ERα17p on the actin network appear once again ERα-independent, since they were not modified by the ERα inhibitor ICI 182,780 (not shown). Of note, these changes in actin organization were not accompanied by significant modifications of the ratio filamentous (polymerized) to globular (monomeric) actin (F/G ratio, Supplemental Fig. 3B), suggesting that the activity of ERα17p is rather related to a redistribution of polymerized actin, than polymerization changes per se. Thus, the action of ERα17p appears atypical, as also observed with other ERα-derived peptides (Kampa et al., in preparation).
Interference of ERα17p With Membrane Acting Steroids
Redistribution and polymerization of actin are known to occur under the rapid action of estrogen and androgen [reviewed in Kampa and Castanas, 2006; Sanchez and Simoncini, 2010]. Similarly to data of E2-BSA action in MCF-7 cells previously reported [Sanchez et al., 2009], in the presence of ERα17p, we observed a shift of actin toward the membrane and the apparition of pseudopodia. Therefore, we investigated whether ERα17p could interfere with membrane-acting steroids on actin cytoskeleton redistribution.
The distribution of actin in the presence of Testosterone-BSA or E2-BSA (10−6 M each), was modified by 10−6 M ERα17p, in T47D or MDA-MB-231 cell lines (Fig. 5A and B). In T47D cells, as expected, Testosterone-BSA induced an increase of the cortical actin [Kampa et al., 2005, 2006]. ERα17p partially reversed this effect, with the reoccurrence of cytoplasmic actin fibers (Fig. 5A). A partial reversion was also observed when cells were incubated with E2-BSA, even though the latter induced mainly the formation of filopodia and membrane ruffles. Similar effects, with an intracellular redistribution of polymerized actin were observed in MDA-MB-231 cells (Fig. 5B).
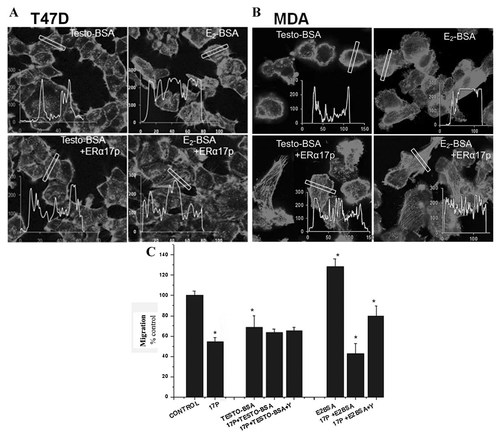
Effects of ERα17p on the actin cytoskeleton changes induced by membrane acting steroids. A and B: Cells were treated with 10−6 M ERα17p and E2-BSA or T-BSA for 15 min. Filamentous actin was visualized by rhodamine–phalloidin staining. The intensity and the distribution of fluorescence (corresponding to F-actin) across a cell (white box) was quantified as described in Material and Methods section and results are presented in insert graphs (fluorescence versus distance). C: The effects of ERα17p on migration in the presence of membrane acting steroids. A linear scratch was performed on a cell monolayer. T47D cells were treated with ERα17p (10−6 M) alone or with membrane acting steroids (10−6 M, E2-BSA or T-BSA) in the presence or in the absence of the ROCK kinase inhibitor Y27632 (Y) (10 µM). The denuded area was photographed before treatment. After 48 h, the migration rate was calculated. The results were normalized with the vehicle treated cells (100%) and were given as the Mean ± SEM of three independent experiments. *p < 0.05.
Moreover, ERα17p inverted the action of membrane-acting estradiol (E2-BSA) by decreasing its ability to promote cell migration in T47D cells (Fig. 5C). On the other hand, ERα17p did not modify the anti-migratory action of Testosterone-BSA, since both agents induce such cytoskeletal changes that do not favor migration (Fig. 5C). Remarkably, data recorded after a simultaneous incubation with ERα17p and E2-BSA in the presence of the ROCK inhibitor Y27632, support the concept of a ROCK-dependent mechanism, as shown in Figure 5C. Finally, ERα17p and E2-BSA shared similar changes on actin cytoskeleton in MCF-7 and SK-BR-3 cells; the peptide did not modify E2-BSA migratory action (data not shown). Thus, even, if the effects mediated by ERα17p are ERα-independent, an indirect role of the latter on a membrane form of estrogen receptor, seems likely.
DISCUSSION
The necessity of new approaches of ER modulation has been advanced, SERMs being accompanied by tumor resistance [see Jordan, 2002; Peng et al., 2009, for reviews]. In this context, the identification of alternative interaction sites, beyond the ER ligand-binding pocket and the synthesis of novel specific endocrine disruptors, interfering with such sites, gained an increased interest [Leclercq et al., 2006; Sengupta and Jordan, 2008]. Accordingly, a number of natural or modified peptide analogs have been synthesized and claimed to act as peptide estrogen-related modulators (PERMs). ERα17p subscribes in this context, as: (i) it corresponds to a site of ERα, targeted by various post-translational modifications [Gallo et al., 2007b], (ii) is subjected to conformational changes [Gallo et al., 2007b], (iii) interacts with calmodulin and Hsp70 [Gallo et al., 2008c], (iv) elicits pseudo-estrogenic responses, under steroid starvation [Gallo et al., 2007b], (v) associates with ERα in vitro, at a site distinct from the hormone-binding pocket [Gallo et al., 2007b], (vi) interferes in vitro with the recruitment of p160 co-activator LxxLL binding motifs [Gallo et al., 2008c], and (vii) induces apoptosis (in vitro and in vivo) and xenograft tumor regression in animals [Pelekanou et al., 2011].
According to our data, ERα17p exerts membrane-initiated actions, strongly compatible with its peptidic nature. Even though its membrane counterpart remains unspecified, the activity displayed by ERα17p seems to be ERα-independent. In addition, we show that ERα17p does not compete with membrane-acting estradiol. Nevertheless, the interaction of the peptide with another ER-related membrane protein or site, important for estrogenic action, is possible. Likewise, we cannot totally exclude a passive association of ERα17p to membranes, in a way that it could modify its interaction properties. Furthermore, we present preliminary data suggesting a possible involvement of GPR30 in the action of ERα17p, which, if further verified, could provide a possible endogenous ligand for this site and explain some reported discrepancies of E2 effects on it [Levin, 2009].
In light of the previously reported activities displayed by ERα17p on cell proliferation and apoptosis [Gallo et al., 2007b; Pelekanou et al., 2011], we also found specific effects of the peptide on cell migration. Our data show two ERα-independent tendencies: In T47D (ERα+) and MDA-MB-231 (ERα−) cells, ERα17p decreased migration; in contrast, it enhanced the migratory activity of MCF-7 (ERα+) and SK-BR-3 (ERα−) cells. Even if these opposite effects indicate an ERα-independent action of ERα17p, a hypothesis related to the cellular receptor stoichiometry (ERα and β+/− and GPR30+/−) and related pathways cannot be excluded. The fact that the strongest ERα17p-mediated inhibitory effects are observed in T47D cells could be relevant to the peptide ability to decrease the rate of phospho-Akt, or its ability to interfere with GPR30 sites, which are abundant in these cells. Likewise, the low inhibitory effects of ERα17p on MDA-MB-231 could be explained by the low level of GPR30 in this cell line [Filardo et al., 2002 and data presented here], a result needing additional confirmation. However, the increased migration of MCF-7 and SKBR3, which share a high level of GPR30, is more surprising and suggests that other factors, not determined in the present study, should be additionally implicated. Moreover, the ERα17p effects in SK-BR-3 cells could be related to its ability to interfere with the p38 MAPK in this cell line, which is a key actor of cell migration [Yu et al., 2004]. Therefore, the observed discrepancies could be attributed (even partially) to particular phenotype and signaling characteristics of the different cell lines.
Recent data suggest that small GTPases, such as Rho, Rac, and Cdc42, are involved in the nucleation of new fibrilar actin, via specific pathways, in which a primordial role of Rho/ROCK is documented [reviewed in Schmitz et al., 2000; Heasman and Ridley, 2008]. Here, we show that in the presence of ERα17p, Rho/ROCK inhibition enhances the motility in all tested cell lines, with the notable exception of SK-BR-3 cells. In addition, the inhibition of PI3k/Akt decreases its migratory potency, in all cell lines. Therefore, a modulation of PI3K/Akt by ERα17p is likely. In this regard, it should be stressed that actin cytoskeleton dynamics-modification, through PI3K/Akt/Cdc42/actin pathways, has been reported to modify the motility and survival of breast and prostate cancer cells [Papakonstanti et al., 2003; Kampa et al., 2005; Kampa et al., 2006]. Our results concerning the existence of an interaction of ERα17p with membrane binding counterparts, in all studied cell lines, are relevant to published data of estrogen-induced changes in the motility of thyroid [Rajoria et al., 2010], melanoma [Matsuoka et al., 2009], ovary [Hua et al., 2008], endometrial [Acconcia et al., 2006], or breast cancer cells [Hua et al., 2008], through classical and extranuclear membrane-initiated actions [Giretti et al., 2008]. Interestingly, in contrast to its inhibitory effect on the E2-BSA-induced migration, ERα17p had no effect on Testosterone-BSA anti-migratory action, in accordance to the changes in the distribution pattern of intracellular actin fibers. In this context and even if previous studies have outlined that the rate of ERα in breast cancer cells has an inverse relation with respect to cell invasion [Rochefort et al., 1998], the present work shows unambiguously that the nuclear ERα or β is not a prerequisite for the action of ERα17p on motility. It is noteworthy that the AF-2 domain of ERα has already been reported to be involved in the motility of breast cancer cells [Platet et al., 2000]. Accordingly, we show that ERα17p, which corresponds to the extremity of the N-terminal tail of the AF2, modulates positively or negatively the migration of breast cancer cells, independently from the constitutive presence of ERα.
ERα17p-mediated migration effects were accompanied by a specific reorganization of the actin cytoskeleton, but not by significant modification of its synthesis or polymerization rate. Hence, the effects of the peptide on actin cytoskeleton are rather qualitative than quantitative. In this regard, it should be stressed that, in cells in which the motility was enhanced, a peripheral redistribution of actin accompanied by the apparition of filopodia and lamelipodia was observed, in line with previous reports [Insall and Machesky, 2009]. In contrast, in cell lines characterized by an ERα17p-induced decrease of motility, an intense intracellular stress fibers network was found. Even if in the present work, our interest in morphologic changes was limited in actin cytoskeleton remodeling, an eventual involvement of ERα17p in mesenchymal-to-epithelial Transition (MET), a crucial counterpart in tumorigenesis and strongly associated with cell motility, could be envisaged. Recently, MET has been reported to be regulated by estrogen-related (orphan nuclear) receptors (ERR), which supports the idea of steroid or steroid-like ligands acting on other targets than the classical nuclear ERα [Tiraby et al., 2011].
Cell migration is a prerequisite for cancer metastasis [Brooks et al., 2010] and migration-modifying agents could constitute potential candidates for new cancer therapeutics. As shown here, ERα17p, a synthetic peptide corresponding to the P295-T311 sequence of ERα, may open new roads for the synthesis of innovative therapeutics for breast cancer, including hormone-resistant ones, which are of poor prognosis. Especially, as ERα17p or an ERα17p-containing fragment of ERα could be produced within the proteasome [Gallo et al., 2008b] to control, in an autocrine or paracrine way, cell migration and, therefore, the dissemination of malignant breast cancer cells and metastasis, this peptide is a good candidate to build up new PERMs for breast cancer control.




