The activation state of the inositol 1,4,5-trisphosphate receptor regulates the velocity of intracellular Ca2+ waves in bovine aortic endothelial cells
Abstract
Ca2+ is a highly versatile second messenger that plays a key role in the regulation of many cell processes. This versatility resides in the fact that different signals can be encoded spatio-temporally by varying the frequency and amplitude of the Ca2+ response. A typical example of an organized Ca2+ signal is a Ca2+ wave initiated in a given area of a cell that propagates throughout the entire cell or within a specific subcellular region. In non-excitable cells, the inositol 1,4,5-trisphosphate receptor (IP3R) is responsible for the release of Ca2+ from the endoplasmic reticulum. IP3R activity can be directly modulated in many ways, including by interacting molecules, proteins, and kinases such as PKA, PKC, and mTOR. In the present study, we used a videomicroscopic approach to measure the velocity of Ca2+ waves in bovine aortic endothelial cells under various conditions that affect IP3R function. The velocity of the Ca2+ waves increased with the intensity of the stimulus while extracellular Ca2+ had no significant impact on wave velocity. Forskolin increased the velocity of IP3R-dependent Ca2+ waves whereas PMA and rapamycin decreased the velocity. We used scatter plots and Pearson's correlation test to visualize and quantify the relationship between the Ca2+ peak amplitude and the velocity of Ca2+ waves. The velocity of IP3R-dependent Ca2+ waves poorly correlated with the amplitude of the Ca2+ response elicited by agonists in all the conditions evaluated, indicating that the velocity depended on the activation state of IP3R, which can be modulated in many ways. J. Cell. Biochem. 112: 3722–3731, 2011. © 2011 Wiley Periodicals, Inc.
Ca2+ is a highly versatile second messenger that plays a key role in the regulation of many cellular processes, including secretion, contraction, proliferation, motility, gene expression, and cell death (Foskett et al., 2007). This versatility resides in the fact that different signals can be encoded spatio-temporally by varying the frequency and amplitude of the Ca2+ response (Berridge et al., 2000). A typical example of an organized Ca2+ signal is a Ca2+ wave initiated in a given area of a cell that propagates throughout the entire cell or within a specific subcellular region (Thomas et al., 1996; Dupont et al., 2007). Ca2+ waves have been observed in a wide variety of eukaryotic cell types, and their velocity generally ranges from 1 to 35 µm/s (Jaffe, 2010). Cells use both extracellular and intracellular Ca2+ pools to modulate the intracellular Ca2+ concentration. In non-excitable cells, the inositol 1,4,5-trisphosphate receptor (IP3R) is responsible for the release of Ca2+ from the endoplasmic reticulum, the main intracellular Ca2+ store by which the concentration of cytosolic Ca2+ is modulated (Clapham, 1995). Three IP3R subtypes have been identified to date (IP3R-1, IP3R-2, and IP3R-3). They associate into tetramers to form functional Ca2+ selective ligand-gated cation channels (Foskett et al., 2007). IP3R is activated by signaling cascades that generate IP3. Briefly, an extracellular agonist binds to its specific receptor, which activates phospholipase C (PLC) via a G-protein or tyrosine kinase. PLC then catalyzes the cleavage of phosphatidylinositol-4,5-bisphosphate into diacylglycerol and IP3, which diffuses into the cytosol and activates IP3R, its receptor/channel (Berridge et al., 2003). When it is released from the endoplasmic reticulum, Ca2+ is rapidly buffered by a number of cytosolic proteins. As such, Ca2+ wave propagation cannot be due to simple Ca2+ diffusion (Allbritton et al., 1992). IP3 has a higher diffusion coefficient than Ca2+ that has been evaluated at 283 µm2/s in Xenopus oocytes (Allbritton et al., 1992). However, simple IP3 diffusion is not sufficient to permit the propagation of a Ca2+ wave and would require positive feedback to occur (Rooney and Thomas, 1993). Ca2+ can participate in this positive feedback by regulating IP3R activity in a biphasic manner. This regulation confers some Ca2+-induced Ca2+ release (CICR) properties on IP3R, allowing the successive activation of IP3R clusters by Ca2+ released from clusters in close proximity in the presence of a minimal concentration of IP3 (Berridge, 1997; Dupont et al., 2007). IP3R activity can also be directly modulated in other ways, including via interacting molecules, proteins, and kinases (Choe and Ehrlich, 2006). PKA, PKC, and mTOR have been shown to affect IP3R activity (Ferris et al., 1991; Hajnóczky et al., 1993; Matter et al., 1993; Cameron et al., 1995; Wojcikiewicz and Luo, 1998; Giovannucci et al., 2000; Poirier et al., 2001; Wagner et al., 2003; Soulsby and Wojcikiewicz, 2005; Arguin et al., 2007; Caron et al., 2007; Chaloux et al., 2007; Regimbald-Dumas et al., 2007, 2011; Betzenhauser et al., 2009; Frégeau et al., 2011).
The endothelium is no longer seen as a passive inner lining of blood vessels, but rather as a multifunctional organ that is actively involved in vital functions of the cardiovascular system, including the modulation of arterial pressure and the maintenance of blood flow (Tran and Watanabe, 2006). As in other tissues, Ca2+ plays an important role in many endothelium functions. In endothelial cells, an IP3R-dependent Ca2+ wave is generated in response to ATP, bradykinin (BK), and thrombin (Isshiki et al., 1998; Isshiki et al., 2002; Béliveau and Guillemette, 2009). Endothelial cells express all three IP3R subtypes and constitute a good model for studying IP3R-dependent Ca2+ wave propagation (Mountian et al., 1999; Laflamme et al., 2002; Grayson et al., 2004; Béliveau and Guillemette, 2009).
While Ca2+ waves have been observed in a wide variety of cell types, little attention has been paid to their velocity. There is a lack of information on how the velocity of Ca2+ waves can be actively modulated and on which elements of the Ca2+ signaling toolkit may modulate it. The velocity of Ca2+ waves is certainly an important aspect of the spatiotemporal distribution of Ca2+ within cells. Intuitively, the velocity of Ca2+ waves should also be an important determinant of the rate of Ca2+ oscillations.
In the present study, we showed that the velocity of IP3R-dependent Ca2+ waves induced by ATP or BK increases with the intensity of the stimulus, that extracellular Ca2+ does not have a significant impact on Ca2+ wave velocity, and more importantly, that endogenous enhancers or inhibitors of IP3R activity can modulate IP3R-dependent Ca2+ wave velocity. Forskolin increased whereas PMA and rapamycin decreased the velocity of IP3R-dependent Ca2+ waves. Our results also suggested that the velocity of IP3R-dependent Ca2+ waves is not directly correlated with the amplitude of the Ca2+ response elicited by an agonist but, rather, that the velocity of IP3R-dependent Ca2+ waves depends on the sensitivity of IP3R, a state that can be modulated in many ways.
MATERIALS AND METHODS
Materials
Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), and penicillin–streptomycin–glutamine were from Gibco Life Technologies (Gaithersburg, MD). Fura-2/AM and phorbol 12-myristate 13-acetate (PMA) were from Calbiochem (San Diego, CA). Rapamycin was from USBiological (Swampscott, MA). ATP, BK, and forskolin were from Sigma–Aldrich (Oakville, ON, Canada).
Cell Cultures
Bovine aortic endothelial cells (BAECs) were isolated from bovine thoracic aortas and were characterized as previously described (Briand et al., 1999). The cells were maintained in low-glucose DMEM containing 2 mM L-glutamine, 10% FBS, 100 U/ml penicillin, and 100 µg/ml streptomycin at 37°C in a humidified atmosphere containing 5% CO2. They were used between the 5th and 20th passages.
Dynamic Video Imaging of Cytosolic Ca2+
BAEC grown on glass coverslips were washed twice with HBSS and loaded with 0.4 µM fura 2-AM for 30 min at room temperature in the dark. The cells were then washed and bathed in fresh HBSS for 30 min to ensure complete hydrolysis of the fura-2/AM before placing the coverslips in a circular open-bottom chamber mounted on the stage of a Olympus IX71 microscope fitted with a MetaFluor digital imaging and photometry system (Olympus, Markham, ON, Canada). Fluorescence from isolated fura-2-loaded cells was monitored by videomicroscopy using 334 nm and 380 nm excitatory wavelengths, and emitted fluorescence was recorded at 510 nm. All experiments were performed at room temperature. The data are expressed as the intracellular free Ca2+ concentration (nM) calculated from the 334/380 fluorescence ratio according to Grynkiewicz et al. (1985). Under some conditions, cells were pretreated for 3 min with 10 µM forskolin, 5 min with 2 µM PMA, or 5 min with 10 µM rapamycin.
Data Analysis
All experiments were performed at least three times. Results are expressed as means ± standard deviations (SD). When needed, the data were analyzed using an analysis of variance, and pairwise comparisons were performed using Dunnet's test. In all cases, results were considered statistically significant when P < 0.05 (*).
Scatter plot analyses and the Pearson's correlation test were used to analyze the relationship between the amplitudes of the Ca2+ signals and the velocities of the Ca2+ waves. A Pearson's correlation coefficient (r) higher than 0.8 indicates a strong direct correlation, an r between 0.5 and 0.8 indicates a moderate correlation, an r between 0.3 and 0.5 indicates a weak correlation, an r between 0.1 and 0.3 indicates a poor correlation, and an r below 0.1 indicates no correlation.
RESULTS
Velocities of ATP-Induced Ca2+ Waves in Single BAECs
ATP is a Ca2+-mobilizing hormone that activates a functional purinergic receptor on BAECs. We used a videomicroscopic system to monitor the fluorescence of fura-2 in real-time in single cells stimulated with ATP. Figure 1 shows typical Ca2+ responses of two BAECs (A,A′) stimulated with 200 nM ATP. Pseudocolored images taken at different times showed that the Ca2+ responses are organized as Ca2+ waves that propagated gradually from a focal point near the edges of the cells through the cytosol to the opposite side of the cells. The experiment was performed in a nominally Ca2+-free extracellular medium, indicating that the Ca2+ response was exclusively due to intracellular Ca2+ release via IP3R. The intracellular Ca2+ concentration was monitored in three different regions in each cell along the axis of propagation of the wave (white squares in Fig. 1B,B′) in order to measure the velocities of the Ca2+ waves. The first region was located near the initiation site of the wave, the second in the perinuclear region (midway between regions 1 and 3), and the third in region 3 where the wave ended. In cell A, the distance between regions 1 and 2 (d1) was 19.8 µm, and the distance between regions 2 and 3 (d2) was 19.9 µm. Within a single cell, peak amplitude variations of ∼150 nM Ca2+ were frequently observed among the different regions. Figure 1C shows that the peak amplitude of the intracellular Ca2+ response was different in the three regions in cell A. Figure 1C′ also shows different peak amplitudes in the different regions of cell A′. While not true for all cells, we generally observed the highest Ca2+ amplitude in the perinuclear region and the lowest in the region where the wave ended (as seen in cell A′). The average peak amplitude measured in all the BAECs (146 cells) stimulated with 200 nM ATP was 319 ± 62 nM Ca2+. Figure 1D shows a time-scale expansion of the results shown in Figure 1C, except that the intracellular Ca2+ levels are expressed as percentages of the maximal Ca2+ amplitude in each region. This conversion was done to facilitate the comparison of results obtained in the different regions. To determine the exact time at which a Ca2+ wave reached a selected region, we chose an arbitrary amplitude set at 50% of the maximal amplitude in this region. For the wave shown in Figure 1A, the time needed to cross-distance d1 (Δt1) was 1.00 s and the time needed to cross-distance d2 (Δt2) was 1.17 s. The wave velocity between regions 1 and 2 was thus 19.9 µm/s, while it was 16.9 µm/s between regions 2 and 3 (Fig. 1E). While slightly slower in the second portion of the cell, the velocity remained relatively constant from beginning to end. To further verify whether the wave velocity remained constant across the entire cell, we measured it between five other regions (along the propagation axis) in cell A. The average wave velocity was 18.8 ± 2.0 µm/s (data not shown). Figure 1C′–E′ shows that the wave velocity was not identical from cell to cell. The same approach was used to measure Ca2+ wave velocity in cell A′ (Fig. 1E′). As with cell A, the wave velocity in cell A′ remained relatively constant. To further verify whether the wave velocity remained constant across the entire cell, we measured it between five other regions (along the propagation axis) in cell A′. The average velocity was 50.9 ± 2.5 µm/s (data not shown). This was approximately 2.5 times higher than in cell A, indicating that, in cells of similar size, the same concentration of ATP can induce intracellular Ca2+ waves that propagate at significantly different velocities. Ca2+ wave velocities must thus be measured in a large number of cells to obtain results that are representative of a cell population tested under a given experimental condition. In subsequent experiments, Ca2+ amplitudes and wave velocities were measured in at least 40 cells. Similar results were obtained when individual cells were stimulated with 10 nM BK, another Ca2+-mobilizing hormone that activates a functional receptor on BAECs (data not shown).
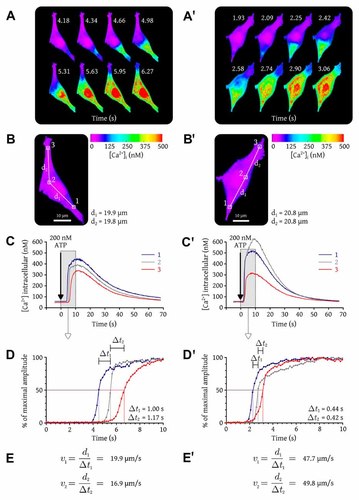
Measurement of intracellular Ca2+ wave velocity. BAECs were loaded with fura-2/AM and were imaged using an Olympus IX71 microscope (100× oil immersion objective) coupled to a MetaFluor imaging system. A,A′: Serial pseudocolored images taken at specific times of two selected cells stimulated with 200 nM ATP (at 0 s) in the absence of extracellular Ca2+. B,B′: Three discrete regions delimited by the white squares were selected in each cell to monitor fura-2 fluorescence in real time. C,C′: Real-time free Ca2+ concentrations in the selected regions identified in B,B′. D,D′: Scale time expansion of the early Ca2+ response (delimited by the shaded rectangle) in the selected regions in cells A,A′. The time needed for the Ca2+ wave to cross-distances d1 and d2 is indicated respectively by Δt1 and Δt2. E,E′: Calculation of the intracellular Ca2+ wave velocities measured for distances d1 and d2 in each cell. These results are representative of experiments performed with 24 BAEC-coated coverslips in three independent experiments.
Modulation of ATP- and BK-Induced Ca2+ Responses by Forskolin, Rapamycin, and PMA
The intensity of the intracellular Ca2+ signal depends on the concentration of ATP, BK, or other Ca2+-mobilizing hormone used to generate IP3. Kinases such as PKA, PKC, and mTOR can modulate IP3R-induced Ca2+ release activity and, as such, the intensity of the Ca2+ signal. However, the relationship between the intensity of the Ca2+ signal and the velocity of the Ca2+ wave is not clear. Figure 2A shows that, in the absence of extracellular Ca2+, 200 nM ATP produced a Ca2+ response with a peak amplitude of 280 ± 64 nM. Interestingly, 2 mM Ca2+ in the extracellular medium did not significantly affect the magnitude of the Ca2+ response elicited by 200 nM ATP (peak amplitude of 319 ± 62 nM). In the absence of extracellular Ca2+, a near-maximal concentration of ATP (1 µM) produced a Ca2+ response with a peak amplitude of 593 ± 50 nM. Forskolin (10 µM), a PKA activator, significantly increased the peak amplitude of the Ca2+ response elicited by 200 nM ATP to 400 ± 51 nM, while 10 µM rapamycin (mTOR inhibitor) and 2 µM PMA (PKC activator) significantly decreased the peak amplitude of the Ca2+ response produced by 1 µM ATP to 481 ± 47 nM and 338 ± 22 nM, respectively.
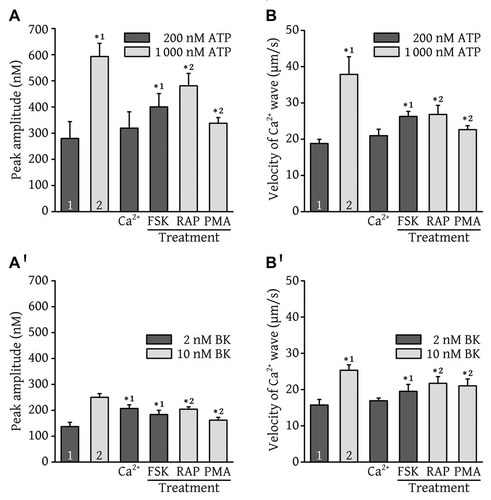
Modulation of intracellular Ca2+ wave velocity. BAECs were loaded with fura-2/AM and were stimulated with 200 nM ATP (panels A and B, black columns), 1 µM ATP (panels A and B, white columns), 2 nM BK (panels A′,B′, black columns), or 10 nM BK (panels A′,B′, white columns) in a nominally Ca2+-free extracellular medium (except for the condition (Ca2+) in which the extracellular medium contained 2 mM Ca2+). The intracellular Ca2+ concentrations of the cells were monitored using the MetaFluor imaging system as described in the legend of Figure 1. The average Ca2+ peak amplitudes and Ca2+ wave velocities measured under control conditions or following a pretreatment with 10 µM forskolin (FSK), 10 µM rapamycin (RAP), or 2 µM PMA, are shown. These results are expressed as the means ± SDs of three different experiments, each conducted with at least 40 cells. In panels A and B, * indicates that the results are significantly different (P < 0.05) from those obtained when the cells were stimulated with 200 nM ATP (*1) or 1 µM ATP (*2). In panels A′,B′, * indicates that the results are significantly different (P < 0.05) from those obtained when the cells were stimulated with 2 nM BK (*1) or 10 nM BK (*2).
Ca2+ wave velocity was measured in parallel with peak amplitude in the same ATP-stimulated BAECs. Figure 2B shows that, in the absence of extracellular Ca2+, 200 nM ATP induced Ca2+ waves that propagated with an average velocity of 18.8 ± 1.1 µm/s. The presence of extracellular Ca2+ did not significantly affect the average wave velocity (21.0 ± 1.8 µm/s). In the absence of extracellular Ca2+, a near-maximal concentration of ATP (1 µM) significantly increased the average wave velocity to 37.9 ± 4.9 µm/s. Forskolin (10 µM) significantly increased the wave velocity obtained with 200 nM ATP to 26.3 ± 1.4 µm/s, while 10 µM rapamycin and 2 µM PMA significantly decreased the wave velocity obtained with 1 µM ATP to 26.8 ± 2.5 µm/s and 22.6 ± 1.1 µm/s, respectively. These results showed that wave velocity increases with increasing concentrations of ATP, and that forskolin, rapamycin, and PMA modulate the Ca2+ wave velocity in the same direction as they modulate the Ca2+ peak amplitude.
Similar experiments were performed with BAECs stimulated with BK. Figure 2A′ shows that, in the absence of extracellular Ca2+, 2 nM BK produced a Ca2+ response with peak amplitude of 137 ± 16 nM. Interestingly, the presence of 2 mM Ca2+ in the extracellular medium significantly increased the magnitude of the Ca2+ response elicited by 2 nM BK (peak amplitude of 207 ± 14 nM), suggesting that BK is a good activator of an efficient Ca2+ entry pathway in BAECs. In the absence of extracellular Ca2+, a near-maximal concentration of BK (10 nM) produced a Ca2+ response with a peak amplitude of 250 ± 14 nM. Forskolin significantly increased the peak amplitude of the Ca2+ response elicited by 2 nM BK to 184 ± 16 nM, while rapamycin and PMA significantly decreased the peak amplitude of the Ca2+ response elicited by 10 nM BK to 204 ± 9 nM and 162 ± 11 nM, respectively.
Ca2+ wave velocity was measured in parallel with peak amplitude in the same BK-stimulated BAECs. Figure 2B′ shows that, in the absence of extracellular Ca2+, 2 nM BK elicited Ca2+ waves that propagated with an average velocity of 15.7 ± 1.5 µm/s. While the presence of 2 mM Ca2+ in the extracellular medium increased the amplitude of the Ca2+ response, it did not significantly modify the velocity of the Ca2+ waves elicited with 2 nM BK (16.9 ± 0.7 µm/s), suggesting that there is no direct correlation between the amplitude of the Ca2+ response and the velocity of the Ca2+ wave. In the absence of extracellular Ca2+, a near-maximal concentration of BK (10 nM) significantly increased the average Ca2+ wave velocity to 25.3 ± 1.5 µm/s. Forskolin (10 µM) increased the Ca2+ wave velocity obtained with 2 nM BK to 19.5 ± 1.9 µm/s, while 10 µM rapamycin and 2 µM PMA significantly decreased the Ca2+ wave velocity obtained with 10 nM BK to 21.7 ± 1.8 µm/s and 21.0 ± 1.9 µm/s, respectively, showing that the Ca2+ wave velocity increased with increasing concentrations of BK, as was observed with ATP. These results further confirmed that forskolin, rapamycin, and PMA modulate the Ca2+ wave velocity in the same direction as they modulate the Ca2+ peak amplitude. However, the significant Ca2+ entry into BK-stimulated cells did not significantly modify the Ca2+ wave velocity.
Relationship Between the Amplitude of the Ca2+ Signal and the Velocity of the Ca2+ Wave
Increasing concentrations of Ca2+-mobilizing agonists increased the intensity (amplitude) of the IP3R-dependent Ca2+ signal and the velocity of the Ca2+ wave, suggesting that these two parameters are directly related. On the other hand, in the presence of extracellular Ca2+, which increases the intensity of the Ca2+ signal elicited by BK, the velocity of the Ca2+ wave was not significantly modified, indicating that there is no direct relation between the amplitude of the Ca2+ signal and the velocity of the Ca2+ wave. To better address this question, we re-evaluated our results using scatter plots and Pearson's correlation test in order to visualize and quantify the relation between the peak amplitude of the Ca2+ signal and the velocity of Ca2+ wave.
Figure 3A shows a scatter plot that graphically locates each cell that generated a Ca2+ wave in response to 2 nM BK (cells that did not respond or that responded so weakly that a Ca2+ wave could not be detected were not included) based on their peak amplitude and wave velocity. The graph is divided into quadrants delimited by the median value of the wave velocity and the median value of the peak amplitude. The upper left quadrant (UL) groups together cells that responded with a high peak amplitude and a slow wave velocity, the upper right quadrant (UR) groups together cells that responded with a high peak amplitude and a fast wave velocity, the lower right quadrant (LR) groups together cells that responded with a low peak amplitude and a fast wave velocity, and the lower left quadrant (LR) groups together cells that responded with a low peak amplitude and a slow wave velocity. In the absence of extracellular Ca2+, 28% of the cells stimulated with 2 nM BK were in the UL quadrant, 22% in the UR quadrant, 28% in the LR quadrant, and 22% in the LL quadrant. In addition, Pearson's correlation test indicated that there is a poor correlation between peak amplitude and wave velocity (r = 0.157, n = 182, P < 0.05).
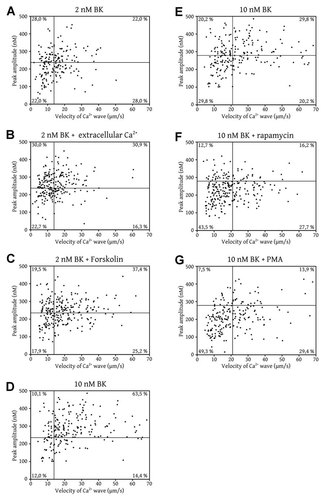
Weak correlation between the Ca2+ wave velocity and the peak amplitude of the Ca2+ response. Panel A: Scatter plot where BAECs stimulated with 2 nM BK in the absence of extracellular Ca2+ were located based on their peak Ca2+ response and Ca2+ wave velocity. The lines represent the median values for both parameters. Panel B: BAECs were stimulated with 2 nM BK in the presence of extracellular Ca2+. They were located in the quadrants set in panel A based on their individual responses. Panel C: Following a pretreatment with 10 µM forskolin, the BAECs were stimulated with 2 nM BK in the absence of extracellular Ca2+ and were located in the quadrants set in panel A based on their individual responses. Panel D: BAECs were stimulated with 10 nM BK in the absence of extracellular Ca2+ and were located in the quadrants set in panel A based on their individual responses. Panel E: BAECs stimulated with 10 nM BK in the absence of extracellular Ca2+ were located in the quadrants based on their peak Ca2+ response and Ca2+ wave velocity. The lines represent the median values for both parameters. Panel F: Following a pretreatment with 10 µM rapamycin, the BAECs were stimulated with 10 nM BK in the absence of extracellular Ca2+ and were located in the quadrants set in panel E based on their individual responses. Panel G: Following a pretreatment with 2 µM PMA, the BAECs were stimulated with 10 nM BK in the absence of extracellular Ca2+ and were placed in the quadrants set in panel E based on their individual responses. Each point represents a single cell positioned at its Ca2+ peak amplitude value and Ca2+ wave velocity value following a stimulation with BK. The percentage of cells in each quadrant is indicated on the plots. The cell populations are the same as those in Figure 2.
Using the same quadrants as set in Figure 3A, cells stimulated with 2 nM BK in the presence of extracellular Ca2+, were graphically located. Figure 3B shows that 30% of these cells were in the UL quadrant, 31% in the UR quadrant, 23% in the LR quadrant, and 16% in the LL quadrant. In the presence of extracellular Ca2+, 11% of the cells relocated to the high amplitude quadrants whereas 3% of the cells relocated to the slow wave velocity quadrants. This further suggested that the Ca2+ entry pathway does not directly influence the velocity of the Ca2+ wave. In addition, Pearson's correlation test indicated that there is a poor correlation between peak amplitude and wave velocity (r = 0.247, n = 233, P < 0.05).
Using the same quadrants as set in Figure 3A, cells stimulated with 2 nM BK after a pretreatment with forskolin, were graphically located. Figure 3C shows that 19.5% of these cells were in the UL quadrant, 37.4% in the UR quadrant, 17.9% in the LR quadrant, and 25.2% in the LL quadrant. Following the treatment with forskolin, 6.9% of the cells relocated to the high peak amplitude quadrants whereas 12.6% of the cells relocated to the fast wave velocity quadrants, indicating that forskolin had a stronger potentiating effect on wave velocity than on peak amplitude. In addition, Pearson's correlation test indicated that there is a poor correlation between peak amplitude and wave velocity (r = 0.166, n = 246, P < 0.05).
Using the same quadrants as set in Figure 3A, cells stimulated with a high concentration of BK (10 nM) were graphically located. Figure 3D shows that 10.1% of these cells were in the UL quadrant, 63.5% in the UR quadrant, 14.4% in the LR quadrant, and 12.0% in the LL quadrant. This higher concentration of BK relocated 23.6% of the cells to the high amplitude quadrants and 27.9% to the fast wave velocity quadrants, indicating that both peak amplitude and wave velocity increased when the cells were stimulated with a higher concentration of BK. Once again, Pearson's correlation test indicated that there is a poor correlation between peak amplitude and velocity (r = 0.139, n = 208, P < 0.05).
Figure 3E shows the same data as Figure 3D except that the graph is divided into quadrants delimited by the median values of cells stimulated with 10 nM BK in the absence of extracellular Ca2+. This scatter plot is the reference for the inhibitory effects of rapamycin and PMA. Figure 3E shows that 20.2% of the cells were in the UL quadrant, 29.8% in the UR quadrant, 29.8% in the LR quadrant, and 20.2% in the LL quadrant. Figure 3F shows that, following a pretreatment with rapamycin and a stimulation with 10 nM BK, 12.7% of the cells were in the UL quadrant, 16.2% in the UR quadrant, 27.7% in the LR quadrant and 43.5% in the LL quadrant. Rapamycin caused a relocation of 21.2% of the cells to the low peak amplitude quadrants whereas it relocated only 6.2% of the cells to the slow wave velocity quadrants. While it affected both parameters, mTOR inhibition had a stronger inhibitory effect on Ca2+ peak amplitude than on Ca2+ wave velocity. Once again, Pearson's correlation test indicated that there is a poor correlation between the Ca2+ peak amplitude and the Ca2+ wave velocity (r = 0.241, n = 260, P < 0.05).
Figure 3G shows that 7.5% of the cells stimulated with 10 nM BK following a pretreatment with 10 µM forskolin were in the UL quadrant, 13.9% in the UR quadrant, 29.4% in the LR quadrant, and 49.3% in the LL quadrant. PMA (2 µM) relocated 28.7% of the cells to the low peak amplitude quadrants and 6.8% to the slow wave velocity quadrants. While it affected both parameters, PKC had a stronger inhibitory effect on peak amplitude than on wave velocity. In addition, Pearson's correlation indicated that there is a poor correlation between peak amplitude and wave velocity (r = 0.382, n = 201, P < 0.05).
These findings showed that, under all the conditions tested, the correlation between peak amplitude and wave velocity is relatively poor. The magnitude of the Ca2+ release was thus not directly responsible for the velocity of Ca2+ waves.
DISCUSSION
Intracellular Ca2+ waves have been observed in various cell types in humans and other species. Depending on the cell type and experimental conditions, different propagation velocities have been reported for intracellular Ca2+ waves (Jaffe, 2010). In the present study, we showed that the velocity of a Ca2+ wave is relatively constant in a single BAEC despite significant variations in the Ca2+ peak amplitude in isolated areas of the cell. These results are consistent with those obtained with confluent human endothelial cells, where intracellular Ca2+ waves induced by a wound in the monolayer propagated at a relatively constant velocity from cell to cell while Ca2+ peak amplitudes tended to diminish as the waves got farther away from the initiation site (Sammak et al., 1997). Isshiki et al. (1998) also observed that repetitive stimulations of a single BAEC with ATP induced intracellular Ca2+ waves with the same initiation site and propagation axis, while the velocities of the waves remained relatively constant during their propagation through the cell. The propagation of Ca2+ waves does not rely exclusively on the cytosolic diffusion of Ca2+ since Ca2+ is rapidly buffered by cytosolic proteins after being released from the endoplasmic reticulum (Allbritton et al., 1992). Since IP3 has a diffusion coefficient of 283 µm2/s in Xenopus oocytes, IP3 diffusion is a more plausible mechanism underlying the propagation of Ca2+ waves (Allbritton et al., 1992). However, in and of itself, IP3 diffusion is not sufficient to explain the constant velocity and high Ca2+ levels during the propagation of Ca2+ waves (Rooney and Thomas, 1993), suggesting that the propagation of intracellular Ca2+ waves does not rely exclusively on the diffusion of IP3 and Ca2+ but requires a feed-forward mechanism in order to propagate with a constant velocity. This feed-forward mechanism is likely related to the CICR property of IP3R, which allows the successive activation of IP3R clusters by Ca2+ released from clusters in close proximity (Berridge, 1997; Dupont et al., 2007). A recent study using caged IP3 and Ca2+ buffers clearly demonstrated that IP3 and Ca2+ are both required for IP3R-dependent Ca2+ wave propagation in smooth muscle cells, which provides support for the CICR mechanism (McCarron et al., 2010). In the present study, we investigated how some elements known to modulate the intracellular Ca2+ response can affect the velocity of Ca2+ waves.
We showed that, in the absence of extracellular Ca2+, increasing the concentration of ATP and BK increases the amplitude of the Ca2+ response and the velocity of the Ca2+ wave. A stronger stimulation should increase the production of IP3, which should increase the open probability of IP3R, which in turn should cause the release of more Ca2+ (Foskett et al., 2007). In terms of the CICR mechanism, IP3 and Ca2+ can contribute to generating higher velocity intracellular Ca2+ waves, by increasing the open probability of IP3R.
Typically, in non-excitable cells, Ca2+ signals have two distinct phases: Ca2+ release from the endoplasmic reticulum and Ca2+ influx from the extracellular environment (Putney, 2009). Ca2+ entry might thus contribute to shaping the propagation of intracellular Ca2+ waves. Under our experimental conditions, the presence of extracellular Ca2+ did not significantly modify the amplitude of the Ca2+ response to ATP, indicating that ATP does not efficiently activate the Ca2+ entry pathway in BAECs. However, the presence of extracellular Ca2+ significantly increased the amplitude of the Ca2+ response induced by BK, suggesting that BK is an efficient activator of a Ca2+ entry pathway in BAECs. Sustained Ca2+ entry is important for BK-induced NO production by endothelial cells (Leung et al., 2006). Interestingly, while the presence of extracellular Ca2+ increased the amplitude of the intracellular Ca2+ response to BK, it did not significantly modify the velocity of the Ca2+ wave induced by BK, suggesting that extracellular Ca2+ and the mechanism of Ca2+ entry do not influence the velocity of the Ca2+ wave. They further suggest that the velocity of the Ca2+ wave is not directly related to the amplitude of the Ca2+ response.
We also verified whether the direct modulation of IP3R activity can regulate the velocity of the intracellular Ca2+ wave. PKA phosphorylates the three IP3R isoforms and increases their apparent affinity for IP3 (Ferris et al., 1991; Hajnóczky et al., 1993; Wojcikiewicz and Luo, 1998; Giovannucci et al., 2000; Wagner et al., 2003; Soulsby and Wojcikiewicz, 2005; Chaloux et al., 2007; Regimbald-Dumas et al., 2007; Betzenhauser et al., 2009). Forskolin, an indirect activator of PKA, significantly increased the amplitude of the ATP- and BK-induced Ca2+ responses. Forskolin also significantly increased the velocity of the ATP- and BK-induced Ca2+ waves. While it is well documented that forskolin is an efficient activator of PKA, it remains that the increased cytosolic level of cAMP induced by forskolin may activate other signaling pathways that could contribute to the effect observed. For example, in excitable cells such as cardiomyocytes and pancreatic beta cells, cAMP enhances the Ca2+ response via the activation the guanine nucleotide exchange factor Epac, leading to an increased ryanodine receptor activity in these cells (for review see Gloerich and Bos, 2010). If a similar mechanism also exists in non-excitable cells, forskolin would increase the velocity of Ca2+ waves by increasing the activity state of both the IP3R and the ryanodine receptor, two Ca2+ release channels located on the endoplasmic reticulum.
Conventional PKCs are activated by Ca2+ and diacylglycerol and thus are activated concomitantly with IP3R (Gallegos and Newton, 2008), which they phosphorylate (Matter et al., 1993; Cameron et al., 1995; Poirier et al., 2001; Vermassen et al., 2004; Arguin et al., 2007; Caron et al., 2007). Unlike PKA, the effects of PKC depend on the IP3R isoform phosphorylated. In cells predominantly expressing IP3R-1, PKC increases IP3R activity (Cameron et al., 1995; Poirier et al., 2001), whereas in cells predominantly expressing IP3R-2 or IP3R-3, PKC decreases IP3R activity (Arguin et al., 2007; Caron et al., 2007). PMA, a direct activator of PKC, decreased the amplitude of ATP- and BK-induced Ca2+ responses in BAECs, which express all three IP3R isoforms. PMA also significantly decreased the velocity of ATP- and BK-induced Ca2+ waves. Although PKC may have substrates other than IP3R, and these substrates may directly or indirectly influence intracellular Ca2+ responses, our results nonetheless show that the velocity of Ca2+ waves is modulated under conditions where it is known that PKC modulates the activity state of the IP3R.
mTOR is a kinase that is activated by diverse signaling pathways under the control of growth factors, cellular stresses, and nutrients (Wullschleger et al., 2006). IP3R-dependent Ca2+ mobilization decreases under conditions where mTOR is inhibited (Dargan et al., 2002; MacMillan et al., 2005; MacMillan and McCarron, 2009). We recently showed that mTOR phosphorylates IP3R and increases its apparent affinity for IP3 (Frégeau et al., 2011; Regimbald-Dumas et al., 2011). In the present study, we showed that rapamycin, a selective inhibitor of mTOR, decreased the amplitudes of ATP- and BK-induced Ca2+ responses in BAECs. Rapamycin also significantly decreased the velocity of ATP- and BK-induced Ca2+ waves.
Our results suggested that endogenous kinases known to modulate the activity state of IP3R can modulate the velocity of intracellular Ca2+ waves in BAECs. All the conditions that affected the velocity of the Ca2+ waves also affected the peak amplitudes of the Ca2+ responses. However, it remains to be determined whether the peak amplitude of the Ca2+ response is directly responsible for the velocity of the Ca2+ wave. Some studies using Ca2+ chelators and caged IP3 have shown that Ca2+ released via IP3R acts as a positive feedback for further Ca2+ release and thus plays a critical role in the initiation and propagation of Ca2+ waves (Wang and Thompson, 1995; Dargan and Parker, 2003; Dargan et al., 2004; McCarron et al., 2010). Since the release of some Ca2+ is essential for initiating a Ca2+ wave, intuitively, the release of more Ca2+ should increase the velocity of the Ca2+ wave. However, our results indicated that the correlation between the peak amplitude of the Ca2+ response and the velocity of the Ca2+ wave is weak at best. Under all the conditions tested, some cells responded with a high Ca2+ peak amplitude and generated a slow wave while others responded with a low Ca2+ peak amplitude and generated a fast wave. This indicates that amplitude and velocity are poorly correlated since a more sensitive IP3R population should necessarily release more Ca2+. However, the sensitivity of IP3R is not the only mechanism that can modulate the peak amplitude of Ca2+ responses. Apart from the Ca2+ entry pathway and the activity of IP3Rs, the amplitude of the Ca2+ signal can be modulated by several other components, including cytosolic Ca2+-binding proteins that modulate the spatial and temporal aspects of increases in cytosolic Ca2+ levels (Schwaller, 2010). The activity of Ca2+ pumps and Ca2+ exchangers that displace Ca2+ from the cytosol to the endoplasmic reticulum or extracellular milieu may also contribute to modulating the amplitude of the Ca2+ response (Carafoli et al., 2001; Bobe et al., 2005; Dong et al., 2006). These mechanisms may explain why, in the absence of extracellular Ca2+, the Ca2+ peak amplitude does not depend exclusively on the activity of IP3R.
Our results suggested that the state of activity of IP3R is more important than the magnitude of the Ca2+ response in modulating the velocity of Ca2+ waves in BAECs stimulated with a Ca2+-mobilizing agonist. Our results also showed that the velocity of an intracellular Ca2+ wave in a given cell remains relatively constant during its propagation, despite variations in Ca2+ peak amplitude. Different mechanisms have been proposed to explain the transition of a local Ca2+ increase into a Ca2+ wave. Some investigators have suggested that the Ca2+ level in a particular region of a cell needs to reach a threshold to initiate a Ca2+ wave. Others have suggested that a sufficient frequency of elementary Ca2+ events in a particular region of the cell is required to initiate a Ca2+ wave [for a review, see Dupont et al. (2007)]. Our results with BAECs provide support for these suggestions, in that once the conditions needed to initiate a Ca2+ wave are reached, the resulting wave propagates in the cytosol with a sustained velocity. Moreover, the velocity of the Ca2+ wave can be modulated by setting conditions that increase or decrease the open probability of IP3R. Since the velocity of the Ca2+ wave and the amplitude of the Ca2+ response appear to be independent Ca2+ signals, it remains to be determined which of these Ca2+ signals is associated with which cellular activity.
Acknowledgements
This work was supported by funding from the Canadian Institutes of Health Research and by the Heart and Stroke Foundation of Quebec and is part of the Ph.D. thesis of E.B.




