ADAMTS-4 and ADAMTS-5: Key enzymes in osteoarthritis†
Competing interests: The authors have no conflicts of interest to declare.
Abstract
Osteoarthritis (OA) is a progressive disease of the joints characterized by degradation of articular cartilage. Although disease initiation may be multi-factorial, the cartilage destruction appears to be a result of uncontrolled proteolytic extracellular matrix destruction. A major component of the cartilage extracellular matrix is aggrecan, a proteoglycan that imparts compressive resistance to the tissue. Aggrecanase-mediated aggrecan degradation is a significant event in early stage OA. The relative contribution of individual ADAMTS-4 and ADAMTS-5 proteinases to cartilage destruction during OA has not been resolved completely. This review reveals that both ADAMTS-4/ADAMTS-5 are responsible for aggrecan degradation in a human model of OA, and is expected to list down the rational strategies which are being focussed for therapeutic intervention in OA. J. Cell. Biochem. 112: 3507–3514, 2011. © 2011 Wiley Periodicals, Inc.
Abbreviations:
ADAMTS, a disintegrin and metalloproteinase with thrombospondin motifs; COMP, cartilage oligomeric matrix protein; ECM, extracellular matrix; GAG, glycosaminoglycan; G1 First (N-terminal) globular domain of aggrecan; G2 Second (N-terminal) globular domain of aggrecan; IGD, interglobular domain; MMP, matrix metalloproteinase; OA, osteoarthritis; TIMP, tissue inhibitor of matrix metalloproteinase; TS, thrombospondin.
More than 15% of the world population older than 18 years are affected by arthritic disorders, including osteoarthritis (OA) and rheumatoid arthritis (RA) [Kevorkian et al., 2004]. The aetiology of OA is still not completely understood, but it appears that the mechanical, biochemical, and enzymatic factors, all together result in triggering the OA. A better approach to understand the factors that contributes to the disease and disability in OA is therefore of high priority in current perspective. Moreover, in OA there is still a lack of disease-modifying treatment options. It has been shown that the final common pathway of all these factors is the failure of the chondrocytes to maintain a homeostatic balance between matrix synthesis and degradation [Martel-Pelletier et al., 2001; Klimiuk et al., 2004]. Accumulating evidences suggest that proteinases perform an important function in OA and RA [Salzet, 2002] by degrading two major structural components of cartilage extracellular matrix, the proteoglycan aggrecan, and type II collagen [Clark and Parker, 2003].
Aggrecan is one of the first matrix components to undergo measurable loss that ultimately leads to a loss of cartilage function. Therefore, it is considered to be a crucial initial event in the development of arthritis, which is followed by essentially irreversible collagen degradation [Mankin and Lippiello, 1970; Hollander et al., 1995]. Aggrecan depletion in osteoarthritic cartilage can be ascribed to increased proteolytic cleavage of the core protein and recent studies reveal that aggrecanases are the principal proteinases responsible for aggrecan degradation in situ in articular cartilage and hence aggrecanases appear as attractive targets for therapeutic intervention in successful treatment of OA.
AGGRECAN: SUBSTRATE OF AGGRECANASES
Aggrecan is a glycoprotein that consists of a protein backbone of 210–250 kDa. The Aggrecan core protein folds into three globular domains: G1, G2, and G3 (see Fig. 1). The first two N-terminal domains G1 and G2 are connected by a short 128–amino acid polypeptide referred to as the aggrecan interglobular domain (IGD), while the second G2 and the third G3 domain at C-terminal are separated by a 1491–amino acid sequence carrying a great number of sulfated glycosaminoglycan chains, also known as GAG region [Doege et al., 1991; Watanabe et al., 1998; Abbaszade et al., 1999; Porter et al., 2005]. In general, glycosaminoglycans belong to the macromolecules family and are highly present in the connective tissues. Due to the negative charges which they deliver, these elements have the properties to fix a large quantity of water molecules onto the cartilage and thus ensure tissue viscoelasticity; and hence they are essential for the proper functioning of the locomotive system. Glycosaminoglycans have also become a promising therapeutic means to protect the cartilage and slow down the tissue degradation process.
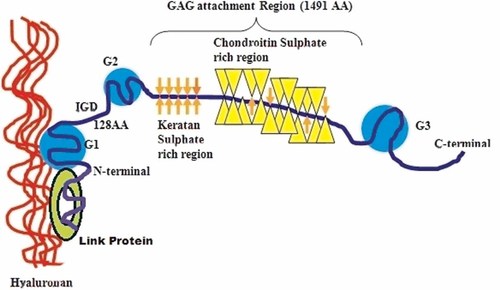
A diagrammatic representation of aggrecan and its known cleavage sites.
Hyaluronan is also reported to be present in cartilage. Hyaluronan is an anionic and only non-sulfated glycosaminoglycan known so far. It is present as a coat around each cell (chondrocytes). The molecular weight of hyaluronan in cartilage decreases with age but its quantity keeps increasing. Individual aggrecan monomers interact with hyaluronic acid to form an aggregate of very high molecular weight. This interaction involves a G1 domain being stabilized by a short protein, called link protein, which interacts with both hyaluronic acid and G1.
In cartilage, aggrecan forms aggregates with link protein and hyaluronan. The aggregates, with their high negative charge, attract water molecules, and endow cartilage with resistibility to compression and deformation. Therefore, aggregate formation is of much significance as it ensures the retention of aggrecan within the collagen network. It has been observed that the major cleavage sites that cause aggrecan depletion from cartilage are in the IGD domain. This is the reason that aggrecan which lacks in the G1 domain, is free to exit the matrix and does not contribute to cartilage function [Sandy et al., 1991]. In IGD domain, two major proteolytic cleavage sites have been identified: one at Asn341–Phe342, at which all Matrix metalloproteinase's (MMPs) present in cartilage primarily act, and the other at Glu373–Ala374. The later one is an aggrecanase cleavage site [Sandy et al., 1992; Lohmander et al., 1993] at which several members of ADAMTS family of metalloproteinase's act [Abbaszade et al., 1999; Tortorella et al., 1999; Kuno et al., 2000; Rodríguez-Manzaneque et al., 2002; Somerville et al., 2003; Collins-Racie et al., 2004; Yamaji et al., 2004]. There are also additional aggrecanase cleavage sites but these occur within the GAG-attachment region between the G2 and G3 globular domains: Glu1480–Gly1481, Glu1667–Gly1668, Glu1771–Ala1772, and Glu1871–Leu1872 [Sugimoto et al., 1999; Tortorella et al., 2000a]. The study of such proteinases along with their cleavage pattern is therefore of great interest from both a pathophysiological and a therapeutic standpoint.
AGGRECANASES
The aggrecanases are the members of the ADAMTS family which are the disintegrin and metalloprotease with thrombospondin (TS) motifs and consist of secreted zinc metalloproteinase with a precisely ordered modular organization. This includes at least one TS type I repeat [Nagase and Kashiwagi, 2003; Apte, 2004; Porter et al., 2005]. Aggrecanases are the main proteinases responsible for aggrecan cleavage in the early events of cartilage remodeling. The MMPs start participating in this process during development of the disease and continue with the degradation of collagen [Nagase and Kashiwagi, 2003]. The aggrecanases activity is therefore considered as a hallmark of cartilage degradation during inflammatory joint diseases like OA.
There are several members of the ADAMTS family which are known to influence development, angiogenesis, coagulation, and progression of arthritis but out of 19 members of ADAMTS family, the following 5 members viz., ADAMTS-1, ADAMTS-4, ADAMTS-5, ADAMTS-8, and ADAMTS-9 have shown to degrade the cartilage proteoglycan–aggrecan in arthritis [Tortorella et al., 1999; Kuno et al., 2000; Tortorella et al., 2000a; Kuno et al., 2004] but with high enzyme-substrate ratio so that they have very weak activity [Porter et al., 2005; Zeng et al., 2006]. It was also shown that ADAMTS-1 null mice are not protected from experimental arthritis [Little et al., 2005] and cleavage by ADAMTS-8 and ADAMTS-9 is highly inefficient.
In the cartilage, two different aggrecanases, i.e., aggrecanase-1 (ADAMTS-4) and aggrecanase-2 (ADAMTS-5) have been isolated. These two proteinases cleave the aggrecan core protein at the aggrecanase-specific Glu373- Ala374 bond in the IGD region [Abbaszade et al., 1999; Tortorella et al., 1999] and thus these proteinases have received most attention in the pathology of arthritic joint diseases, because they are the most efficient aggrecanases in vitro [Kashiwagi et al., 2004; Gendron et al., 2007]. A tight regulation of these aggrecanases activity is critical for maintaining a fine balance between aggrecan anabolism and catabolism. In normal human body, a control mechanism for aggrecan catabolism may involve endogenous inhibitors like, tissue inhibitor of matrix metalloproteinase (TIMP-3) of the aggrecanases but in diseases such as OA, the balance between TIMP-3 and ADAMTS-4 synthesis is disturbed in favor of catabolism. This disturbance could be attributed to de novo synthesis of ADAMTS-4 [Curtis et al., 2002; Malfait et al., 2002] and/or post-translational activation of ADAMTS-4 and ADAMTS-5 [Yamanishi et al., 2002; Pratta et al., 2003].
Several experiments using si-RNA approach [Song et al., 2007], neutralizing antibodies to ADAMTS-4 in pig cartilage [Powell et al., 2007] and immunoprecipitation with anti-ADAMTS-4 and anti-ADAMTS-5 antibodies in bovine cartilage [Tortorella et al., 2001] have demonstrated that ADAMTS-4 plays a significant role in vitro aggrecanolysis. On the other hand, it was revealed that the mice deficient in ADAMTS-5 (but not ADAMTS-4) are protected from early aggrecan loss and cartilage erosion in inflammatory and non-inflammatory models of arthritis [Glasson et al., 2005; Stanton et al., 2005] making recombinant human ADAMTS-5 substantially more active than ADAMTS-4 [Gendron et al., 2007]. But which aggrecanase-1 (ADAMTS-4) or aggrecanase-2 (ADAMTS-5) is mainly responsible for aggrecan degradation during human articular cartilage destruction in vivo, however, remains debatable up to date. Therefore, it is still not clear which aggrecanase plays the main role in pathological cartilage degradation in humans. To identify the exact situation is a marathon task because the final level of enzyme activity is influenced by multiple, independent, and interacting molecular processes, including promoter activity, epigenetic modifications, regulation by non-coding RNA (ncRNA) including microRNA (miRNA) and alternative splicing.
Therefore in order to gain potential drug targets in OA, one should seek synthetic inhibitors for both ADAMTS-4 and ADAMTS-5, as experimental findings indicates that both ADAMTS-4 and ADAMTS-5 contribute to the structural damage of cartilage that characterizes human OA.
DIFFRENCES BETWEEN ADAMTS-4 AND ADAMTS-5
Both aggrecanases (ADAMTS-4 AND ADAMTS-5) have a similar domain arrangement, consisting of a pro-domain, a catalytic metalloproteinase domain, a disintegrin-like (Dis) domain, a cysteine-rich (CysR) domain, and a spacer (Sp) domain. In addition, ADAMTS-5 contains an additional TS domain after the Sp domain (see Fig. 2).
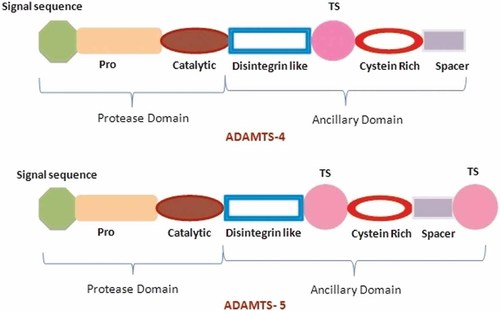
A diagrammatic representation of ADAMTS showing prometalloprotease and ancillary domains. The ancillary domain is further made up of disintegrin-like module, a central TSR (thrombospondin repeat), a cysteine-rich module, a cysteine-free spacer, and a variable number of additional TSRs, 0 (in ADAMTS-4) and 1 (in ADAMTS-5).
Both ADAMTS-4 and ADAMTS-5 proteases are synthesized as zymogens. In order to produce the active and mature enzymes, the prodomains of ADAMTS-4 and ADAMTS-5 containing furin recognition sequences are cleaved constitutively by proprotein convertases prior to their secretion. The catalytic domains of ADAMTS-4 and ADAMTS-5 contain the consensus HEBxHxBGBxH catalytic motif and “met-turn” characteristic of the metzincin family of metalloenzymes. Their enzymatic specificity is heavily influenced by their ancillary domain as schematically shown in Figure 2. This ancillary domain plays a critical role in directing these enzymes to their substrates, the cell surface and the extracellular matrix, respectively.
The CysR and Sp domains of ADAMTS-4 are necessary for the enzymes' interactions with the GAG chains of aggrecan [Flannery et al., 2002]. The TS domain of ADAMTS-4 is critical for aggrecan recognition and cleavage [Tortorella et al., 2000b] and the C-terminal domains of ADAMTS-4 and ADAMTS-5 govern the specificity of the enzymes by modulating substrate binding [Fushimi et al., 2008]. Fushimi et al. used chimeric enzymes in which the catalytic domains of ADAMTS-4 and ADAMTS-5 were exchanged with each other. They found that the catalytic domain of ADAMTS-5 is fourfold more active than the ADAMTS-4 in the IGD region and 2.5-fold in the CS-2 region of aggrecan [Fushimi et al., 2008].
It has also been reported that the cleavage by ADAMTS-4, but not ADAMTS-5, in the IGD was affected by the age of animal and human cartilage [Roughley et al., 2003]. This cleavage may be due to the glycosylation pattern of aggrecan which varies with age [Pratta et al., 2000; Miwa et al., 2006]
Moreover, it has been shown that the ADAMTS-4 can cleave other chondroitin sulfate proteoglycans, including brevican and versican (Table I) after Glu393 and Glu441, respectively [Matthews et al., 2000; Gao et al., 2002]. ADAMTS-4 also cleaves cartilage oligomeric matrix protein (COMP) as well as fibromodulin and decorin, indicating that the proteolytic spectrum of this subgroup of proteinases is not restricted to the cleavage of proteoglycans only as in case of ADAMTS-5[Liu et al., 2006; Apte, 2009].
| Gene name | Protein name | Alternative names | Chromosome location | Known substrates |
|---|---|---|---|---|
| ADAMTS-4 | ADAMTS-4 | aggrecanase-1 | 1q23 | Aggrecan, brevican, versicanV1, fibromodulin, decorin, carboxymethylated transferrin and COMP |
| ADAMTS-5 | ADAMTS-5 | aggrecanase-2 ADAMTS-11 | 21q21 | Aggrecan, brevican and α2-macroglobulin |
REGULATION OF ADAMTS-4 AND ADAMTS-5 EXPRESSION
It has been studied that ADAMTS-4 mRNA was induced by catabolic cytokines such as interleukin-1β (IL-1β) or tumor necrosis factor-α (TNF-α) in human cartilage and chondrocytes but ADAMTS-5 mRNA was not regulated by cytokines and it expressed constitutively [Koshy et al., 2002; Pratta et al., 2003]. Also, the ADAMTS-4 was up regulated upon the influence of transforming growth factor-β (TGF-β) in articular cartilage at the mRNA level [Yamanishi et al., 2002]. Furthermore an exposure to TNF-α or IL-1β blocking agents reduces only the activity of ADAMTS-4 [Bondeson et al., 2006]. This finding (from in vitro studies) deviates from the animal studies which show that ADAMTS-5 mRNA expression is up regulated by catabolic cytokines [Fosang et al., 2008].
An interesting insight has come up also from animal models. ADAMTS-4 and ADAMTS-5 knockout mice expressed no major phenotypical abnormalities, but showed a significant reduction in the severity of surgically induced OA in the ADAMTS-5 in contrast to the ADAMTS-4 knockout groups [Glasson et al., 2004; Glasson et al., 2005; Stanton et al., 2005].
Another relevant point to note concerns the functional sites of the aggrecanases in an organism. Aggrecanase-1 (ADAMTS-4) transcript levels are by far more abundant and ubiquitous in adult tissues. Its expression is particularly prominent in lung, heart, and brain. ADAMTS-5 transcripts are expressed to a significant level only in placenta due to which it is also called by implantin [Hurskainen et al., 1999]. The thyroid hormone tri-iodothyronine (T3) is also known to up-regulate the mRNA expression of ADAMTS-5 with subsequent aggrecan degradation, in growth plate cartilage during endochondral ossification [Makihira et al., 2003]. The functional differences between ADAMTS-4 and ADAMTS-5 are tabulated in Table II.
| Function | ADAMTS-4 | ADAMTS-5 |
|---|---|---|
| Localization in the matrix | By Spacer domain | By Cys-R domain |
| Proteolytic activity | By carboxyl-terminal Cys-R and Sp domains | By second TS domain and little by catalytic domain alone |
| Known substrates | Aggrecan, brevican, versican V1, fibromodulin, decorin, carboxymethylated transferring, and COMP | Aggrecan, brevican, and α2-macroglobulin |
| Optimum NaCl Conc for aggrecan degradation | 12.5–50 mM NaCl | 200 mM NaCl |
| Optimal activity at pH | 7.0–9.5 | 7.0–9.5 |
| Strength of Proteolytic activities | 1X | 1,000-fold greater |
| IL-1 and TNF-α response | mRNA up-regulated | Constitutively expressed |
Moreover ADAMTS-5 activity is reduced by C-terminal processing whereas ADAMTS-4 activity is maintained or increased. From Table II, it is concluded that ADAMTS-4 and ADAMTS-5 are in contrast to each other.
THERAPEUTIC INTERVENTION
In OA, pain, restricted movement, and joint instability often result in the need for total joint replacement. Current therapies alleviate the mild to moderate pain and inflammation associated with OA, but do not protect the cartilage from further damage and have not demonstrated an effect on disease progression [Felson and Kim, 2007]. Therefore, therapeutics that stop or slow down the degradation of joint structure and function, will address a major need of present scenario. Therapies aimed at preventing aggrecan loss by inhibiting aggrecanase activity could slowdown the progressive cartilage erosion and thereby it may delay or even prevent the development of end-stage disease. The recent identification of ADAMTS-4 and ADAMTS-5 as major cartilage aggrecanases in vivo made the aggrecanases inhibitors being much sought targets for therapy of arthritis. There has been increased interest in the design of active-site inhibitors of these enzymes in the recent years. It was found that ADAMTS inhibitors that block the progression of OA might be more successful than inhibitors that block the initial stages [Cooper et al., 2000]. This is because many patients with early stage OA remain stable for long periods of time and some do not progress at all [Massardo et al., 1989; Spector et al., 1992].
Current studies suggest that inhibition of both ADAMTS-4 and ADAMTS-5 may be required to achieve maximum drug efficacy in treating OA. But one should also keep in mind the side-effects which a non-specific ADAMTS inhibitor can cause by inhibiting the non-aggrecanase ADAMTS, which otherwise plays an indispensable physiological role in human body.
With recent elucidation of crystal structures of ADAMTS-4 and ADAMTS-5 [Hörber et al., 2000] at a resolution of 2.8 Å, a starting point for the structure-based design of aggrecanase-specific inhibitors has emerged as a promising aid in the design of the next generation of dual ADAMTS-4 and ADAMTS-5 inhibitors as shown in Table III. By comparing the crystal structures of ADAMTS-5 with that of ADAMTS-5 bounded with broad-spectrum metalloprotease inhibitor marimastat. It was determined that the specificity of the relative inhibitors for ADAMTS-5 was not driven by a specific interaction, such as zinc chelation, hydrogen bonding, or charge interactions, but rather by subtle and indirect factors, such as water bridging, ring rigidity, pocket size, and shape, as well as protein conformation flexibility [Tortorella et al., 2009].
| S. No | Inhibitor compound(s) | Target | Author |
|---|---|---|---|
| 1 | Series of cis-1(S)2(R)-amino-2-indanol | ADAMTS-4 and ADAMTS-5 |
Tortorella et al. [2009 ] |
| 2 | Series of (2R)-N4-hydroxy-2-(3-hydroxybenzyl)-N1-[(1S,2R)-2-hydroxy-,3-dihydro-1H-inden-1-yl]butanediamide derivatives | ADAMTS-4 and ADAMTS-5 |
Yao et al. [2002 ]; Yao et al. [2001 ] |
| 3 | Series of α-glutamic acid scaffold based compounds | ADAMTS-4 and ADAMTS-5 |
Peng et al. [2011 ] |
| 4 | Active achiral N-hydroxyformamide compounds | ADAMTS-4 |
De Savi et al. [2011 ] |
| 5 | (4-keto)-phenoxy)methyl biphenyl-4-sulfonamides | ADAMTS-4 |
Hopper et al. [2009 ] |
| 6 | (1S,2R,3R)-2,3-Dimethyl-2-phenyl-1-sulfamidocyclopropanecarboxylates | ADAMTS-4 and ADAMTS-5 |
Shiozaki et al. [2011 ] |
| 7 | 5-((1H-pyrazol-4-yl)methylene)-2-thioxothiazolidin-4-one derivatives | ADAMTS-4 and ADAMTS-5 |
Gilbert et al. [2007 ] |
| 8 | N-((8-hydroxy-5-substituted-quinolin-7-yl)(phenyl)methyl)-2 phenyloxy/amino-acetamides | ADAMTS-5 |
Gilbert et al. [2008 ] |
From this review, it has also been indicated that the C-terminal domains of ADAMTS-4 and ADAMTS-5 direct the specificity of the enzymes by modulating substrate binding [Curtis et al., 2002]. Agents with ability to block interaction of the C-terminal domains with endogenous substrates have the potential to act as substrate-specific exquisite inhibitors of the aggrecanases.
Another complementary approach to design an inhibitor is to target the exosite interactions of these proteinases with aggrecan. Studies suggest that efficient cleavage of aggrecan depends on multiple interactions with the core protein outside the active site [Mosyak et al., 2008] and with the GAG side chains of aggrecan [Pratta et al., 2000; Tortorella et al., 2000b]. Therefore, any non-proteoglycan substrate lacking the GAG side chains would remain unaffected by inhibitors that target these interactions and provide an additional degree of specificity to such kind of inhibitors.
Till date, several potential disease-modifying agents for OA like glucosamine and chondroitin sulfate, diacerhein, and pentosan polysulfate are already under clinical trials as they are known to inhibit proinflammatory pathways. This, in turn, further leads to down-regulation of ADAMTS enzymes. Also, Glucosamine is known to increase the levels of TIMP-3, the endogenous inhibitor of ADAMTS-4 and ADAMTS-5, possibly through transcriptional and post-transcriptional mechanisms [Chan et al., 2006; Chan et al., 2007].
Another therapeutic intervention suggested by researchers [Pratta et al., 2003] showed that the IL-1 induction of aggrecanase activity is not associated with an increase in ADAMTS-4 generation, but due to the induction of an activator of constitutively produce ADAMTS-4. Thus, the identification of the activator and its inhibitor may provide an alternative target to control aggrecanase-mediated degradation of cartilage aggrecan in arthritic diseases.
One of the major lead in discovering aggrecanase inhibitors comes from Histone deacetylase (HDAC) inhibitors. HDACs potently inhibit IL-1α-induced, or oncostatin M-induced, cartilage degradation. They also suppress the IL-1α-induced, or oncostatin-M-induced, upregulation of ADAMTS-4 and ADAMTS-5 [Young et al., 2005]. Therefore, histone deacetylase inhibitors are also under clinical trials for obtaining the aggrecanase inhibitors. However, these inhibitors may only be considered as potential therapies for OA if their known toxicity on haematopoetic tissue is markedly reduced.
Another approach which could have important implications and complications for therapeutic strategies aimed at inhibiting both aggrecanases is the differential gene regulation of ADAMTS-4 and ADAMTS-5. For example, ADAMTS-4 expression by human synoviocytes is severely inhibited by the TNF-α blocker etanercept and an anti-IL-1β neutralizing antibody, whereas the expression of ADAMTS-5 in the same cells is not affected by neutralization either of IL-1β or TNF-α alone or in combination [Yamanishi et al., 2002].
CONCLUSION
Considerable advances have now been made to further our understanding of the molecular mechanisms contributing to aggrecanase-mediated cartilage catabolism and a number of tools and insights have been developed to aid in the design of inhibitors of these proteases.
But still we have to search upon the potential consequences of aggrecanase inhibition and seek out what other ADAMTS protein cleave aggrecan in vivo and which mediators are involved in up regulating the aggrecanase activity in human arthritis. Also, the activity of these proteases modulation: via transcriptional regulation, post-translational processing or other mechanisms should be looked at before reaching any consensus. Research on these gray areas is critical for the development of aggrecanase inhibitors as therapeutics in arthritic diseases. It may be concluded that the successful OA therapies would require drugs that will inhibit collagenases and aggrecanases and prevent the bone metabolism and cartilage breakdown by arresting the structural damage and hence reducing the pain associated with this debilitating disease. Considerable pre-clinical and clinical researches therefore remain to be undertaken in this area.




