Reactive oxygen species-mediated endoplasmic reticulum stress and mitochondrial dysfunction contribute to polydatin-induced apoptosis in human nasopharyngeal carcinoma CNE cells†‡
Conflict of interest: The authors declare that there are no conflicts of interest.
Huanhai Liu and Shuwei Zhao contributed equally to this work.
Abstract
Previous studies revealed that polydatin, a natural small compound, possessed protective effect against ischemia/reperfusion injury and inflammation. However, the action and molecular mechanism of its potent anti-cancer activity remain poorly understood. In the present study, polydatin significantly killed several human tumor cell lines in a dose- and time-dependent manner. The compound also dose-dependently caused mitochondrial apoptosis in human nasopharyngeal carcinoma CNE cells. In addition, polydatin triggered endoplasmic reticulum (ER) stress and down-regulated the phosphorylation of Akt in CNE cells, while knock-down of CCAAT/enhancer-binding protein homologous protein (CHOP) dramatically abrogated the inactivation of Akt and reversed the pro-apoptotic effect of polydatin. Furthermore, polydatin provoked the generation of reactive oxygen species in CNE cells, while the antioxidant N-acetyl cysteine almost completely blocked the activation of ER stress and apoptosis, suggesting polydatin-induced reactive oxygen species is an early event that triggers ER stress mitochondrial apoptotic pathways in CNE cells. Taken together, these findings strongly suggest that polydatin might be a promising anti-tumor drug and our data provide the molecular theoretical basis for clinical application of polydatin. J. Cell. Biochem. 112: 3695–3703, 2011. © 2011 Wiley Periodicals, Inc.
Apoptosis is a physiological mechanism for eliminating malignant cells, including cancer cells, without eliciting damage to normal cells or surrounding tissues [Elmore, 2007]. The understanding of apoptosis has provided the basis for novel targeted therapies that can induce death in cancer cells or sensitize them to established cytotoxic agents [Ghobrial et al., 2005]. The activation of caspase family proteases is the key step in apoptosis, and several pathways lead to caspase activation [Lavrik et al., 2005]. Up to now, three predominant apoptotic pathways, namely death receptor-mediated extrinsic pathway, mitochondria-mediated intrinsic pathway, and endoplasmic reticulum (ER) stress-mediated apoptotic pathway have been elucidated. The accumulation of unfolded or misfolded protein in ER can induce ER stress. As a protective mechanism, these unfolded protein response (UPR) signaling pathways initiate cell protective mechanisms to protect cells against ER stress-induced damage. However, if protein aggregation is persistent and the stress cannot be alleviated, cell apoptotic pathways will be initiated [Faitova et al., 2006]. CHOP (CCAAT/enhancer-binding protein homologous protein), also known as growth-arrest and DNA-damage inducible gene 153, is a key pro-apoptotic transcription factor that is closely related to ER stress [Oyadomari and Mori, 2004]. Thus, targeting apoptosis pathways in premalignant and malignant cells may be an effective strategy for cancer prevention and treatment.
Plant-derived natural products occupy a very important position in the area of cancer chemotherapy. Molecules such as vincristine, vinblastine, paclitaxel, camptothecin derivatives, epipodophyllotoxin, etc. are invaluable contributions of nature to modern medicine. However, the quest to find out novel therapeutic compounds for cancer treatment and management is a never-ending venture [Li and Vederas, 2009]. Natural compounds from fruits, vegetables, and grains, possess anti-cancer properties and represent a promising therapeutic approach for the prevention and treatment of many cancers [Kawasaki et al., 2008]. The anti-tumor activities of the natural compounds have recently attracted much attention [Diaz et al., 2008; Khan et al., 2008; Kim et al., 2010]. In the present study, we found that polydatin, a natural small compound, exhibited significant cytotoxicity in various human tumor cell lines. Therefore, we investigated the anti-cancer activity and the mechanism of polydatin in human nasopharyngeal carcinoma CNE cells, including ER stress and mitochondrial dysfunction.
Polydatin is an effective small natural molecule from Polygonum cuspidatum Sieb. et Zucc., which possessed protective effect against shock [Zhao et al., 2003], ischemia/reperfusion injury [Cheng et al., 2006], congestive heart failure [Gao et al., 2010], endometriosis [Indraccolo and Barbieri, 2010], and colitis [Yao et al., 2011]. Previously, polydatin was reported to have lipid-lowering effects in hyperlipidemic hamsters [Du et al., 2009] and hyperlipidemic rabbits [Xing et al., 2009]. However, the action and mechanism of anti-cancer effect of polydatin on human tumor cells remain vague. In the present study, we demonstrate that polydatin is a potent anti-cancer agent against human nasopharyngeal carcinoma, and reactive oxygen species-dependent ER stress mitochondrial pathway is involved in the signaling of polydatin-induced apoptosis. Our data provide the molecular theoretical basis for clinical application of polydatin.
MATERIALS AND METHODS
Cell Culture
Human nasopharyngeal carcinoma CNE cell line, cervical carcinoma HeLa cells, hepatoma cell line SMMC-7721 cells, and epidermal carcinoma cell line A-431 cells were purchased from the Cell Bank of Shanghai Institute of Biochemistry and Cell Biology (Chinese Academy of Sciences, China), and they were grown at 37°C in a 5% (v/v) CO2 atmosphere. The cells were maintained in DMEM medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS, Invitrogen) plus 2 mmol/l glutamine and 50 U/ml penicillin. Human peripheral blood mononuclear cells (PBMC) were isolated from fresh heparinized whole blood donated by healthy volunteers who gave informed consent. Separation of blood cells was performed using density gradient centrifugation (Hao-Yang Bio. Co. Ltd., Tianjin, China). The buffy coat layer containing PBMC at the interface was carefully removed, washed twice with PBS and centrifuged at 200g for 5 min. The cells were suspended in RPMI 1640 supplemented with 10% FCS, and incubated in the presence of 5% CO2 in air at 37°C.
Drugs and Reagents
Polydatin (3,4',5-trihydroxystilbene-3-β-mono-D-glucoside, purity > 99%, obtained from Suzhou Baozetang Biotechnology Co., Ltd., Suzhou, China) was dissolved at a concentration of 20 mmol/l in 100% DMSO as a stock solution, stored at −20°C, and diluted with medium before each experiment. The final DMSO concentration did not exceed 0.1% throughout the study (all the control groups are composed of 0.1% DMSO). 3-(4,5-dimethyl-2-thiazyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) and N-acetyl cysteine (NAC) were purchased from Sigma Chemical Co. (St. Louis, MO). NAC was freshly dissolved in medium at a stock concentration of 40 mmol/l. The pH adjusted to 7.4, and then the NAC was sterilized by 0.22 µm filtration and diluted to different concentrations. All the NAC treatments mentioned in this article are pretreatments. Annexin V-FITC (fluorescein isothiocyanate)/PI (propidium iodide) kit was purchased from BD Biosciences (San Jose, CA). ProteoExtract Cytosol/Mitochondria Fractionation Kit was purchased from Merck Bioscience (Bad Soden, Germany). Anti-cleaved caspase-3, anti-caspase-8, anti-cleaved caspase-9, anti-cleaved caspase-4, anti-phospho-Akt (Thr 308), and anti-Akt were purchased from Cell Signaling Technology (Beverly, MA). Anti-ATF4 (CREB2), anti-CHOP (GADD153), anti-XBP-1S, anti-cytochrome c, anti-cytochrome c oxidase subunit IV (COX IV), anti-lamin B, and anti-α Tubulin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The 5-(and-6)-darboxy-2′-7′-dichlorofluorescin diacetate (carboxy-DCFDA) were purchased from Invitrogen. All other chemicals were purchased from Sigma Chemical Co.
Cytotoxicity Assay
The cytotoxic effect of polydatin was measured using the MTT assay. Cells were seeded in 96-well microtiter plates with polydatin for indicated time, then MTT solution (5 mg/ml in RPMI 1640 medium; Sigma-Aldrich) was added (10 µl/well), and plates were incubated for a further 4 h at 37°C. The purple formazan crystals were dissolved in 100 µl of DMSO. After 5 min, the plates were read on an automated microplate spectrophotometer (Sunrise, Tecan, Austria) at 570 nm. Assays were performed in triplicate on three independent experiments.
Cell Apoptosis Assay
The detachment of CNE cells was performed by 0.25% trypsin in phosphate buffered saline prior to FACS analysis. Importantly, the detached cells must be neutralized with medium (DMEM + 10% FBS) as soon as possible after the treatment of trypsin. To avoid the damage of the treatment, the washing medium (DMEM + 0.5% FBS) was used in the centrifugation. Then the detached cells were stained with Annexin V-FITC/PI and measured by FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA). Annexin V+/PI− cells were considered as apoptotic cells.
Subcellular Fractionation
The proteins in the cells were separated into cytosolic and mitochondrial fractions using the ProteoExtract Cytosol/Mitochondria Fractionation Kit (Merck Bioscience) according to the procedures provided by the manufacturer. To check the selectivity of proteins from subcellular fractionation, Tubulin, and COX IV were used as marker proteins representing the cytosolic and mitochondrial fractions, respectively.
Western Blot
Proteins were extracted in lysis buffer (30 mmol/l Tris, pH 7.5, 150 mmol/l sodium chloride, 1 mmol/l phenylmethylsulfonyl fluoride, 1 mmol/l sodium orthovanadate, 1% Nonidet P-40, 10% glycerol, and phosphatase and protease inhibitors). The proteins were then separated by SDS-PAGE and electrophoretically transferred onto polyvinylidene fluoride membranes. The membranes were probed with antibodies overnight at 4°C, and then incubated with a horse radish peroxidase-coupled secondary antibody. Detection was performed using a LumiGLO chemiluminescent substrate system (KPL, Guildford, UK).
Small Interference RNA
The CHOP-specific siRNA (sense 5′-CCAGGAAACGGAAACAGA-3′; antisense 5′-UCUGUUUCCGUUUCCUGG-3′) [Guan et al., 2009], and non-silencing scrambled siRNA were synthesized and purchased from Genescript (Nanjing, China). The siRNA transfection was performed as follow. Briefly, CNE cells were plated in 6-well culture plates at density of 3 × 105 and transfected with siRNA by using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's protocol. Briefly, for each well, 5 µl Lipofectamine 2000 was diluted in 250 µl Opti-MEMI medium (Invitrogen). This mixture was carefully added to a solution containing 200 nmol/l siRNA in 250 µl Opti-MEMI medium. The solution was incubated for 20 min at room temperature, and then gently dripped into the CNE cells in 2 ml antibiotic free medium. Regular growth medium was added 6–12 h after transfection. Twenty-four hours after transfections, cells were treated with 10 µmol/l polydatin for 12 h. Then cells were harvested and performed to RT-PCR or Western blot or apoptosis analysis.
Detection of Intracellular Reactive Oxygen Species
The production of intracellular reactive oxygen species was measured in the CNE cell line using the oxidation-sensitive fluorescent dye 5-(and-6)-darboxy-2′-7′-dichlorofluorescin diacetate (carboxy-DCFDA). An increase in green fluorescence intensity is used to quantify the generation of intracellular reactive oxygen species. After adding carboxy-DCFDA at a final concentration of 15 µmol/l to the culture medium, the cells were incubated at 37°C for an additional 30 min, and then they were harvested, washed with PBS, and measured immediately by FACSCalibur flow cytometer (Becton Dickinson).
Statistical Analysis
Data are expressed as mean ± SEM. Statistical significance was determined by one-way analysis of variance followed by the Bonferroni post-hoc test for multiple comparisons or the two-tailed Student's t-test. A P value of less than 0.05 was considered statistically significant.
RESULTS
Polydatin Killed Various Human Tumor Cells
The cytotoxic effect of polydatin was determined in various human tumor cells including human cervical carcinoma HeLa cells, hepatoma cell line SMMC-7721 cells, epidermal carcinoma A-431 cells, and nasopharyngeal carcinoma CNE cells. As shown in Figure 1A, human nasopharyngeal carcinoma CNE cells exhibited time- and dose-dependent sensitivity to polydatin, with IC50 values (the concentration of drug inhibiting 50% of cells) around 7 µmol/l at 24 h. In addition, polydatin also killed human cervical carcinoma HeLa cells, hepatoma cell line SMMC-7721 cells, and epidermal carcinoma cell line A-431 cells in a dose-dependent manner (Fig. 1B). However, polydatin at the concentrations of 5–20 µM did not affect the survival of human keratinocyte HaCaT cells or human peripheral blood mononuclear cells (PBMC) (Fig. 1C), indicating that high concentration of polydatin did not influence the normal tissue cells or blood cells. These results suggest that polydatin has promising anti-tumor activity against human carcinoma cells with low cytotoxic effect on normal cells.
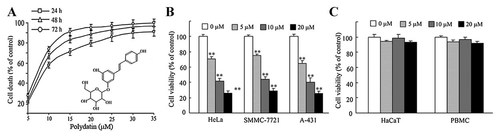
Polydatin killed various human tumor cells. A: Polydatin time-dependently and dose-dependently inhibited the viability of human nasopharyngeal carcinoma CNE cells. Cells were treated with different concentrations of polydatin for 24, 48, and 72 h, respectively. Insert: Molecular structure of polydatin. B: Polydatin dose-dependently suppressed the viability of human cervical carcinoma HeLa cells, human hepatoma SMMC-7721 cells and human epidermal carcinoma cell line A-431 cells. Cells were treated with different concentrations of polydatin for 24 h. **P < 0.01 versus drug-untreated group. C: The cytotoxic effect of polydatin on human keratinocyte HaCaT cells and human peripheral blood mononuclear cells (PBMC). Cells were treated with different concentrations of polydatin for 24 h. Cell viability was estimated by the MTT assay. Data represent the mean ± SEM of three different experiments with triplicate sets in each assay.
Polydatin Induced Significant Apoptosis in Human Nasopharyngeal Carcinoma CNE Cells
Because the chemotherapeutic agent against nasopharyngeal carcinoma in the clinical setting was still lack, the nasopharyngeal carcinoma cell line CNE cells were focused. To assess whether the cytotoxic effect of polydatin was caused by apoptotic cell death, we determined apoptosis of CNE cells using Annexin V/PI binding assay followed by flow cytometry. As illustrated in Figure 2A, polydatin induced a dose-dependent increase in apoptotic CNE cells: approximately 41.1% of the cells were at early apoptosis in 10 µmol/l polydatin-treated group, compared with drug-untreated group. Annexin V- and PI-double positive cells were observed with about 24.0% of total cells in the polydatin-treated groups, indicating a certain level of necrosis induced by polydatin. To assess the role of mitochondria in polydatin-induced cell death, we tested whether polydatin caused a loss of mitochondrial membrane potential. Compared with the corresponding control, polydatin caused an obvious decrease of mitochondrial membrane potential in CNE cells in a time-dependent manner (Fig. 2C).
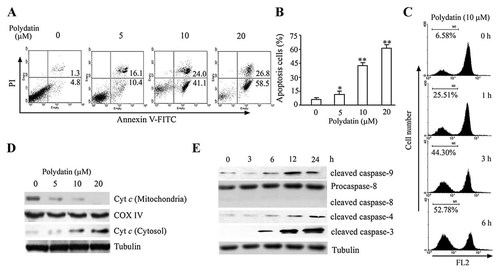
Polydatin caused significant apoptosis in human nasopharyngeal carcinoma CNE cells. A: Apoptosis in CNE cells was assessed at 12 h after treatment with polydatin by Annexin V-FITC/PI binding and measured by flow cytometry. Numbers indicate the percentage of cells in each quadrant. B: The statistical data of three independent experiments. *P < 0.05, **P < 0.01 versus drug-untreated group. C: CNE cells were treated with or without 10 µM polydatin for indicated time, then cells were harvested and the disruption of mitochondrial transmembrane potential was measured using fluorochrome dye JC-1 by flow cytometry. The histogram shown here is one of three different experiments. D: Polydatin dose-dependently induced cytochrome c release to cytosol. CNE cells were treated with or without polydatin for 24 h, then the cells were harvested and separated into cytosolic and mitochondrial fractions using the commercial fractionation kit. The expressions of cytochrome c (Cyt c) in cytosol and mitochondria were analyzed by Western blotting. The data shown here is one of three different experiments. E: Polydatin triggered caspase cascades in CNE cells. Cells were treated with or without 10 µmol/l polydatin for indicated times, then the cells were harvested and lysed. The expressions of apoptosis-related caspase proteins were analyzed by Western blotting. The data shown here are one of three different experiments.
Polydatin Triggered Cytochrome c Release From Mitochondria to Cytosol and Activated Caspase Cascades in Human Nasopharyngeal Carcinoma CNE Cells
The mitochondrial apoptosis pathway is one of the critical pathways of apoptosis. In this study, mitochondrial protein and cytosolic protein were isolated from polydatin-treated CNE cells, respectively, and then these proteins were subjected to Western blotting. As shown in Figure 2D, polydatin remarkably increased the cytosolic cytochrome c and notably decreased the mitochondrial cytochrome c as compared with the drug-untreated group.
We next assessed the effects of polydatin on caspases activation in CNE cells by Western blot. The results showed that the activated cleavage fragment of caspase-9 increased in response to polydatin, whereas the activated cleavage fragment of caspase-8 was not detected (Fig. 2E), suggesting that mitochondria-mediated intrinsic apoptosis pathway but not death receptor-mediated extrinsic apoptosis pathway might be involved in polydatin-induced apoptosis. ER stress leads to proteolytic cleavage of caspase-12 in mouse and caspase-4 in human, both of which localize to the cytoplasmic side of the ER membrane. In our study, caspase-4 was also activated by treatment with polydatin, as shown from the increase of cleavage fragment of caspase-4 after 12 h exposure to polydatin. In parallel with the activation of caspase-9 and -4, there were also increases in the cleavage of effector caspase, namely caspase-3 (Fig. 2E). The activation of caspase-9 and -4 indicated that mitochondria and ER stress-mediated apoptotic pathways might be involved in polydatin-induced cell apoptosis in CNE cells. Next, we asked whether polydatin could induce ER stress-mediated apoptotic pathway.
Polydatin Induced ER Stress in Human Nasopharyngeal Carcinoma CNE Cells
To determine whether polydatin could induce ER stress, we investigated several crucial ER stress markers, including ATF4, XBP-1S, and CHOP. As a result, the expressions of these ER stress-related proteins in CNE cells were time-dependently (Fig. 3A) and dose-dependently (Fig. 3B) induced after polydatin treatment.
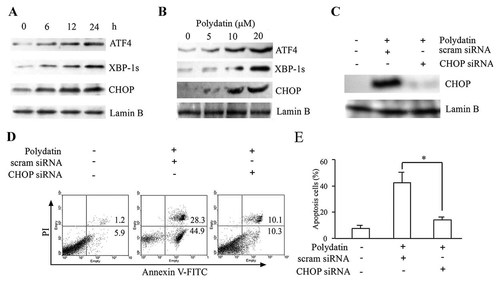
Polydatin induced CHOP-dependent cell apoptosis in human nasopharyngeal carcinoma CNE cells. (A,B) Polydatin triggered activation of ER stress. Representative immunoblots against CHOP, ATF-4, XBP-1 and lamin B from the nuclear extracts of CNE cells treated with 10 µmol/l polydatin for indicated times (A). Lamin B was used as a loading control of nuclear protein. The data shown here are one of three different experiments. (B) CNE cells were treated with polydatin for 24 h, then subjected to immunoblots. (C–E) Cells were transfected with CHOP-specific siRNA and scrambled siRNA. Twenty-four hours after transfection, cells were treated with 10 µmol/l polydatin for 24 h. The protein level of CHOP was determined by Western blot (C). The results shown are representative of at least three independent experiments. (D) Apoptosis was assessed by Annexin V-FITC/PI binding and measured by flow cytometry. (E) The statistical data of three independent experiments. *P < 0.05.
CHOP and AKT Were Involved in ER Stress-Mediated Cell Apoptosis Induced by polydatin
Having confirmed the initiation of ER stress by polydatin treatment, we next investigated whether polydatin-induced ER stress actually contributed to CNE cell apoptosis. CHOP is induced during ER stress and regularly participates in ER stress-mediated apoptosis [Oyadomari and Mori, 2004]. To further establish a functional role of CHOP in polydatin-induced apoptosis of human nasopharyngeal carcinoma CNE cells, CHOP-specific siRNA was used to inhibit CHOP expression. A scrambled RNA duplex was used as a negative control. CHOP-specific siRNA effectively down-regulated CHOP mRNA (data not shown) and protein levels (Fig. 3C) and significantly reduced polydatin-induced CNE cell apoptosis (Fig. 3D,E) compared with scrambled siRNA. The similar result was also seen in human hepatoma SMMC-7721 cells (Supplementary Fig. 1). These results indicate that CHOP participates in ER stress-mediated apoptosis induced by polydatin in human tumor cells.
What is the downstream signal of CHOP-mediated cell apoptosis? AKT plays a critical role in controlling cell survival by resisting ER stress-induced apoptotic signal [Guan et al., 2009]. Western blot result showed that phospho-Thr 308-AKT (the activated form of AKT) decreased in a time-dependent manner after polydatin treatment; while no apparent change of AKT level was observed (Fig. 4A,B). This finding indicates that down-regulation of AKT activation probably contributes to the pro-apoptotic effect of polydatin in CNE cells. Silencing of CHOP gene expression prevented the reduction of phospho-Thr 308-AKT in response to polydatin (Fig. 4C,D). Thus CHOP-induced cell apoptosis is probably mediated by inhibiting the activation of anti-apoptotic kinase AKT.
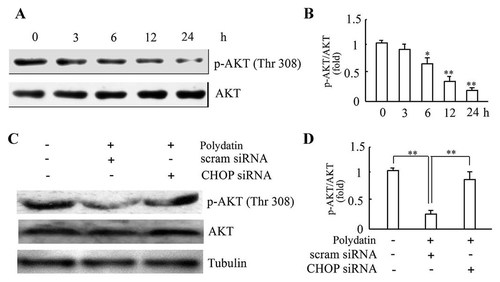
CHOP-siRNA inhibited the inactivation of AKT induced by polydatin. A: Time-dependent effect of polydatin on the activity of AKT. CNE cells were treated with 10 µmol/l polydatin for indicated time, and the protein levels of AKT or phospho-AKT were determined by Western blot. The results shown are one of three independent experiments. B: The statistical data of protein expression from three independent experiments. *P < 0.05, **P < 0.01 versus 0 h. C: CHOP-specific siRNA prevented dephosphorylation of AKT in response to polydatin. Cells were transfected with CHOP-specific siRNA and scrambled siRNA, respectively. Twenty-four hours after transfection, cells were treated with 10 µmol/l polydatin for 12 h. The protein levels of AKT or phospho-AKT were determined by Western blot. The results shown are representative of three independent experiments. D: The statistical data of protein expression from three independent experiments. **P < 0.01.
Polydatin-Induced ER Stress Required Reactive Oxygen Species Generation
Next, we examined the upstream regulatory mechanisms leading to polydatin-induced ER stress. As a result, there was a significant increase in intracellular reactive oxygen species levels in CNE cells treated with 10 µmol/l polydatin (Fig. 5A). Pretreatment with 1 mmol/l antioxidant NAC for 1.5 h, abrogated the increase in reactive oxygen species and almost completely inhibited cell apoptosis induced by polydatin in human nasopharyngeal carcinoma CNE cells (Fig. 5B,C) and human hepatoma SMMC-7721 cells (Supplementary Fig. 2), suggesting that reactive oxygen species play an important role in polydatin-induced cell apoptosis. It was noted that polydatin hardly induced reactive oxygen species generation in HaCaT cells and PBMC cells (Supplementary Fig. 3). To test the relationship between polydatin-induced reactive oxygen species production and ER stress, we incubated CNE cells with NAC, prior to polydatin treatment. The results showed that NAC significantly attenuated polydatin-induced activations of ATF-4, XBP-1S, and CHOP following polydatin stimulation (Fig. 5D). Furthermore, we also investigated the effect of scavenging reactive oxygen species by NAC on polydatin-induced mitochondrial dysfunction. Pretreatment with NAC prevented polydatin-induced the activation of caspase-9, -4, and -3 (Fig. 5E). These results indicate that reactive oxygen species play a major role in polydatin-induced ER stress and mitochondrial dysfunction in CNE apoptosis process.
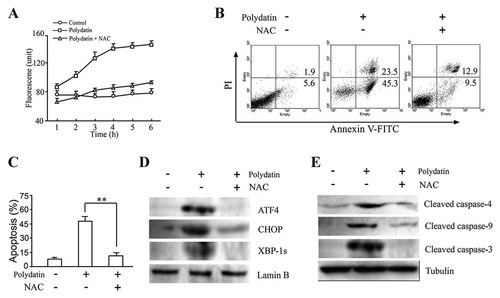
Polydatin-induced reactive oxygen species generation triggered ER stress and mitochondrial dysfunction. A: Polydatin triggered reactive oxygen species generation in human nasopharyngeal carcinoma CNE cells. Cells were pretreated with or without 1 mmol/l NAC for 1.5 h and then treated with 10 µmol/l polydatin for indicated time. The level of reactive oxygen species in cells was detected by carboxy-DCFDA-dependent assay. Data represent the mean ± SEM of three different experiments with triplicate sets in each assay. B: CNE cells were pretreated with or without 1 mmol/l NAC for 1.5 h and then treated with 10 µmol/lol/l polydatin for 12 h. The effect of NAC on polydatin-induced apoptosis was determined by flow cytometry. C: The statistical data of three independent experiments. **P < 0.01. D: The effect of NAC on ER stress markers. The nuclear extracts of CNE cells at 24 h following polydatin exposure were isolated and subjected to Western blot. Lamin B was used as a loading control of nuclear protein. The data shown here are one of three different experiments. E: The effect of NAC on activation of caspase-9, -4, and -3. Tubulin was used as a loading control. The results shown are representative of at least three independent experiments.
DISCUSSION
There has been growing interest to explore novel anti-cancer property from natural herbal medicines [Yamada, 1991]. Traditional Chinese medicine that has been practiced for thousands of years in clinic often provides a vast source of pharmaceutical material for the development of effective drugs [Zhang and Tang, 2006; White, 2008]. The substantial obstacle, however, is that the detailed mechanisms of action of many of these natural active ingredients are largely unknown [Ren et al., 2008; Sun et al., 2009]. So finding effective anti-cancer natural compounds will not only facilitate our understanding of regulation for the network of cancer, but also provide some useful small probes for investigating the interactions of critical signaling molecules involved in cancer. To our interest, in the present study, polydatin was clearly demonstrated to kill various human tumor cell lines, such as SMMC-7721, CNE, A-431 cells. However, it was noted that polydatin at the concentrations mentioned above hardly affected the viability of the benign non-tumor human keratinocyte HaCaT cells and PBMC cells, suggesting a selective anti-tumor action of polydatin to some degree. Similarly, Yang et al. had previously reported that gambogic acid could selectively induce apoptosis of human hepatoma SMMC-7721 cells while had relatively less effect on human normal embryon hepatic L02 cells due to the higher distribution and longer retention time of gambogic acid in tumor cells compared to the normal cells [Yang et al., 2007]. The detailed mechanisms of polydatin killing tumor cells but not benign cells need further investigation. Apoptosis is a major mechanism to eliminate cancer cells. Many chemopreventive compounds appear to target signaling molecules in apoptosis-related pathways. Thus, targeting apoptosis pathways in premalignant and malignant cells may be an effective strategy for cancer prevention [Sun et al., 2004; Ghobrial et al., 2005]. The present study indicates that polydatin, a small natural compound, is a novel anti-cancer agent through inducing the production of reactive oxygen species which triggers ER stress and mitochondrial apoptotic pathways in human nasopharyngeal carcinoma CNE cells.
Increasing evidence suggests that ER stress responses account for the chemoprevention of human cancer by chemopreventive agents [Guan et al., 2009; Lai et al., 2009; Quan et al., 2010; Liu et al., 2011]. Disruption of ER homeostasis in tumor cells by these agents triggers cellular stress responses including the UPR, the ER specific stress response. The signaling pathways of the UPR are important for maintenance of normal cellular homeostasis. However, inappropriate activation of the UPR can lead to cellular dysfunction and ultimately cell death [Kadowaki et al., 2004]. The results from our functional studies demonstrated that polydatin not only activated the UPR, but also triggered mitochondrial apoptosis in human CNE cells. To further demonstrate our notion that polydatin-induced ER stress response is critical to its pro-apoptotic effect, we examined the effect of knocking down CHOP expression on polydatin-induced apoptosis in human CNE cells. CHOP is a UPR-induced transcription factor that mediates ER stress-mediated apoptosis [Malhotra and Kaufman, 2007]. Previously, Feng et al. [2003] reported that macrophages from CHOP−/− mice were highly protected from free cholesterol-induced cell death. Our current study also clearly showed that down-regulation of CHOP expression significantly blocked polydatin-induced apoptosis in CNE cells (Fig. 3D), suggesting the critical role of CHOP in polydatin-induced apoptosis. However, the downstream effectors of CHOP are poorly understood. CHOP expression can result in the down-regulation of Bcl-2 expression, depletion of cellular glutathione, and exaggerated production of reactive oxygen species [Li et al., 2007]. Moreover, Guan et al. [2009] reported that AKT inactivation was the downstream event of CHOP-mediated human acute promyelocytic leukemia NB4 cell apoptosis induced by sodium selenite. In this study, we found that silencing of CHOP gene expression by siRNA prevented polydatin-induced the inactivation of anti-apoptotic kinase AKT. Activated AKT can induce the phosphorylation of Bad and cause a decrease of cytochrome c release [McCullough et al., 2001]. These data suggest that CHOP acts as a critical negative regulator of AKT in polydatin-induced CNE cell apoptosis. Therefore, polydatin-induced ER stress and subsequent activation of the UPR represents an important cellular mechanism underlying polydatin-induced apoptosis of human CNE cells.
A growing body of data implicates reactive oxygen species are the known mediators of intracellular signaling cascades, which trigger a series of mitochondria-associated events including apoptosis [Moon et al., 2009; Wang et al., 2009]. Excessive production of reactive oxygen species nevertheless leads to oxidative stress, loss of cell function, and ultimately apoptosis or necrosis [Wang et al., 2008; Moon et al., 2009]. In the present study, we found that polydatin induced reactive oxygen species generation in human nasopharyngeal carcinoma CNE cells (Fig. 5A) as well as hepatoma SMMC-7721 cells (Supplementary Fig. 2A). However, polydatin hardly induced reactive oxygen species generation in normal cells such as human keratinocyte HaCaT cells and human peripheral blood mononuclear cells (Supplementary Fig. 3). The detailed mechanisms of this compound inducing reactive oxygen species generation in human tumor cells but not benign cells need further investigation. Furthermore, we demonstrated that polydatin-induced CNE cell apoptosis was associated with the generation of reactive oxygen species, ER stress and mitochondrial damage. What are the relationships between these three signals? Polydatin induced the activation of UPR at early times and then activated CHOP, which may initiate and amplify mitochondrial membrane permeabilization by dephosphorylation of AKT. Thus CHOP may mediate the apoptotic signals from ER to mitochondria. The activation of UPR is probably earlier than mitochondrial dysfunction. Previous studies showed that high levels of reactive oxygen species could be an effective inducer of cell apoptosis, probably through activation of ER stress-mediated apoptotic pathway [Das et al., 2007; Quan et al., 2010]. Removal reactive oxygen species by antioxidant NAC could attenuate polydatin-induced the activation of UPR and at the same time abolish the expression of CHOP induced by polydatin. NAC also completely prevented polydatin-induced the activation of caspase-4, -9, and -3. Taken together, these results show that the production of reactive oxygen species is an early event that initiates mitochondria and ER stress-mediated apoptotic pathways in CNE cells.
In conclusion, our results demonstrate that polydatin-induced apoptosis in human nasopharyngeal carcinoma cells is mediated by the activation of ER stress and the dysfunction of mitochondria that require reactive oxygen species generation. The proposed apoptotic pathways induced by polydatin are depicted in Figure 6. Our study thus provides a rationale for the development of polydatin as chemotherapeutic agent against nasopharyngeal carcinoma in the clinical setting. Taken together, these findings strongly suggest that polydatin might be a promising anti-tumor drug.
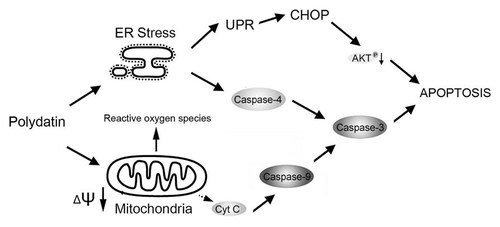
Overview of pathways for polydatin-induced apoptosis in human nasopharyngeal carcinoma CNE cells. Polydatin induces the production of reactive oxygen species which triggers ER stress and mitochondrial apoptotic pathways in CNE cells. Polydatin induces the activation of UPR first and then activates CHOP, which contributes to cell apoptosis by inhibition of anti-apoptotic kinase AKT activity. Inactivated AKT may cause mitochondrial dysfunction by dephosphorylation of Bad. ER stress and mitochondrial dysfunction induce activation of caspase-4 and -9, respectively and in concert mediate polydatin-induced CNE cell apoptosis.




